Abstract
We examined the early effects of infection by CCR5-using (R5 human immunodeficiency virus [HIV]) and CXCR4-using (X4 HIV) strains of HIV type 1 (HIV-1) on chemokine production by primary human monocyte-derived macrophages (MDM). While R5 HIV, but not X4 HIV, replicated in MDM, we found that the production of the C-X-C chemokine growth-regulated oncogene alpha (GRO-α) was markedly stimulated by X4 HIV and, to a much lesser extent, by R5 HIV. HIV-1 gp120 engagement of CXCR4 initiated the stimulation of GRO-α production, an effect blocked by antibodies to CXCR4. GRO-α then fed back and stimulated HIV-1 replication in both MDM and lymphocytes, and antibodies that neutralize GRO-α or CXCR2 (the receptor for GRO-α) markedly reduced viral replication in MDM and peripheral blood mononuclear cells. Therefore, activation of MDM by HIV-1 gp120 engagement of CXCR4 initiates an autocrine-paracrine loop that may be important in disease progression after the emergence of X4 HIV.
Isolates of human immunodeficiency virus type 1 (HIV-1) are classified according to their ability to infect cells bearing the chemokine receptors CCR5 and CXCR4 (37). CCR5-using isolates of HIV-1 (R5 HIV) are transmitted most frequently and predominate during the asymptomatic stages of infection with HIV-1 (52, 63). R5 HIV can infect monocyte-derived macrophages (MDM), as well as CD4+ T lymphocytes (2, 11, 17, 19, 20). MDM serve as a reservoir for HIV-1, since they are relatively resistant to the cytopathic effects of HIV-1 and live for weeks, and perhaps longer, despite infection (24, 27). CXCR4-using strains of HIV-1 (X4 HIV) are associated with disease progression, a decline in peripheral CD4+ T-lymphocyte levels, and the onset of clinical symptoms of AIDS (16). X4 HIV efficiently infects T lymphocytes, as well as CXCR4+ T-cell lines, but does not infect MDM under most circumstances (25). Interestingly, MDM do express CXCR4 but seem to exhibit a postentry block to viral replication (43, 50, 54, 58).
Chemokine receptors are seven transmembrane domain-containing G-protein-coupled receptors (GPCR) that transmit signals induced by a family of small, secreted polypeptides collectively known as chemokines. Chemokines attract and activate leukocytes and are divided into subgroups according to the position of the first two cysteine residues (60). C-C chemokines act primarily on mononuclear cells, including MDM, lymphocytes, and eosinophils (60). While initially thought to act principally on neutrophils, some C-X-C chemokines have been shown to attract activated T cells, act as angiogenic regulators, and stimulate monocyte adherence (29, 55, 60). The C-X-C chemokine growth-regulated oncogene alpha (GRO-α), also called melanoma growth stimulatory activity (MGSA), was initially identified as an autocrine growth factor for malignant melanoma cells (49). Subsequent studies have shown that the receptor for GRO-α is CXCR2, and GRO-α attracts cells that express this receptor, including both neutrophils and dendritic cells (1, 4, 44). GRO-α also has been demonstrated to have direct angiogenic activity in several in vivo assays and to activate ORF 74 of the Kaposi's sarcoma-associated herpesvirus (KSHV), a GPCR that has been implicated in the transformation and angiogenic phenotype of Kaposi's sarcoma (KS) lesions (6, 28, 38, 55). GRO-α and the two other GRO chemokines, GRO-β and GRO-γ, are encoded by distinct genes, are 88% identical at the amino acid level, signal through CXCR2, and are thought to be largely functionally redundant (1, 38).
Aberrant function of both infected and uninfected MDM has been implicated in the pathogenesis of HIV dementia and AIDS-associated opportunistic infections (26, 35, 42, 53). MDM infected with HIV-1 are known to produce a host of inflammatory mediators, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6, less than 48 h after infection (8, 12, 31, 42, 47). In addition, macrophages exposed to HIV-1 contribute to the death of T lymphocytes by TNF-α- and FasL-dependent pathways (5, 32). Following infection, macrophages also produce the C-C chemokines RANTES, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β (9, 51). These three chemokines inhibit HIV replication by nature of their ability to ligate the HIV coreceptor CCR5 and prevent HIV entry, both by blocking binding sites and inducing receptor internalization (13, 14, 39, 56, 61). Subsequent reports have demonstrated that RANTES can also stimulate the replication of X4 HIV by activating, and increasing virion attachment to, target cells (18, 30, 34, 57). Similarly, the CXCR4 ligand stromal cell-derived factor 1 (SDF-1) can both prevent X4 HIV entry by inducing receptor internalization and stimulate HIV proviral gene expression (3, 7, 40, 45). While much is known about the roles of RANTES, MIP-1α, and MIP-1β in HIV pathogenesis, relatively little is known about the role of other chemokines produced by HIV-infected leukocytes.
Here we demonstrate a striking increase in the production of the C-X-C chemokine GRO-α following exposure of MDM to HIV-1. Stimulation of GRO-α production by HIV-1 is dependent on gp120 ligation of CXCR4. Further, GRO-α itself stimulates the replication of HIV-1 in both macrophages and lymphocytes, thus creating an autocrine-paracrine loop that may contribute to HIV-1 pathogenesis. Because GRO-α production is more markedly enhanced following encounter with X4 HIV than with R5 HIV, GRO-α may help activate HIV-1 replication following the emergence of CXCR4-using isolates in the late stages of AIDS.
MATERIALS AND METHODS
Reagents.
The following reagents were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): HIV-1BaL from Suzanne Gartner, Mikulas Popovic, and Robert Gallo; pHXB2-env from Kathleen Page and Dan Littman; HIV-193TH975 gp120 from Steve Showalter, Maria Garcia-Moll, and the DAIDS, NIAID; HIV-1CM235 gp120 from Protein Sciences Corporation; HIV-1IIIB and HIV-1MN gp120 from the DAIDS, NIAID and ImmunoDiagnostics, Inc.; and HIV-1 Tat from John Brady.
Recombinant GRO-α, SDF-1α, and RANTES and monoclonal antibodies to CXCR2 (MAb331), GRO (MAb276), and GRO-α (MAb275) were obtained from R&D Systems (Minneapolis, Minn.) and added as indicated in the figure legends. Antibodies capable of blocking HIV binding to CD4 (RPA-T4), CCR5 (2D7), and CXCR4 (12G5) were added as indicated (No Azide/Low Endotoxin Format; PharMingen, San Diego, Calif.). Lipopolysaccharide (LPS) from Escherichia coli strain O55:B5, E-TOXATE reagents, and all other chemicals were obtained from Sigma (St. Louis, Mo.).
Isolation and preparation of PBMC, PBL, and PBM.
Peripheral blood mononuclear cells (PBMC) were collected by venipuncture of healthy volunteers as described previously (36). Cellular proliferation, viability, and activation were assayed by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)-based colorimetric assay according to the manufacturer's instructions (Boehringer Mannheim, Mannheim, Germany) and as described previously (36). PBMC contained approximately 20% CD14+ monocytes as determined by flow cytometry (data not shown). In order to separate the PBMC into subpopulations composed mainly of monocytes or lymphocytes, PBMC were subjected to a plate adherence step for 2 h. Adherent cells were consistently >90% peripheral blood monocytes (PBM) as determined by Diff-Quik analysis and >85% CD14+ by flow cytometric staining with a phycoerythrin (PE)-conjugated mouse anti-human monoclonal antibody to CD14 (M5E2; PharMingen), as well as >99% viable as determined by trypan blue exclusion. PBM were differentiated into monocyte-derived macrophages (MDM) by culture in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 U of penicillin and 100 μg of streptomycin per ml (complete DMEM) for up to 2 weeks (typically 3 days).
The nonadherent cells following the plate adherence step were enriched for lymphocytes and contained less than 2% CD14+ monocytes (data not shown). These monocyte-depleted PBMC (peripheral blood lymphocytes [PBL]) were cultured at 1 × 106 to 2 × 106/ml in RPMI 1640 supplemented with 10% FBS, 2 mM glutamine, and 100 U of penicillin and 100 μg of streptomycin per ml (complete RPMI). PBL were stimulated with 5 μg of phytohemagglutinin (PHA; Sigma) per ml for 1 to 3 days and then maintained in IL-2 (Hoffmann-La Roche, Nutley, N.J.; 40 U/ml). In some experiments, PBL were also depleted of CD8+ cells with magnetic Dynabeads M-450 CD8 according to the manufacturer's instructions (Dynal, Lake Success, N.Y.).
Preparation of viruses.
All of the HIV-1 isolates used in this report were originally obtained from the NIH AIDS Research and Reference Reagent Program. Stocks of HIV-1BaL were prepared by infection of HOS-CD4-CCR5 cells and of HIV-1BRU by infection of CEM-SS cells. For some experiments, viral stocks were prepared by infection of PHA-activated, CD8-depleted PBL; results were identical to those obtained using the cell line-derived viral isolates (data not shown).
In order to generate replication-incompetent HIV-1, 293 cells were transfected with HXBePLAP (10 μg), an HXB2-based HIV-1 provirus in which the env gene was replaced with the human placental alkaline phosphatase (PLAP) gene (10). Virions were envelope pseudotyped by cotransfecting 1 μg of a plasmid encoding the vesicular stomatitis virus (VSV) glycoprotein (VSV-G) or the env gene from the X4 isolate HIV-1HXB2, by the calcium-phosphate method (15). Supernatants were collected 2 days after transfection and found to contain infectious virus by a reverse transcriptase (RT) assay and infection of T1 cells. The supernatants contained no detectable GRO-α (data not shown) and were used at a 1:2 dilution to infect MDM.
HIV-1 infection of MDM, PBL, and PBMC.
For each experiment, multiple wells of MDM, PBL, or PBMC were infected with equal RT counts of HIV-1 (30 × 106 to 300 × 106 cpm of RT used per 105 cells). This amount of RT activity per cell corresponds to a multiplicity of infection (MOI) of between 0.01 and 0.1, as determined by titration on HOS-CD4-CCR5 and CEM-SS cell lines and quantitation of proviral DNA in PBMC 24 h after infection (data not shown). MDM were infected with HIV-1BaL for 16 h, washed, and then cultured in complete DMEM. PBL and PBMC were infected with HIV-1BaL or HIV-1BRU for 4 h, washed, and then incubated in complete RPMI plus IL-2. A portion of the media (25%) was removed from the MDM and PBMC cultures and replaced twice weekly.
Cytokine ELISAs.
Cellular supernatants were collected and stored at −70°C until analysis. Extracellular immunoreactive GRO-α was measured using a sandwich-type enzyme-linked immunosorbent assay (ELISA) with capture (MAb275) and biotinylated detection (BAF275) antibodies according to the manufacturer's instructions (R&D Systems). The lower limit of detection for this assay is 8 pg/ml. GRO-α, as well as the other cytokines tested, was also evaluated using an in-house ELISA protocol as previously described (23, 36). Dilutions of recombinant human MCP-1, MIP-1α, MIP-1β, RANTES, IL-8, GRO-α, GRO-γ, ENA-78, NAP-2, IP-10, MIG, IL-1β, IL-1ra, IL-6, IL-10, IL-12, TGF-β, and TNF-α (R&D Systems), ranging from 1 pg/ml to 100 ng/ml, were used as standards. This ELISA method consistently detected cytokine levels of >50 pg/ml.
RT assay.
HIV replication was determined by quantification of the RT activity present in the supernatants using a poly(A)-oligo(dT) template primer as previously described (48). RT activity was quantified using either a Series 400 PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.; see Fig. 5) or a Betascope radioisotope imaging system (see Fig. 1). The absolute values varied between experiments, but the peak activity consistently occurred 7 to 14 days after infection of MDM and 4 to 9 days after infection of PBL.
FIG. 5.
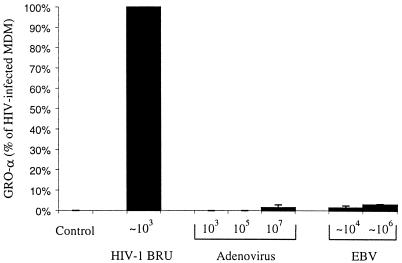
MDM produce greater amounts of GRO-α in response to HIV-1 than to adenovirus or EBV. MDM were infected with adenovirus strain DL7001, type I EBV Marmoset strain, or HIV-1BRU. The approximate number of viral particles added to 105 MDM is indicated. The data shown are the mean values of multiple wells of MDM from two donors.
FIG. 1.
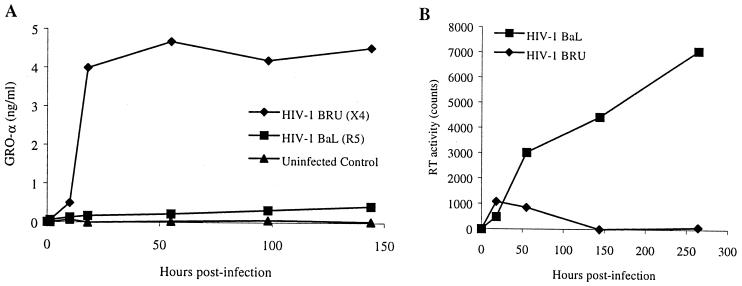
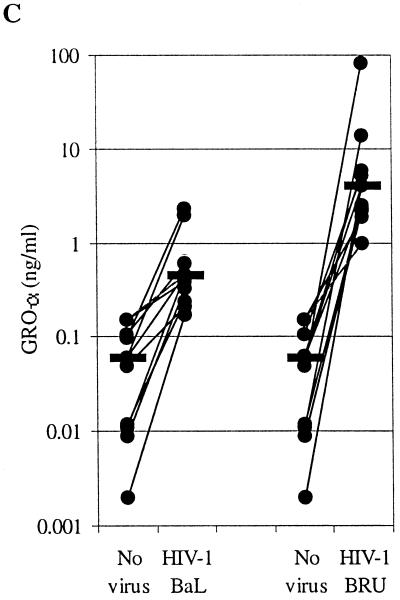
Increased production of GRO-α by MDM following encounter with HIV-1 is independent of productive infection. MDM were infected with equal counts of RT activity of HIV-1BaL or HIV-1BRU. (A) Extracellular immunoreactive GRO-α was measured by ELISA. (B) HIV replication was determined by quantitating the RT activity (above the background activity in uninfected controls) present in the supernatants. A portion of the supernatant was collected at several time points as indicated, so that input virus was removed entirely from the cells by day 2, at which point fresh medium was added, and a portion of the media (25%) was removed and replaced twice weekly. These experiments are representative of experiments performed with MDM from three different donors. (C) Exposure to X4 HIV (HIV-1BRU) stimulates GRO-α production by MDM to a greater extent than exposure to R5 HIV (HIV-1BAL). Shown are the results of ELISA for GRO-α performed on the supernatants collected 1 day after exposure of MDM from 11 different donors to HIV-1. Lines connect the values for control (no virus) and HIV-exposed MDM from each donor on this logarithmic scale. The horizontal black bars indicate the median values.
Northern blot analysis of GRO mRNA.
Total cellular RNA was extracted from noninfected and HIV-1BRU-infected MDM with TRIzol (Gibco-BRL, Rockville, Md.) according to the manufacturer's instructions. Equal amounts of RNA were then analyzed for GRO gene expression by Northern (RNA) blot analysis. Ethidium bromide-stained gels were photographed to demonstrate equivalent amounts of 28S and 18S rRNA in each sample. The MGSA-GRO probe was digested with EcoRI and PstI (270 bp), gel purified, and labeled by the random hexamer method with [α-32P]dATP. Probe was annealed for 1 h at 68°C in QuickHyb solution (Stratagene, La Jolla, Calif.). Hybridization and a high-stringency wash were performed in accordance with the manufacturer's instructions. Quantitation of RNA was determined using a Series 400 PhosphorImager and ImageQuant software.
Intracellular chemokine staining.
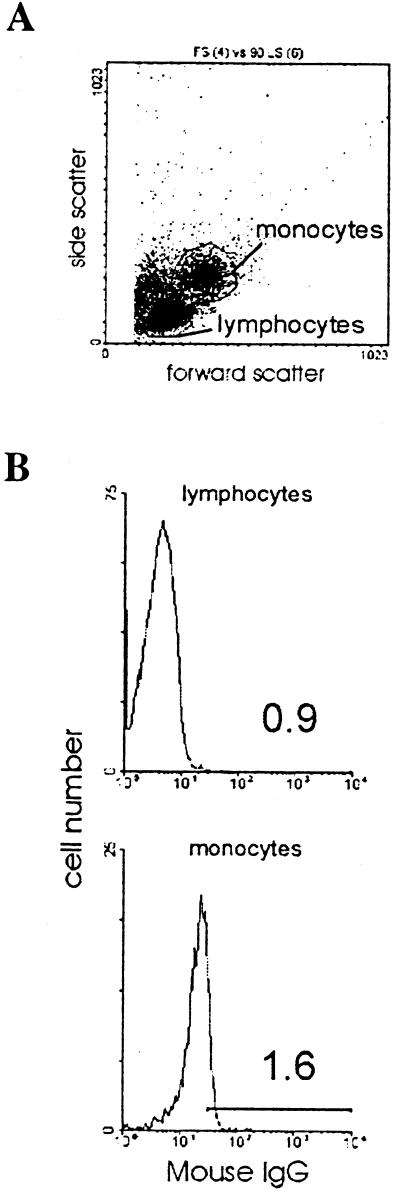
PBMC were collected as described above and cultured in complete RPMI without PHA stimulation. The next day, the PBMC were treated for 6 h with monensin (GolgiStop; PharMingen) to prevent cytokine secretion and incubated with HIV-1, viral proteins, or TNF-α. PBMC were then collected and stained for the presence of intracellular GRO according to the manufacturer's instructions (PharMingen). Adherent cells were collected by incubation in phosphate-buffered saline plus 10 mM EDTA for 30 min at 4°C. PBMC (both adherent and nonadherent cells) were then incubated in staining buffer (DPBS plus 1% FBS plus 0.09% sodium azide) with mouse immunoglobulin G (IgG) for 20 min at 4°C to block nonspecific binding of IgG to target cells. After two washes with staining buffer, PBMC were fixed and permeabilized with Cytofix/Cytoperm (PharMingen). PBMC were washed twice in Perm/Wash solution and then stained with either R-PE-conjugated mouse anti-human GRO monoclonal antibody (GRO-PE) or PE-mouse IgG1, κ isotype control immunoglobulin (PharMingen). Analysis of cell staining was performed using an EPICS Elite cell sorter (Beckman-Coulter, Fullerton, Calif.). The monocyte and lymphocyte subpopulations were gated according to the pattern of forward scatter and side scatter.
Flow cytometry for detecting CXCR2.
PBMC were collected as described above, cultured in complete RPMI without PHA stimulation, and then stained using the CXCR2 monoclonal antibody (MAb331), biotin-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.), and dichlorotriazinyl amino fluorescein (DTAF)-conjugated Streptavidin (Jackson ImmunoResearch Laboratories). Cells were incubated in flow buffer (Hanks balanced salt solution plus 2% FBS plus 0.05 % sodium azide) with anti-CXCR2 or mouse IgG for 30 minutes at 4°C. PBM were then washed with flow buffer and incubated in flow buffer with the secondary antibody (biotin-conjugated goat anti-mouse IgG) for 30 min at 4°C. PBM were then washed with flow buffer and incubated in flow buffer with the staining reagent (DTAF-SA) for 30 min at 4°C. Cell staining analysis was performed using an XL Z14107 cytometer. The monocyte and lymphocyte subpopulations were gated according to the patterns of forward scatter and side scatter.
Statistics.
The data are expressed as the mean of triplicate wells (± the standard deviation) throughout unless otherwise noted. Statistical significance was evaluated by t test for normally distributed data and by the Wilcoxon signed rank test for nonparametric data. A P value of <0.05 or <0.005 was regarded as statistically significant.
RESULTS
Production of GRO-α by MDM exposed to HIV-1.
MDM play a critical role in HIV pathogenesis as both producers of infectious HIV and sources of immunoregulatory factors (27, 31, 41). In early experiments, we examined the pattern of cytokine secretion by primary human MDM when exposed to HIV-1. We detected only modest increases in the cytokines TNF-α and the IL-1 receptor antagonist (IL-1ra) 1 to 3 days after infection and no significant change in 12 other immunoregulatory molecules at this point in time. The levels of RANTES, MIP-1α, and MIP-1β were not elevated during the first 72 h after infection but increased in parallel with virus production by MDM at later time points (data not shown). In contrast, we measured a substantial increase in the production of the C-X-C chemokines GRO-α, GRO-γ, and IL-8 and the C-C chemokine MCP-1 within 24 h, which was maintained for more than 1 week after exposure to HIV-1 (data not shown).
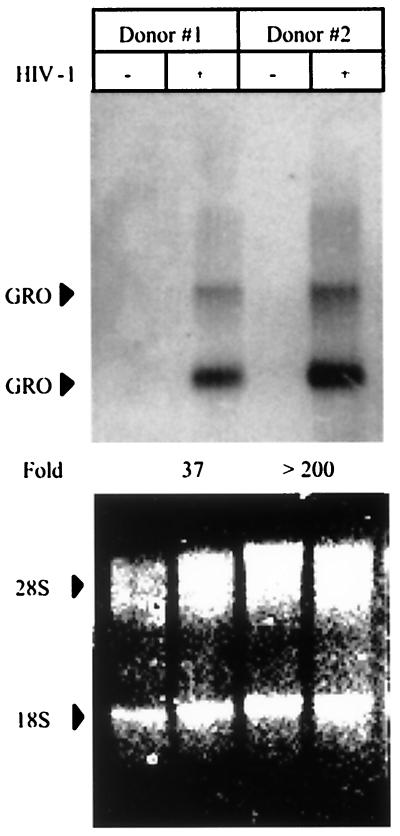
We next compared the chemokine production by MDM exposed to a prototypical R5 isolate of HIV (HIV-1BaL) with that by MDM exposed to the X4 isolate HIV-1BRU. While exposure to R5 HIV or X4 HIV had similar effects on MCP-1 and IL-8 production by MDM, there was a striking difference in the production of the C-X-C chemokine GRO-α by MDM exposed to the X4 isolate HIV-1BRU, compared with MDM exposed to the R5 isolate HIV-1BaL (Fig. 1A and data not shown). Secreted GRO-α was elevated as early as 75 min after incubation with HIV-1BRU and remained elevated throughout the period of culture (up to 2 weeks), while MDM infected with HIV-1BaL produced only slightly more GRO-α than uninfected controls. Indeed, we found all X4 isolates of HIV-1 and HIV-2 to be more potent inducers of GRO-α production than any of the R5 isolates tested (data not shown). MDM from 11 different healthy donors exposed to HIV-1BRU produced significantly more GRO-α (median value, 4.00 ng/ml) than MDM infected with HIV-1BaL (0.45 ng/ml, P = 0.019) and uninfected controls (0.06 ng/ml, P < 0.001) (Fig. 1C). Northern blot analysis revealed that exposure of MDM to HIV-1 increased steady-state GRO mRNA levels greater than 35-fold (Fig. 2). While HIV-1BRU stimulated far more GRO-α production than did HIV-1BaL, no virus production was detectable in MDM exposed to HIV-1BRU even as late as 2 weeks after infection, while substantial HIV-1 was detected in MDM infected with HIV-1BaL a few days after infection (Fig. 1B). GRO-α production appeared to be sustained, despite the lack of active viral replication in these cells, since the increases in GRO-α protein were maintained even when the media were changed after the first 2 days (Fig. 1A). These data indicate that GRO-α production is independent of productive infection with HIV-1 and is more pronounced following exposure to viral isolates that use CXCR4 for entry.
FIG. 2.
HIV-1 increases GRO mRNA levels in MDM. Total cellular RNA was extracted from control MDM (−) and MDM exposed to HIV-1BRU (+) after 2 days. RNA from two different donors was then analyzed for GRO gene expression by Northern (RNA) blot analysis using a GRO-specific probe (top panel). The total amount of RNA was determined by quantitating the intensity of the 28S and 18S rRNA bands (bottom panel). The amount of GRO mRNA was normalized to the amount of rRNA in each sample and the fold increase in HIV-exposed MDM relative to control MDM is indicated.
GRO chemokine production by PBMC.
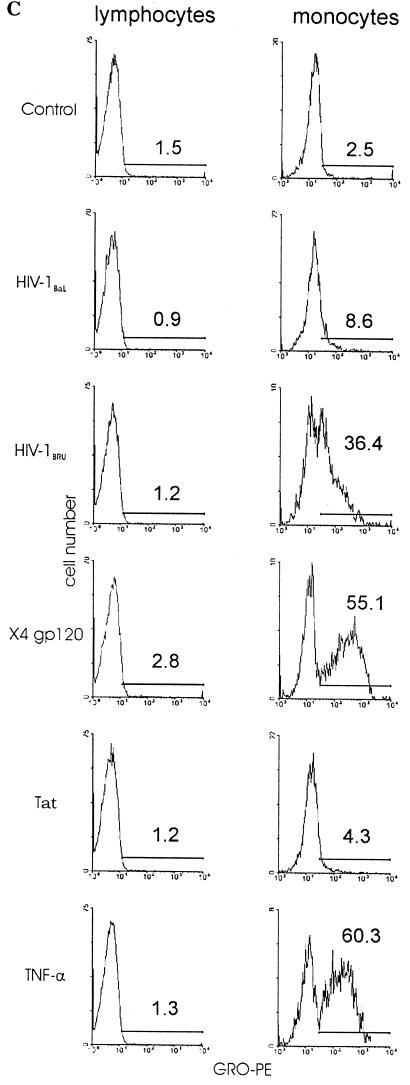
In order to analyze the production of GRO by individual cells, we looked by flow cytometry for the presence of GRO in PBMC stimulated with HIV-1 virions or viral proteins. Fresh PBMC were cultured overnight without PHA stimulation and then exposed to small numbers of HIV-1BaL or HIV-1BRU virions or the viral proteins gp120 or Tat. Staining after 6 h revealed that GRO was present in a large percentage of monocytes treated with HIV-1BRU, X4 gp120, or TNF-α and in less than 10% of untreated monocytes or those treated with HIV-1BaL or Tat (Fig. 3). GRO proteins were detected in less than 5% of PBL in all treatment conditions (Fig. 3). In addition, no increase in GRO-α production by activated PBL was detectable by ELISA following exposure to either R5 or X4 isolates of HIV-1 (data not shown). Thus, as GRO-α production occurred well before the detection of virus in MDM infected with R5 HIV and in the absence of infection of MDM by X4 HIV (Fig. 1), and in more cells than could be infected by the titers of virus used, we conclude that GRO-α production occurs by a mechanism that does not depend on completion of the viral life cycle within the MDM. Moreover, because X4 isolates of HIV-1 stimulated GRO-α production to a far greater extent than R5 isolates, we hypothesized that the interaction between HIV-1 gp120 and CXCR4 induces the signals necessary to upregulate GRO-α production.
FIG. 3.
Intracellular GRO protein is present in monocytes exposed to HIV-1 and gp120. PBMC were treated with GolgiStop (Control) along with HIV-1BaL, HIV-1BRU, X4 gp120 (1 μg/ml, from HIV-1IIIB), Tat (100 ng/ml), or TNF-α (100 ng/ml). PBMC were harvested after 6 h and analyzed for intracellular GRO proteins by flow cytometry. (A) Lymphocyte and monocyte subpopulations were gated according to the pattern of forward scatter and side scatter. (B) Background staining in lymphocytes and monocytes was determined by incubation with PE-mouse IgG1 isotype control (mouse IgG). The histograms show fluorescence intensity on a logarithmic scale along the x axis and the number of events on the y axis. The percentage of cells staining positive in each condition is indicated. (C) Staining of lymphocytes and monocytes with a PE-conjugated mouse anti-human GRO antibody (GRO-PE) for each treatment condition is shown. The data shown are representative of four independent experiments.
Specificity of GRO-α production by MDM exposed to HIV-1.
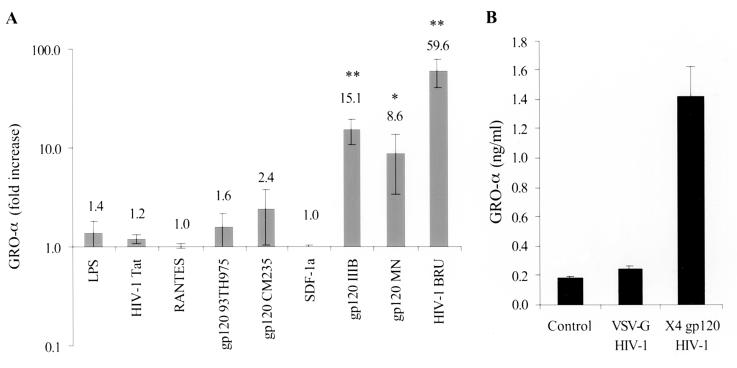
We next examined the ability of bacterial and viral antigens to stimulate the production of GRO-α by MDM in order to further investigate the specificity of the response to HIV-1. As positive controls for these experiments, we stimulated MDM with TNF-α and phorbol 12-myristate 13-acetate (PMA). TNF-α did induce the production of GRO-α by monocytes and MDM, while PMA did not (Fig. 3C and data not shown). In addition, while stimulation with LPS from E. coli strain O111:B4 (up to 2 μg/ml) activated MDM as determined by an MTT-based assay for cellular activation (data not shown), MDM treated with LPS did not produce increased amounts of GRO-α protein (Fig. 4A). Treatment with polymixin B (10 μg/ml) prevented the activation of MDM by LPS but did not prevent the HIV-1BRU-induced increases in GRO-α, indicating that GRO-α production was not due to LPS contamination of the viral stocks (data not shown). In addition, viral stocks were found to be negative for detectable endotoxin using a Limulus amebocyte lysate test (data not shown). Exposure to neither adenovirus nor Epstein-Barr virus (EBV) resulted in a significant increase in GRO-α production (Fig. 5). Thus, GRO-α is produced specifically in response to encounter with HIV and not simply because the MDM are activated nonspecifically by viral antigens or in response to contamination with LPS.
FIG. 4.
HIV-1 gp120 stimulates GRO-α production. (A) MDM were exposed to HIV-1BRU or treated with recombinant gp120 (1 μg/ml) from isolates HIV-193TH975 (R5), HIV-1CM235 (R5), HIV-1IIIB (X4), HIV-1MN (X4), or HIV-1 Tat (1 μg/ml), SDF-1α (0.5 μg/ml), RANTES (1.35 μg/ml), or LPS (1 μg/ml) as indicated. Supernatants were collected after 1 day and analyzed by ELISA for GRO-α. The data are shown on a logarithmic scale and are the mean (± the standard error of the mean) of the results of 3 to 13 independent experiments for each treatment. The data and data labels show the fold increase relative to untreated controls for each set of experiments. The absolute amount (in nanograms per milliliter) of GRO-α in each set of experiments ranged from: untreated controls, 0.002 to 4.0; LPS, 0.19 to 0.74; HIV-1 Tat, 0.015 to 0.051; gp12093TH975, 0.001 to 4.1; gp120CM235, 0.06 to 2.1; SDF-1α, 3.4 to 4; gp120IIIB, 0.5 to 30.9; gp120MN, 1.1 to 14.6; and HIV-1BRU, 0.851 to 114. (B) Pseudotyped, replication-incompetent HIV-1 was prepared by cotransfection of 293 cells with an env(−) HIV-1 provirus (HXBePLAP) and either VSV-G or gp120 from HIV-1HXB2 (X4 gp120). MDM were treated with 50% cell-free supernatants from transfected or mock-transfected 293 cells. ELISA was performed on supernatants collected 2 days after treatment. The data shown are representative of three experiments.
Engagement of CXCR4 by HIV-1 gp120 stimulates GRO-α production by MDM.
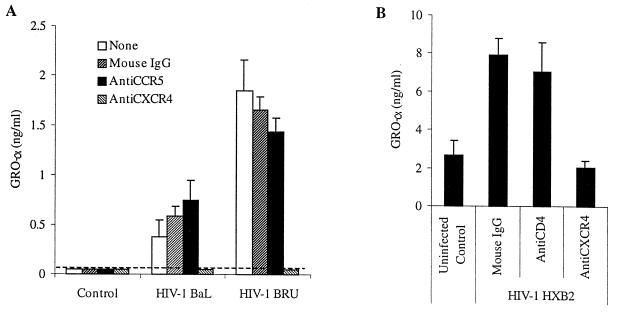
Although MDM are not infected by X4 HIV under most circumstances, these cells do express CXCR4, in addition to CD4 and CCR5 (43, 50, 54, 58). In order to determine whether binding of HIV-1 to one of these specific receptors is required for the production of GRO-α by MDM, we preincubated MDM with antibodies known to block HIV binding to CXCR4, CCR5, or CD4 prior to infection with HIV-1 (Fig. 6). Neutralization of binding to CXCR4 by the anti-CXCR4 antibody 12G5 prevented the release of GRO-α by MDM exposed to HIV-1BRU, HIV-1HXB2, and HIV-1BaL, while anti-CCR5 (2D7), anti-CD4, and control mouse IgG did not have a significant effect. The ability of anti-CXCR4 to block the small increase in GRO-α production induced by the R5 isolate HIV-1BaL suggests that this isolate has some moderate affinity for CXCR4, although it is not able to use this receptor to infect CXCR4+ T-cell lines (reference 9 and data not shown). This hypothesis is further supported by the finding that HIV-1BaL stimulated slightly more GRO-α production when MDM were preincubated with anti-CCR5 than with control IgG (Fig. 6A), presumably because more viral particles are able to bind to CXCR4 when the binding sites on CCR5 are blocked. Thus, the modest effect observed with HIV-1BaL is most likely attributable to interaction with CXCR4 as well, similar to the recent observation that the R5 isolate HIV-1ADA is able to signal via neuronal CXCR4 (62).
FIG. 6.
GRO-α production is mediated by interaction of HIV-1 with CXCR4. (A) MDM were preincubated with antibodies (20 μg/ml) to CCR5 (2D7) or CXCR4 (12G5), each of which is known to neutralize HIV infection, or else mouse IgG (20 μg/ml) or no antibody as controls, and then exposed to either HIV-1BaL or HIV-1BRU. Supernatants were collected after 24 h and analyzed by ELISA. Several values fell below the limit of detection (dashed line). (B) MDM were preincubated with the same antibodies or with anti-CD4 (15 μg/ml) before exposure to the X4 isolate HIV-1HXB2. The data shown are representative of five independent experiments.
In order to confirm the role of gp120 engagement of CXCR4 in GRO-α production, we determined whether the incubation of monocytes and MDM with gp120 from X4 HIV would stimulate GRO-α production. GRO proteins were detected in more than 55% of monocytes in response to X4-specific gp120, compared with <5% in response to Tat, as determined by intracellular cytokine analysis (Fig. 3). Treatment of MDM with recombinant gp120 from the X4 isolates HIV-1IIIB and HIV-1MN, but not with gp120 from the R5 isolates HIV-193TH975 or HIV-1CM235, nor with HIV-1 Tat, significantly increased the production of GRO-α (Fig. 4A). Subsequent dose-response studies demonstrated that concentrations of gp120 of as low as 100 ng/ml were able to increase GRO-α production by MDM (data not shown). This concentration of envelope protein has previously been found in the serum of HIV-infected individuals, indicating that levels of gp120 present in vivo are sufficient to induce GRO-α production (46). In addition, the stimulation of GRO-α production by HIV-1BRU was resistant to heat inactivation at 56°C for 30 min but sensitive to boiling for 5 min (data not shown), a result consistent with the notion that a relatively heat stable factor (e.g., gp120) is responsible for this effect (12). Preparations of recombinant gp120 were analyzed for LPS content using the Limulus amebocyte lysate test and found to contain fewer than 0.54, 0.23, 1.6, and 1.6 EU/μg for gp120 from HIV-193TH975, HIV-1CM235, HIV-1IIIB, and HIV-1MN, respectively. In addition, polymyxin B treatment did not prevent the increases in GRO-α production observed with gp120 treatment and, as shown above, these and higher amounts of LPS did not stimulate GRO-α production (Fig. 4). Further, HIV-1 gp120-mediated stimulation of GRO-α production was blocked by anti-CXCR4 antibody (Fig. 6) and by a small-molecule inhibitor of CXCR4 (data not shown). Therefore, increased production of Gro-α is due to engagement of CXCR4 by gp120 and is not due to contaminating endotoxin.
Neither the natural ligand for CXCR4, SDF-1α, nor RANTES, which binds to CCR5 and CCR1, was able to stimulate GRO-α production (Fig. 4A). In addition, anti-CXCR4 (12G5) caused only a transient increase in GRO-α production (data not shown). This indicates that it is not simply interaction with CXCR4 but specific aspects of the engagement of CXCR4 by gp120 which lead to the striking increase in GRO-α production. To further address the ability of X4 gp120 to signal the production of GRO-α, replication-incompetent HIV-1 was pseudotyped with the envelope glycoproteins of HIV-1 or VSV (15). Exposure of MDM to HIV-1 pseudotyped with X4 gp120 stimulated GRO-α production, while exposure to HIV-1 pseudotyped with VSV-G did not (Fig. 4B), further demonstrating that the ability of gp120 to signal increased GRO-α production is quite specific.
GRO-α stimulates HIV-1 replication in MDM and T lymphocytes.
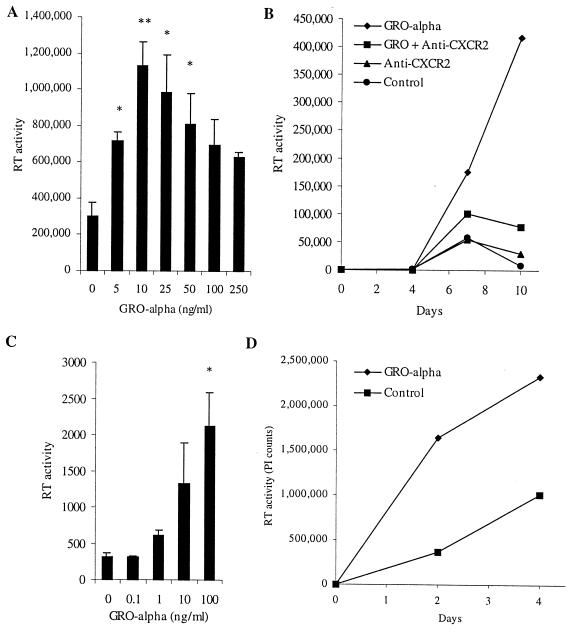
Increased chemokine production triggered by the ligation of CXCR4 on MDM by HIV-1 gp120 could either benefit or compromise the ability of the virus to replicate in its target cells (or have no effect). Since certain chemokines (RANTES, MIP-1α, MIP-1β, and SDF-1α) have been demonstrated to play important roles in HIV-1 pathogenesis, mainly as inhibitors of viral entry (7, 13, 14, 45), we studied the effect of GRO-α, and signaling through its receptor, CXCR2, on HIV replication in MDM and T lymphocytes. Replication of the macrophage-tropic R5 isolate HIV-1BaL in MDM was augmented by the addition of exogenous GRO-α (Fig. 7A and B). The dose-response curve was bell-shaped, with maximal effect at 10 to 25 ng/ml (Fig. 7A). In experiments with MDM from five different donors, HIV-1BaL replication was increased 14-fold on average (range, 2- to 40-fold). The addition of an antibody that prevents GRO-α from interacting with CXCR2 blocked the stimulatory effect of GRO-α on HIV-1BaL replication in MDM (Fig. 7B). On its own, the anti-CXCR2 antibody had no significant effect on viral replication in MDM from some donors (Fig. 7B) and decreased replication in others (data not shown). No viral replication was detected when MDM from multiple donors were infected with HIV-1BRU in the presence or absence of exogenous GRO-α, indicating that GRO-α does not confer susceptibility to productive infection with X4 HIV on MDM (Fig. 1B and data not shown). In addition to the increase in viral replication seen in MDM with GRO-α treatment, HIV-1BaL replication in PBL was stimulated 5-fold on average (n = 6; range, 2- to 8-fold) by the addition of GRO-α (Fig. 7C). Replication of the X4 isolate HIV-1BRU in PBL was also increased 2.4-fold on average (n = 7; range, 0.9- to 4.2-fold; P = 0.027) (Fig. 7D). GRO-α stimulated the replication of all isolates tested, including both R5 and X4 isolates of HIV-1 and HIV-2 (data not shown).
FIG. 7.
GRO-α stimulates HIV-1 replication in MDM and PBL. (A) MDM were treated with GRO-α at the indicated concentrations for 16 h before infection with HIV-1BaL. Supernatants were analyzed for RT activity 8 days after infection. (B) MDM were treated twice weekly with GRO-α (25 ng/ml) and/or anti-CXCR2 (20 μg/ml) starting 1 day before infection with HIV-1BaL. (C) CD8-depleted PBL were treated with GRO-α at the doses indicated 1 day before and 2 days after infection with HIV-1BaL. RT activity was assayed on day 5. (D) PBL were treated with GRO-α (25 ng/ml) 1 day before and 1 and 4 days after infection with HIV-1BRU. These experiments are representative of infections of cells from five (A), three (B), six (C), and seven (D) different donors.
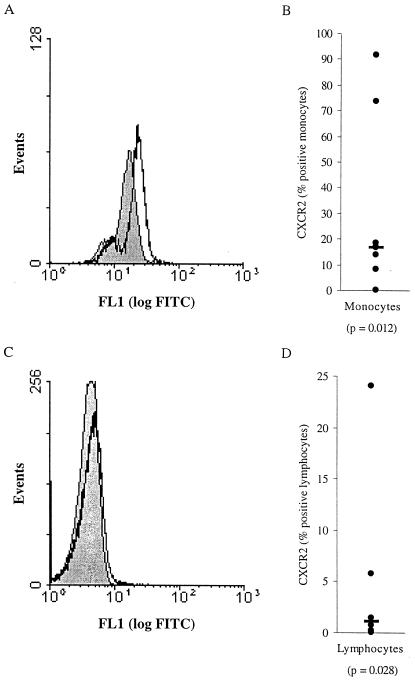
CXCR2, the receptor for GRO-α, is expressed on PBM and PBL.
Although it has been questioned whether functional CXCR2 is present on PBMC, recent studies have indicated that low levels of this receptor may indeed be present and capable of transmitting signals in T lymphocytes and monocytes (29, 60). Our flow cytometric analyses indicate that CXCR2 is consistently expressed on a small percentage of PBL and a greater proportion of PBM (Fig. 8). The greater expression of CXCR2 on PBM than on PBL correlates well with the larger stimulatory effect of GRO-α on HIV-1 replication in MDM than in PBL. In addition, the expression of CXCR2 on MDM may be induced by HIV-1 (data not shown).
FIG. 8.
CXCR2 is expressed on monocytes and lymphocytes. PBMC were analyzed for CXCR2 expression by flow cytometry after 2 days. Monocytes (A and B) and lymphocytes (C and D) were gated according to forward and side scatter and, in some experiments, by the presence of CD4 or CD14. Histograms indicating the amount of staining with the mouse IgG control (filled gray) and with the anti-CXCR2 antibody (black line) are presented for monocytes (A) and lymphocytes (C). Data from multiple donors are presented as the percentage of cells staining positive with the anti-CXCR2 antibody minus the percentage of cells staining positive with the mouse IgG2a isotype control. Each point represents the value for a different donor monocytes, n = 8, (B); and lymphocytes, n = 6 (D). The horizontal black bars indicate the median values, which are 17.0 and 1.2%, respectively. The data were found to be statistically significant using the Wilcoxon signed rank test; P values are indicated below the data labels.
Endogenous GRO-α regulates HIV-1 replication in MDM and PBMC.
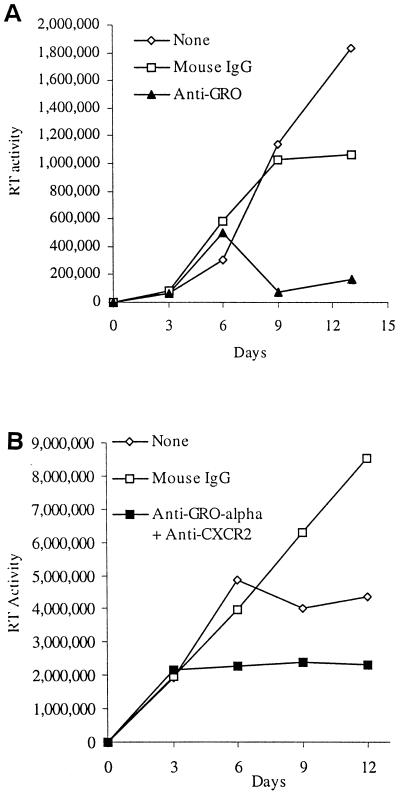
Because HIV-1 stimulates GRO-α production by MDM and exogenous GRO-α stimulated HIV replication in MDM and PBL, we next investigated whether endogenous GRO-α chemokine production and CXCR2-mediated signaling are necessary for HIV-1 to replicate in these primary human cells. Indeed, the addition of an antibody that neutralizes the activity of GRO-α, GRO-β, and GRO-γ(anti-GRO) markedly reduced HIV-1BaL replication in MDM (Fig. 9A). Identical results were obtained using an antibody specific for GRO-α (data not shown). In addition, when anti-GRO-α and an antibody that prevents interaction with CXCR2 were added to HIV-1BRU-infected PBMC, viral replication was substantially diminished (Fig. 9B). Importantly, treatment with these antibodies did not significantly affect cellular activation or viability compared to control cultures, as measured by an MTT-based assay (Fig. 9B). These data indicate that endogenous, HIV-1 stimulated GRO-α production and signaling through CXCR2 play a stimulatory role in viral replication in primary human macrophages and lymphocytes and point to the existence of an autocrine-paracrine loop involving GRO-α and HIV-1 replication.
FIG. 9.
Involvement of endogenous GRO-α and CXCR2 signaling in HIV-1 replication in MDM and PBMC. (A) MDM were treated with mouse IgG1 (20 μg/ml) or anti-GRO (20 μg/ml) every 3 days starting 1 day before infection with HIV-1BaL. Media were collected and replenished every 3 days. (B) PBMC were stimulated with PHA (5 μg/ml) for 2 days and then infected with HIV-1BRU. Media were then aspirated, and PBMC were treated with mouse IgG (40 μg/ml) or anti-GRO-α and anti-CXCR2 (each at 20 μg/ml). Supernatants (25%) were collected every 3 days and replaced with fresh media containing antibodies. Cellular viability was assessed in this experiment on day 12 by MTT assay. The absorbance (A570-A650) was determined to be 0.323 ± 0.045 for no treatment, 0.262 ± 0.075 for mouse IgG, and 0.318 ± 0.026 for anti-GRO-α and anti-CXCR2. These experiments are representative of infections of cells from four (A) and three (B) different donors.
DISCUSSION
The envelope protein gp120 allows HIV-1 to enter and productively infect MDM through interactions with CD4 and CCR5 and, under some circumstances, CXCR4 (43, 50, 54, 58). We now show that HIV-1 gp120 interaction with CXCR4 on MDM, independent of productive infection, induces signals that result in GRO-α production, which subsequently augments viral replication in both macrophages and T lymphocytes. Increases in the amount of GRO-α produced by MDM were detected as early as 75 min after exposure to X4 HIV. GRO-α expression was increased more than 35-fold at the RNA level, an average of 70-fold at the protein level in response to the X4 isolate HIV-1BRU, and only 7.5-fold in response to the macrophage-tropic R5 isolate HIV-1BaL. GRO-α was also induced by recombinant gp120 from X4 HIV, but not from R5 HIV, and by replication-incompetent HIV-1 enveloped with X4 gp120, but not with the envelope protein of VSV (VSV-G). Moreover, GRO was detected intracellularly in the majority of PBM (but not in PBL) when exposed to low titers (MOI <0.01) of HIV-1 or to recombinant HIV-1 gp120. GRO-α production was inhibited by preincubation with an antibody that prevents interaction with CXCR4 (12G5) and was not blocked by anti-CD4 or anti-CCR5 antibodies. HIV-1 gp120-induced signaling through CXCR4 leading to GRO-α production therefore appears to be CD4 independent, in contrast to the interaction leading to viral entry via CXCR4, which is generally CD4 dependent. This is consistent with recent reports which have indicated that gp120 can functionally interact with CXCR4 independent of CD4 in various settings (21, 22, 33). GRO-α was not produced in response to other viral antigens, bacterial LPS, or the natural ligand for CXCR4, SDF-1α. These experiments indicate that GRO-α production is a specific response of MDM to encounter with gp120, particularly from X4 HIV.
Our data indicate that GRO-α, a chemokine not previously suspected to play a role in HIV pathogenesis, is involved in an autocrine-paracine loop controlling HIV-1 replication. We demonstrate here that exogenous GRO-α stimulates HIV-1 replication in both acutely infected primary human MDM and PBL. While the mechanism by which HIV replication is activated is not yet clear, our preliminary data suggest that GRO-α acts to enhance viral entry by increasing the surface expression of CCR5 and CXCR4 (data not shown). Importantly, we show here that disruption of this loop by depletion of endogenous, HIV-1 induced GRO-α greatly reduces HIV-1 replication in MDM and PBMC. As HIV-1 evolves during the course of infection to use CXCR4 in addition to CCR5 for infection, GRO-α induced by X4 HIV could stimulate the replication of both R5 HIV and X4 HIV in infected individuals. The induction of GRO-α by CXCR4-using isolates of HIV-1 may thus contribute to the increased pathogenicity of these isolates and help to explain the increased viral replication and advanced clinical deterioration associated with the emergence of X4 HIV that often occurs some years after initial infection (16). Consistent with this hypothesis, elevated levels of GRO-α have been detected in the bronchoalveolar lavage fluid of HIV-infected individuals with Pneumocystis carinii pneumonia (59). In addition to effects on HIV replication, since GRO-α is angiogenic and can activate the KSHV GPCR ORF 74, an increase in GRO-α following exposure to HIV may also affect the progression of AIDS-associated KS (28, 38, 55). Therefore, interventions targeting GRO-α and signaling through its receptor, CXCR2, have the potential to offer new therapeutic approaches for patients infected with HIV.
ACKNOWLEDGMENTS
This project was supported by NIH grants AI30924, AI36685, and HL57885 and the General Clinical Research Center at the University of Michigan (grant M01-RR00042). B.R.L. was supported in part by the following training programs at the University of Michigan: Medical Scientist Training Program (NIH grant NIGMS T32 GM07863), Cellular and Molecular Biology (NIH grant GM07315), and Molecular Mechanisms of Microbial Pathogenesis (NIH grant AI 07528), and by funds from the Harvey Fellows Program.
We thank Sara Cheng, Steven King, Kathy Collins, Marie Burdick, and Paul Bock for helpful discussions. We also thank Mark Kukuruga (University of Michigan Flow Cytometry Core), Sue Morris, Pam Lincoln, and Holly Evanoff for technical assistance. HIV-1BRU was provided by Steven King and Gary Nabel. The HIV-1 provirus HXBePLAP and the VSV-G expression plasmid were the kind gift of Kathy Collins, and the GRO probe was provided by Ann Richmond. Stocks of adenovirus were prepared by Dennis Hartigan and Jeff Chamberlain, and EBV was provided by Erle Robertson.
REFERENCES
- 1.Ahuja S K, Murphy P M. The CXC chemokines growth-regulated oncogene (GRO)α, GROβ, GROγ, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci USA. 1987;84:7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C V. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A, Gerhengorn M C. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 7.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 8.Borghi P, Fantuzzi L, Varano B, Gessani S, Puddu P, Conti L, Capobianchi M R, Ameglio F, Belardelli F. Induction of interleukin-10 by human immunodeficiency virus type 1 and its gp120 protein in human monocytes/macrophages. J Virol. 1995;69:1284–1287. doi: 10.1128/jvi.69.2.1284-1287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canque B, Rosenzwajg M, Gey A, Tartour E, Fridman W H, Gluckman J C. Macrophage inflammatory protein-1α is induced by human immunodeficiency virus infection of monocyte-derived macrophages. Blood. 1996;87:2011–2019. [PubMed] [Google Scholar]
- 10.Chen B K, Gandhi R T, Baltimore D. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol. 1996;70:6044–6053. doi: 10.1128/jvi.70.9.6044-6053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 12.Clouse K A, Cosentino L M, Weih K A, Pyle S W, Robbins P B, Hochstein H D, Natarajan V, Farrar W L. The HIV-1 gp120 envelope protein has the intrinsic capacity to stimulate monokine secretion. J Immunol. 1991;147:2892–2901. [PubMed] [Google Scholar]
- 13.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 14.Coffey M J, Woffendin C, Phare S M, Strieter R M, Markovitz D M. RANTES inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. Am J Physiol. 1997;272:L1025–L1029. doi: 10.1152/ajplung.1997.272.5.L1025. [DOI] [PubMed] [Google Scholar]
- 15.Collins K L, Baltimore D. HIV's evasion of the cellular immune response. Immunol Rev. 1999;168:65–74. doi: 10.1111/j.1600-065x.1999.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 16.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Dolei A, Biolchini A, Serra C, Curreli S, Gomes E, Dianzani F. Increased replication of T-cell-tropic HIV strains and CXC-chemokine receptor-4 induction in T cells treated with macrophage inflammatory protein (MIP)-1α, MIP-1β and RANTES beta-chemokines. AIDS. 1998;12:183–190. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 20.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 21.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briand P, Hazan U. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 23.Evanoff H L, Burdick M D, Moore S A, Kunkel S L, Strieter R M. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1) Immunol Investig. 1992;21:39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- 24.Fauci A S. AIDS: immunopathogenic mechanisms and research strategies. Clin Res. 1987;35:503–510. [PubMed] [Google Scholar]
- 25.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 26.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 27.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 28.Gershengorn M C, Geras-Raaka E, Varma A, Clark-Lewis I. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J Clin Investig. 1998;102:1469–1472. doi: 10.1172/JCI4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerszten R E, Garcia-Zepeda E A, Lim Y C, Yoshida M, Ding H A, Gimbrone M A, Jr, Luster A D, Luscinskas F W, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 30.Gordon C J, Muesing M A, Proudfoot A E, Power C A, Moore J P, Trkola A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J Virol. 1999;73:684–694. doi: 10.1128/jvi.73.1.684-694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbein G, Keshav S, Collin M, Montaner L J, Gordon S. HIV-1 induces tumour necrosis factor and IL-1 gene expression in primary human macrophages independent of productive infection. Clin Exp Immunol. 1994;95:442–449. doi: 10.1111/j.1365-2249.1994.tb07016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 33.Iyengar S, Schwartz D H, Hildreth J E. T cell-tropic HIV gp120 mediates CD4 and CD8 cell chemotaxis through CXCR4 independent of CD4: implications for HIV pathogenesis. J Immunol. 1999;162:6263–6267. [PubMed] [Google Scholar]
- 34.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, Jackson R, Ostrowski M, Hoxie J, Offord R, Fauci A S. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4+ T cells: role of signal transduction. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koziel H, Eichbaum Q, Kruskal B A, Pinkston P, Rogers R A, Armstrong M Y, Richards F F, Rose R M, Ezekowitz R A. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor down regulation. J Clin Investig. 1998;102:1332–1344. doi: 10.1172/JCI560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane B R, Markovitz D M, Woodford N L, Rochford R, Strieter R M, Coffey M J. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163:3653–3661. [PubMed] [Google Scholar]
- 37.Littman D R. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 38.Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen J D, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson K N, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 39.Mack M, Lucknow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N, Schlondorff D, Proudfoot A E. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marechal V, Arenzana-Seisdedos F, Heard J M, Schwartz O. Opposite effects of SDF-1 on human immunodeficiency virus type 1 replication. J Virol. 1999;73:3608–3615. doi: 10.1128/jvi.73.5.3608-3615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meltzer M S, Skillman D R, Gomatos P J, Kalter D C, Gendelman H E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 42.Merrill J E, Koyanagi Y, Chen I S. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriuchi M, Moriuchi H, Turner W, Fauci A S. Exposure to bacterial products renders macrophages highly susceptible to T-tropic HIV-1. J Clin Investig. 1998;102:1540–1550. doi: 10.1172/JCI4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 46.Oh S K, Cruickshank W W, Raina J, Blanchard G C, adler W H, Walker J, Kornfeld H. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5:251. [PubMed] [Google Scholar]
- 47.Poli G, Fauci A S, editors. Role of cytokines in the pathogenesis of human immunodeficiency virus infection. Cambridge, Mass: Blackwell Scientific; 1995. [Google Scholar]
- 48.Potts B J, Maury W, Martin M A. Replication of HIV-1 in primary monocyte cultures. Virology. 1990;175:465–476. doi: 10.1016/0042-6822(90)90431-p. [DOI] [PubMed] [Google Scholar]
- 49.Richmond A, Thomas H G. Purification of melanoma growth stimulatory activity. J Cell Physiol. 1986;129:375–384. doi: 10.1002/jcp.1041290316. [DOI] [PubMed] [Google Scholar]
- 50.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiratsuchi H, Johnson J L, Toossi Z, Ellner J J. Modulation of the effector function of human monocytes for Mycobacterium avium by human immunodeficiency virus-1 envelope glycoprotein gp120. J Clin Investig. 1994;93:885–891. doi: 10.1172/JCI117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons G, Reeves J D, McKnight A, Dejucq N, Hibbitts S, Power C A, Aarons E, Schols D, De Clercq E, Proudfoot A E, Clapham P R. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–8457. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strieter R M, Polverini P J, Kunkel S L, Arenberg D A, Burdick M D, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 56.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 57.Trkola A, Gordon C, Matthews J, Maxwell E, Ketas T, Czaplewski L, Proudfoot A E, Moore J P. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J Virol. 1999;73:6370–6379. doi: 10.1128/jvi.73.8.6370-6379.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi A G, Vercelli D. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J Immunol. 1998;161:2084–2088. [PubMed] [Google Scholar]
- 59.Villard J, Dayer-Pastore F, Hamacher J, Aubert J D, Schlegel-Haueter S, Nicod L P. GRO alpha and interleukin-8 in Pneumocystis carinii or bacterial pneumonia and adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:1549–1554. doi: 10.1164/ajrccm.152.5.7582292. [DOI] [PubMed] [Google Scholar]
- 60.Ward S G, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 61.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 62.Zheng J, Thylin M R, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y C, Gelbard H A, Shepard R B, Swartz J M, Gendelman H E. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 63.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]