Abstract
Microglia, the brain’s resident macrophages, can be reconstituted by surrogate cells - a process termed “microglia replacement.” To expand the microglia replacement toolkit, we here introduce estrogen-regulated (ER) homeobox B8 (Hoxb8) conditionally immortalized macrophages, a cell model for generation of immune cells from murine bone marrow, as a versatile model for microglia replacement. We find that ER-Hoxb8 macrophages are highly comparable to primary bone marrow-derived (BMD) macrophages in vitro, and, when transplanted into a microglia-free brain, engraft the parenchyma and differentiate into microglia-like cells. Furthermore, ER-Hoxb8 progenitors are readily transducible by virus and easily stored as stable, genetically manipulated cell lines. As a demonstration of this system’s power for studying the effects of disease mutations on microglia in vivo, we created stable, Adar1-mutated ER-Hoxb8 lines using CRISPR-Cas9 to study the intrinsic contribution of macrophages to Aicardi-Goutières Syndrome (AGS), an inherited interferonopathy that primarily affects the brain and immune system. We find that Adar1 knockout elicited interferon secretion and impaired macrophage production in vitro, while preventing brain macrophage engraftment in vivo - phenotypes that can be rescued with concurrent mutation of Ifih1 (MDA5) in vitro, but not in vivo. Lastly, we extended these findings by generating ER-Hoxb8 progenitors from mice harboring a patient-specific Adar1 mutation (D1113H). We demonstrated the ability of microglia-specific D1113H mutation to drive interferon production in vivo, suggesting microglia drive AGS neuropathology. In sum, we introduce the ER-Hoxb8 approach to model microglia replacement and use it to clarify macrophage contributions to AGS.
Keywords: microglia, microglia replacement, ER-Hoxb8, macrophage, Aicardi-Goutières Syndrome
Introduction:
Microglia, the parenchymal tissue-resident macrophages of the brain and spinal cord, play critical roles in development, homeostasis, injury, and disease (Salter and Stevens, 2017; Li and Barres, 2018). When depleted of endogenous microglia, the brain parenchyma can be reconstituted by surrogate macrophages - a process termed “microglia replacement.” Microglia replacement has the potential for precise and personalized delivery of therapeutic payloads or correction of dysfunction. Primary microglia are challenging to manipulate - they rapidly lose transcriptional identity ex vivo, resist viral manipulation, are minimally proliferative, and are highly sensitive to serum (Balcaitis et al., 2005; Jiang et al., 2012; Masuda et al., 2013; Su et al., 2016, Bohlen et al., 2017). These challenges limit the study of microglia replacement, underscoring the need for new, transplantable cell models. Common microglia surrogate cells include myeloid cells from the blood or bone marrow and induced pluripotent stem cell (iPSC)-derived microglia (iMG; Muffat et al., 2016; Pandya et al., 2017; Haenseler et al., 2017; Douvaras et al., 2017; Abud et al., 2017; Takata et al., 2020). Although capable of reconstituting the microglial niche (Priller et al., 2001; Bohlen et al., 2017; Bennett et al., 2018; Hasselmann et al., 2019), primary cells and iPSCs each have limitations that motivated us to consider new microglia replacement tools.
To create an alternative microglia surrogate, we turned to the estrogen-regulated (ER) homeobox B8 (Hoxb8) system, a method for generating and gene-editing unlimited quantities of macrophages from primary murine bone marrow (Wang et al., 2006). When overexpressed, Hoxb8 promotes the expansion of hematopoietic progenitor cells while preventing their differentiation. When transduced with ER-Hoxb8 virus, myeloid progenitor cells from murine bone marrow become immortalized and indefinitely expandable when in the presence of exogenous estrogen, but differentiate upon estrogen removal. The ER-Hoxb8 approach has been used to generate so-called “conditionally immortalized” progenitors with lymphoid and myeloid potential (Wang et al., 2006; Rosas et al., 2011; Redecke et al., 2013; Gurzeler et al., 2013; Zach et al., 2015; Fites et al., 2018; Ma et al., 2020; Lail et al., 2022), including macrophages.
We recently showed that ER-Hoxb8 cells can reconstitute the microglial niche (Chadarevian, Lombroso, et al., 2023), supporting their potential as a new cell model for microglia replacement. Here, we aimed to deeply characterize the identity, function, and application of ER-Hoxb8s as microglia surrogates to test hypotheses about microglia-specific gene functions in health and disease.
Results:
Comparison of ER-Hoxb8 to bone marrow-derived macrophages in vitro
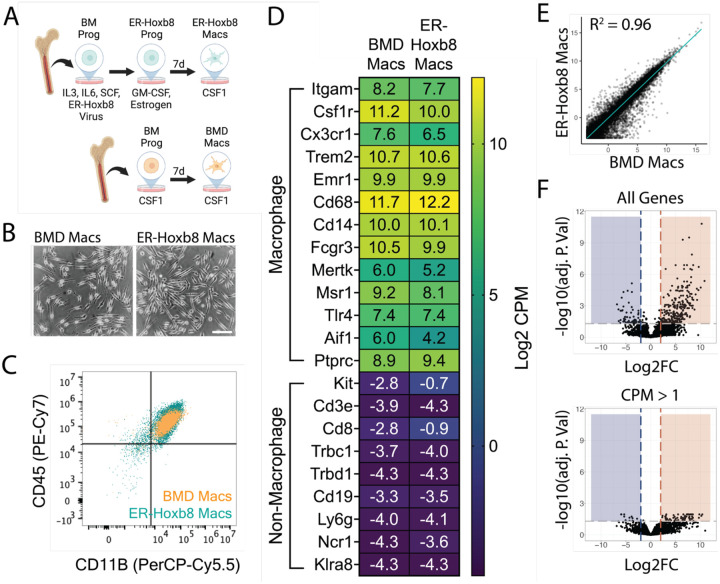
To directly compare primary bone marrow-derived (BMD) and ER-Hoxb8 cells, we created three biologically independent lines of conditionally immortalized macrophage progenitors as described previously (Wang et al., 2006). ER-Hoxb8 progenitor cells were plated, differentiated for seven days in CSF1, and compared to BMD macrophages (Stanley and Heard 1977; Murray et al., 2014; Figure 1A). We found that ER-Hoxb8 macrophages were highly similar to BMD macrophages morphologically (Figure 1B) and by CD11B/CD45 expression (Figure 1C, Supplemental Figures 1A/B).
Figure 1: Comparison of ER-Hoxb8 to BMD macrophages in vitro.
(A) Schematic for creation of bone marrow-derived (BMD) and ER-Hoxb8 cells (B) Brightfield images of BMD and ER-Hoxb8 macrophages plated in the presence of 30ng/mL mouse CSF1 and differentiated for seven days (scale bar = 100um) (C) Dot plot representing CD45/CD11B levels (pre-gated on live, singlet, leukocyte) by flow cytometry (D) Heatmap showing Log2 CPM of canonical macrophage (top) and non-macrophage (bottom) immune cell genes (E) Whole transcriptome comparison between BMD and ER-Hoxb8 macrophages, depicting best fit line and coefficient of determination (one dot = one gene) (F) Volcano plots comparing all genes or those with CPM > 1 (Log2FC >= 2, FDR < 0.05); blue = upregulated in ER-Hoxb8 macrophages, red = upregulated in BMD macrophages
To expand upon previous studies (Redecke et al., 2013; Roberts et al., 2019; Accarias et al., 2020), we performed RNA sequencing of both cell types before and after seven days of differentiation. We generated high-quality transcriptomes (Phred Score > 35, n = 3 biological replicates) for BM progenitors, BM monocytes, seven-day differentiated BMD macrophages, ER-Hoxb8 progenitors, four-day differentiated ER-Hoxb8 cells, and seven-day differentiated ER-Hoxb8 macrophages (top 100 expressed genes in Supplemental Table 1). In agreement with previous literature (Wang et al., 2006), ER-Hoxb8 progenitors express canonical macrophage genes (Supplemental Figure 1C) and, interestingly, ER-Hoxb8 cells at all time points were more similar to BMD macrophages than to BM progenitors or monocytes (Supplemental Figure 1D).
Transcriptionally, seven-day differentiated ER-Hoxb8 macrophages were highly similar to BMD macrophages as demonstrated by expression of macrophage-specific genes and lack of expression of common progenitor, lymphocyte, neutrophil, and natural killer cell-specific genes (Figure 1D). Linear regression analysis between the two cell types (Figure 1E), revealed a strong correlation (R2 = 0.96) between expression levels of all 29,625 expressed genes. We identified 85 genes as differentially expressed between ER-Hoxb8 and BMD macrophages (FDR < 0.05, Log2 Fold Change >= 2, counts per million (CPM) > 1; Figure 1F). Of these, 74 were upregulated in BMD macrophages, 11 were upregulated in ER-Hoxb8 macrophages, and no statistically significant Gene Ontology (GO) terms were found for either group (top ten DEGs by Log2FC in Supplemental Figure 1E, all DEGs in Supplemental Table 2). These data demonstrate that ER-Hoxb8 macrophages are highly similar to primary BMD macrophages in vitro.
ER-Hoxb8 cells engraft the brain parenchyma after intracranial transplantation and attain a microglia-like identity
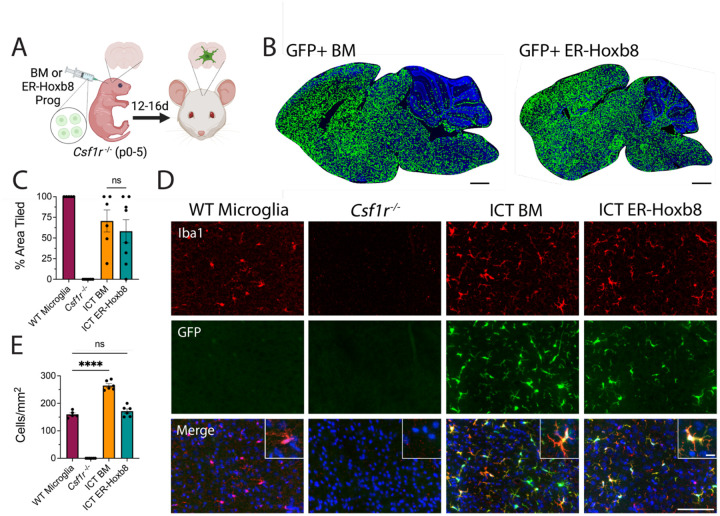
To extend the utility of the ER-Hoxb8 model, we next studied their engraftment and identity following intracranial transplantation into Csf1r−/− hosts, which lack microglia and readily permit donor cell engraftment (Bennett et al., 2018; Figure 2A). Using BMD cells as a primary cell control, early postnatal transplantation at days zero to five (P0–5) led to robust and comparable parenchymal engraftment by 16 days (Figure 2B–D). ER-Hoxb8 macrophages engrafted at equivalent densities (171.7 cells/mm2 +/− 8.0) as endogenous, wild-type (WT) microglia (160.0 cells/mm2 +/− 5.5), while BMD macrophages engrafted at higher densities (264.6 cells/mm2 +/− 6.9; Figure 2E).
Figure 2: Engraftment potential of ER-Hoxb8 compared to BMD macrophages after intracranial transplantation in Csf1r−/− hosts.
(A) Schematic for in vivo Csf1r−/− transplant experiments (B) Rendered tile stitches of Csf1r−/− brains after intracranial injection of GFP+ bone marrow (left) or ER-Hoxb8 (right) progenitor cells (C) Percent of total brain area tiled by donor cells; n = five to seven biological replicates per group; each dot = one biological replicate (average area across three matched sagittal sections) (D) Immunostaining of cortical brain region 12–16 days post-intracranial injection (red = IBA1, green = endogenous GFP, blue = DAPI; scale bar = 100um; inset scale bar = 5um) (E) Cortical density calculations (cells per mm2) between groups; n = five to seven biological replicates per group; each dot = one biological replicate (average density across three regions of interest across three matched sagittal sections). All p-values calculated via one-way ANOVA with multiple comparisons; ns = not significant or p >= 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
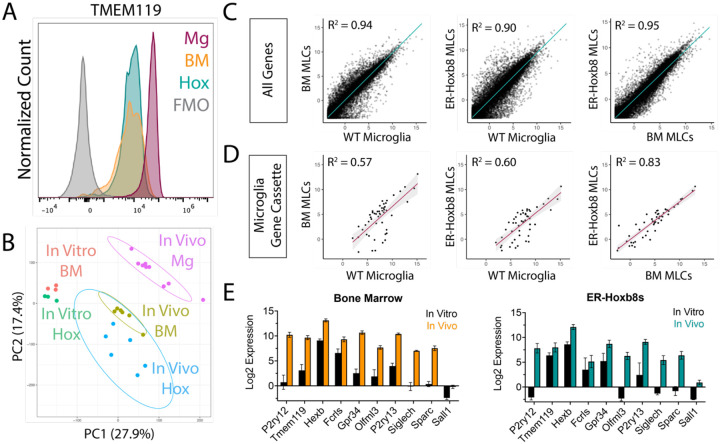
BMD macrophages become “microglia-like” after engraftment in the Csf1r−/− brain (Bennett et al., 2018). Like BMD cells, ER-Hoxb8 cells lack expression of the microglia signature protein TMEM119 in vitro (Supplemental Figure 2A). After brain engraftment, we found comparably high levels of TMEM119 in BMD and ER-Hoxb8 macrophages by flow cytometry, though both had lower TMEM119 expression than endogenous microglia, consistent with prior studies (Bennett et al., 2018; Cronk et al., 2018; Shemer et al., 2018; Figure 3A, Supplemental Figure 2B).
Figure 3: ER-Hoxb8 macrophages become microglia-like cells (MLCs) after engraftment in the Csf1r−/− brain.
(A) Histogram of TMEM119 surface staining by flow cytometry (pre-gated on live, singlet, leukocyte, CD45+/CD11B+) for brain-engrafted cells 14 days post-intracerebral transplantation; Mg = WT Microglia, BM = BMD MLCs, Hox = ER-Hoxb8 MLCs (B) PCA plot comparing in vitro macrophages from Figure 1 with in vivo macrophages; Mg = WT Microglia, BM = BMD MLCs, Hox = ER-Hoxb8 MLCs (C) Whole transcriptome comparison between WT microglia, BMD, and ER-Hoxb8 macrophages in vivo, depicting best fit line and the coefficient of determination (D) Comparison of microglia signature genes (Cronk, et al. JEM (2018)) depicting best fit line and coefficient of determination (E) In vitro and in vivo Log2 CPM gene expression of ten canonical microglia/myeloid genes for bone marrow (left) and ER-Hoxb8s (right)
To more comprehensively assess microglial identity, we generated high-quality bulk RNA sequencing transcriptomes (Phred Score > 35; n = 6–9 biological replicates) from isolated brain-engrafted BMD macrophages, ER-Hoxb8 macrophages, and endogenous microglia from age-matched WT hosts. Principal component analysis (PCA) and unsupervised hierarchical clustering revealed that brain residence induces both BMD and ER-Hoxb8 macrophages to become more similar to endogenous microglia (Figure 3B, Supplemental Figure 2C). We saw no evidence for batch effects between harvest days, cell sorter used, or host mouse sex (Supplemental Figure 2D). BMD and ER-Hoxb8 microglia-like cells (MLCs) were transcriptionally similar, by both PCA and linear regression analysis (Figures 3B/C/D). ER-Hoxb8 MLCs upregulated expression of canonical microglia signature genes akin to BMD controls (P2ry12, Tmem119, Hexb, Fcrls, Gpr34, Olfml3, P2ry13, Sparc), including genes highly downregulated in vitro (Figure 3E). Like BMD macrophages, ER-Hoxb8 macrophages lack Sall1 expression in vivo (Figure 3E). Taken together, these data show that, similar to BMD macrophages, ER-Hoxb8 macrophages become “microglia-like” with exposure to signals in the brain microenvironment.
Despite their similarities, BMD MLCs and ER-Hoxb8 MLCs have 650 differentially expressed genes (FDR < 0.05, Log2FC >= 2, and CPM > 1 in at least six of the samples (the “n” of each group); Supplemental Table 3). We performed statistical overrepresentation tests to identify relevant GO terms (Supplemental Table 4). Of particular interest were terms relating to biological processes as they concerned immune activity and reactivity. We discovered that the 34 overrepresented genes for these GO terms (Supplemental Table 5), were unique to ER-Hoxb8 MLCs, suggesting that the brain environment differentially affects engrafted BMD and ER-Hoxb8 cells, and that ER-Hoxb8 MLCs may be more primed to activation.
To gauge how significantly these transcriptomic increases in inflammation-associated genes impact the brain environment, we stained for GFAP, a marker of reactive astrocytosis and a sensitive marker for central nervous system (CNS) perturbations. We found no increase in GFAP expression across brain regions between any groups, including in brains engrafted with ER-Hoxb8 cells compared to BMD cells (Supplemental Figure 2E).
Adar1 mutation reduces macrophage number and induces interferon responses, effects mitigated by JAK inhibition or Ifih1 mutation
Having established ER-Hoxb8 macrophages as microglia-like cells, we explored their potential for study of microglia in health and disease. Building on prior work (Gran et al., 2018; Roberts et al., 2019; Accarias et al., 2021; Bromberger et al., 2022; Shen et al., 2022; Xu et al., 2022; Möller et al., 2023), we used CRISPR-Cas9 to knockout (KO) Tlr4 in Cas9+/− ER-Hoxb8 progenitors, using fluorescence-activated cell sorting (FACS) to establish lines of guide-transduced ER-Hoxb8 progenitors (Supplemental Figure 3A). We confirmed editing by TIDE analysis (Supplemental Figure 3B, Brinkman et al., 2014) and protein loss by flow cytometry (Supplemental Figures 3C/D). We then verified phenotypic knockout by treating unmodified, non-targeting control (NTC) guide-transduced, and Tlr4 guide-transduced ER-Hoxb8 macrophages with lipopolysaccharide (LPS, a Tlr4 agonist) or R848 (a Tlr7/8 agonist). As expected, Tlr4 KO blunted TNF-α production in response to Tlr4 but not Tlr7/8 agonism (Supplemental Figure 3E).
Having validated effective gene targeting, we next leveraged strengths of the ER-Hoxb8 system to study the contribution of microglia to a monogenic CNS disease. Aicardi-Goutières Syndrome (AGS) is a rare, brain-predominant genetic interferonopathy with nine known causal genes (Gavazzi et al., 2024). As many cells respond to interferon, it is unclear which cells drive AGS brain pathology, particularly in cases where global gene knockout is embryonic lethal. We therefore tested the hypothesis that microglia, which produce and react strongly to interferons (Zheng et al., 2015; Aw et al., 2020; Escoubas et al., 2024), are a main contributor to AGS pathology. We first confirmed that the seven coding AGS-causal genes are expressed by ER-Hoxb8 cells at similar levels to those of BMD cells and true microglia (Supplemental Figure 4A). As ADAR1 loss disrupts hematopoiesis (Wang et al., 2004; Hartner 2004), hematopoietic progenitor differentiation (XuFeng et al., 2009), and is embryonically lethal in mice (Wang et al., 2004; Hartner et al., 2004), the impact of ADAR1 loss in macrophages is unknown. We leveraged the ER-Hoxb8 model to study Adar1 loss in macrophages by creating independent Adar1 KO lines using two distinct sgRNAs targeting different exons (Figure 4A, Supplemental Figure 4B).
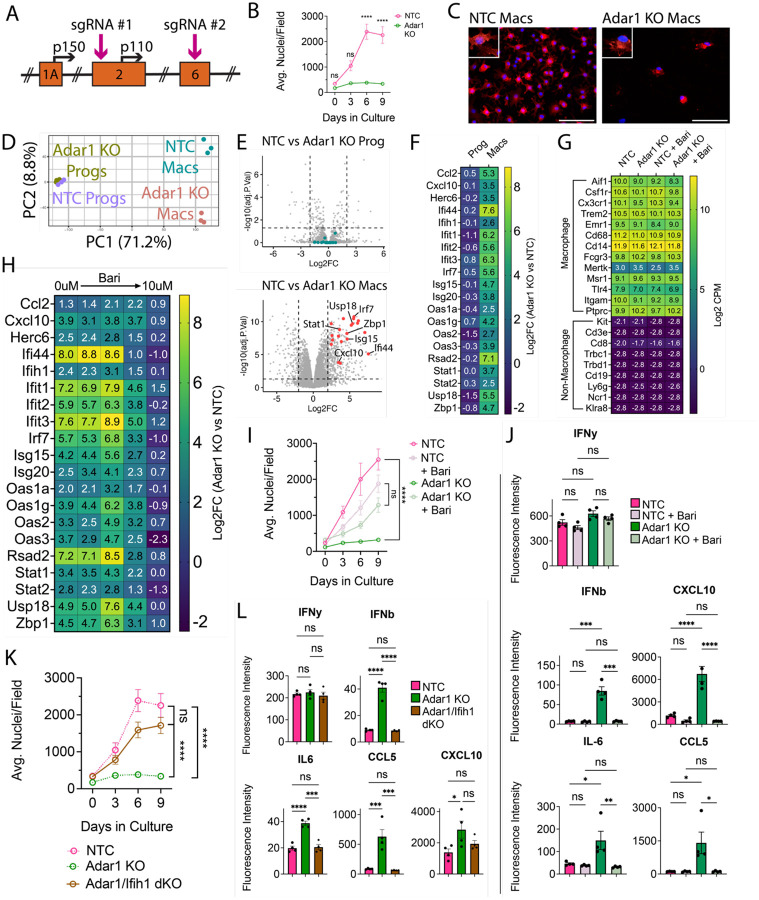
Figure 4: Adar1 mutation prevents macrophage-lineage cell expansion and causes interferon induction, rescued by JAKi or Ifih1 mutation.
(A) Schematic of ADAR1 locus, depicting exons, alternative start sites for p150 and p110 isoforms, and sgRNA targets (B) ER-Hoxb8 cell counts over differentiation time course (C) Immunostaining of in vitro, eight-day differentiated macrophages comparing control (NTC) and Adar1 guide-transduced macrophages (red = CD11B, blue = DAPI; scale bar = 100um) (D) PCA plot of progenitors and macrophages in vitro (E) Volcano plots showing differentially expressed genes between Adar1 KO and NTC progenitors and macrophages (CPM > 1, Log2FC >= 2, FDR < 0.05) (F) Heatmap showing the Log2FC (Adar1 KO values over NTC values) for relevant interferon-stimulated genes (G) Heatmap showing Log2 CPM of canonical macrophage (top) and non-macrophage (bottom) immune cell genes (H) Heatmap showing Log2FC (Adar1 KO over NTC expression) for interferon-stimulated genes in macrophages treated with baricitinib (I) ER-Hoxb8 cell counts over differentiation time course, comparing the effect of baricitinib on Adar KO and NTC lines (dosages = 0uM, 0.00064uM, 0.16uM, 0.4uM, and 10uM) (J) Interferon, cytokine and chemokine production after treatment with baricitinib via cytokine bead array (K) ER-Hoxb8 cell counts over differentiation time course, comparing NTC, Adar1 KO, and Adar/Ifih1 double KO (dKO) lines - dotted NTC and Adar1 KO lines are equivalent to those shown in panel (B) (L) Interferon, cytokine and chemokine production via cytokine bead array, comparing NTC, Adar KO, and Adar/Ifih1 dKO lines. All p-values calculated via one-way ANOVA with multiple comparisons; ns = not significant or p >= 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
We first explored differences during differentiation of Adar1 KO ER-Hoxb8 cells in vitro. Despite normal numbers at three days, there were significantly fewer Adar1 KO cells during the later stages of differentiation as compared to NTC cells (Figures 4B/C, Supplemental Figures 4C/D). This suggests that either Adar1 KO inhibits progenitor differentiation or macrophage proliferation and survival. We next performed RNA sequencing of Adar1 KO progenitors and differentiated macrophages. Adar1 KO progenitors were highly similar to NTC control progenitors (Figure 4D/E/F; all DEGs in Supplemental Table 6), and Adar1 KO macrophages expressed comparable levels of macrophage-identity genes to NTC (Figure 4G), suggesting that Adar1 KO ER-Hoxb8 progenitors can generate macrophages. At the macrophage stage, however, we found 547 DEGs (Figure 4D/E/F; Supplemental Table 7), remarkable for interferon-stimulated gene (ISG) upregulation and GO term enrichment for responses to virus, stress, cytokine stimulation, and innate immunity (Figure 4E/F; Supplemental Figure 4E, Supplemental Table 8).
To test whether Adar1 KO-mediated interferon production underlies this macrophage phenotype, we used the Janus kinase (JAK) inhibitor Baricitinib to inhibit interferon signaling. Baricitinib, a treatment for AGS (Vanderver et al., 2020; Han et al., 2022; Kanazawa et al., 2023), caused a dose-dependent reduction in ISG expression (Figure 4H) and normalized cell counts during macrophage differentiation (Figure 4I, Supplemental Figure 4F). We validated increased type I-specific interferon (IFN-β) and ISG (CXCL10, IL6, CCL5) production by Adar1 KO ER-Hoxb8 macrophages, and their suppression by baricitinib treatment using multiplex bead array (Figure 4J). As interferons inhibit hematopoiesis and differentiation (Demerdash et al., 2021), we confirmed that the baricitinib-treated cells were indeed macrophages (Figure 4G) and that the ISG reduction improved macrophage production.
Melanoma differentiation associated protein 5 (MDA5, or Ifih1) is an epistatic modifier of Adar1 such that deletion rescues some Adar1 phenotypes (Liddicoat et al., 2015; Guo et al., 2022). We introduced a guide targeting Ifih1 (BFP+) to Adar1 KO cells (GFP+). We created single cell clones and validated double KO (dKO, GFP/BFP+) by TIDE analysis (Supplemental Figure 4H). Ifih1 loss completely rescued the Adar1 KO cell growth deficit during differentiation (Figure 4K), and normalized production of IFN-β, IL-6, and CCL5 (Figure 4L), but not CXCL10. As with baricitinib treatment (Supplemental Figure 4G), we found that multiple chemokines/cytokines are downregulated in Adar1-mutant cells as compared to NTC (TNF-α, IL-17, MIP-1a, MIP-1B, MIP-2, M-CSF), and are partially rescued by Ifih1 deletion (Supplemental Figure 4I).
Together, these data show that Adar1 deletion impairs macrophage but not progenitor health and is associated with interferonopathy, demonstrating the utility of the ER-HoxB8 model system for experimental isolation and study of macrophage dysfunction in a genetic disease.
Adar1 mutation prevents ER-Hoxb8 engraftment
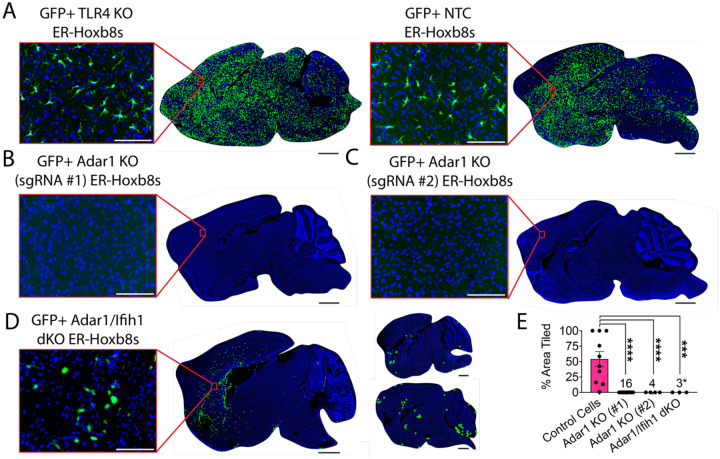
We next attempted to study how Adar1 KO macrophages affect the CNS. To our surprise, although TLR4 KO gene edited macrophages engrafted robustly (n = 10; Figure 5A/E), mice injected with Adar1 KO cells died early and showed no engraftment in the brains of any transplanted mice that survived to endpoint (n = 3; Figure 5B/C).
Figure 5: Adar1 mutation prevents ER-Hoxb8 engraftment in the Csf1r−/− mouse, partially rescued by Ifih1 deletion.
(A) Representative rendering of donor cell engraftment (scale bar = 1000um) with inset microscopy of GFP+ donor cell engraftment (green = endogenous GFP, blue = DAPI; scale bar = 100um) for control cells (TLR4 KO and NTC) harvested 7–15 days post-injection (dpi), (B) Adar1 KO (sgRNA #1) cells (harvest details in (E)), (C) Adar1 KO (sgRNA #2) cells harvested 9–12dpi, and (D) Adar1/Ifih1 double KO (dKO) cells harvested 10–15dpi (rendered dots in two right brains enlarged 5x for visualization) (E) Percent of total brain area tiled with cells between groups (numbers denote “n” per group); Adar1 KO (#1) cells include pooled data (brains injected with 300k cells/hemisphere, harvested at 10–15 days post-injection (dpi; n = 3); brains injected with 300k cells/hemisphere, harvested at 4–8dpi (n=8); brains injected with 50k cells/hemisphere, harvested at 13dpi (n = 4); and brains injected with 100k cells/hemisphere pre-treated with 0.5uM Baricitinib, mice treated daily with 1mg/kg Baricitinib, harvested at 5dpi (n = 1)); asterisk indicates samples where engraftment is present but does not meet criteria for tiled brain area, as exemplified in (D); p-values calculated via one-way ANOVA with multiple comparisons
Because blocking interferon signaling with baricitinib or Ifih1 deletion correlated with better macrophage survival in vitro, we wondered if modifying our approach to limit interferon tone would permit Adar1 KO cell engraftment. We first reduced the number of injected cells (n = 4) and harvested tissues earlier, between 4–8 days post-transplant (n = 8), and did not observe engraftment. We then tried to pre-treat donor cells and host mice with Baricitinib and likewise did not observe engraftment in surviving mice (n = 1; Figure 5E). Lastly, we transplanted Adar1/Ifih1 dKO cells (n = 3), which showed partial rescue of Adar1 KO phenotypes in vitro. Although we detected no areas of engraftment meeting the stringent criteria applied for our “percent area” quantification method, we noted diffuse patches of engrafted cells with an unramified, rounded morphology, in three of three experimental replicates (Figure 5D/E), potentially consistent with a mild partial rescue of engraftment.
Csf1r−/− mice have severe constitutional, skeletal, and CNS abnormalities, typically succumbing by weaning age (Dai et al., 2002). To extend our findings about Adar1 KO macrophages in vivo we applied an inducible microglia depletion model using healthy Cx3cr1CreERT; Csf1rfl/fl hosts (Bennett et al., 2018). After depleting endogenous microglia via subcutaneous tamoxifen injection at age P1 and P2, we intracerebrally transplanted cells into Cx3cr1CreERT; Csf1rfl/fl brains (Supplemental Figure 5A). After 7–15 days in vivo, we saw engraftment of control (TLR4 KO and NTC; n = 4; Supplemental Figure 5B), but not Adar1 KO cells (n = 5; Supplemental Figure 5C). Further mirroring the Csf1r−/− data, we saw small, diffuse patches of engrafted Adar1/Ifih1 dKO cells, but none reached quantifiable levels of engraftment (n = 11; Supplemental Figure 5D). Lastly, we harvested transplanted Cx3cr1CreERT; Csf1rfl/fl hosts early, at day three, and saw a diffuse pattern of non-quantifiable but parenchymally engrafted Adar1 KO cells (n = 4; Supplemental Figure 5E). These data suggest that Adar1 KO cells can enter the brain parenchyma but do not persist, consistent with the reduction in cell numbers observed in vitro during macrophage differentiation.
Overall, these results show that genetically modified ER-Hoxb8 cells robustly engraft in the brain parenchyma, which is prevented by Adar1 KO, independent of interferon production.
Adar1 D1113H mutant ER-Hoxb8 macrophages drive brain ISG expression
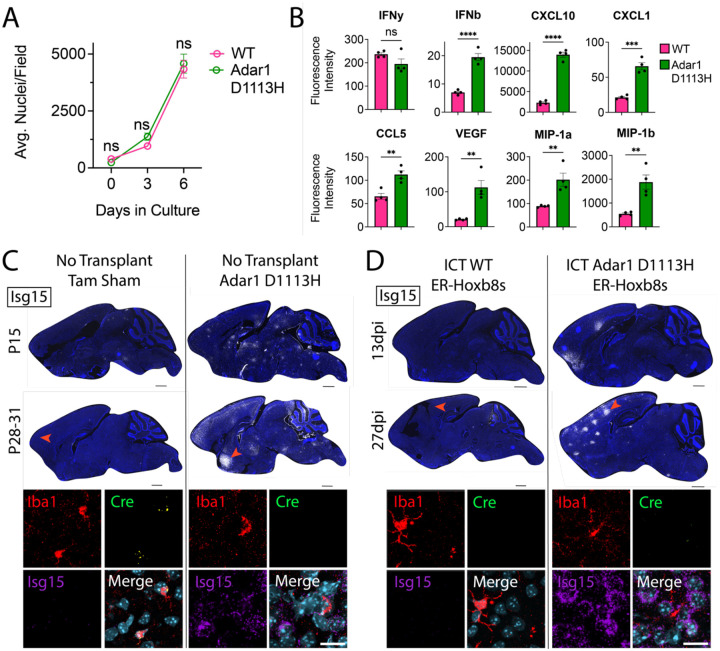
Most available mouse models of AGS are either embryonic lethal (Wang et al., 2004; Hartner et al., 2004; Liddicoat et al., 2015) or fail to produce CNS phenotypes (Morita et al., 2004; Ward et al., 2011; Hiller et al., 2012; Pereira-Lopes et al., 2013; Behrendt et al., 2013; Rehwinkel et al., 2013; Ohto et al., 2022). We recently created a viable mouse model harboring a patient-derived mutation (D1113H) in the catalytic domain of ADAR1, which displays robust brain ISG expression, astrocytosis, microgliosis, and white matter calcifications (Guo et al., 2022). Since AGS patient mutations are typically hypomorphic (rather than knockout), this more authentically models AGS (Rice et al., 2014). Given the non-engraftment of Adar1 KO cells, we created ER-Hoxb8 cells from Adar1 D1113H mice to explore the impact of AGS-specific mutations in microglia. Unlike Adar1 KO cells, D1113H ER-Hoxb8s showed normal growth and expansion during in vitro differentiation (Figure 6A), despite a similarly increased production of IFN-β, ISGs, and other cytokines, including CXCL10, CXCL1, CCL5, VEGF, MIP-1a, and MIP-1b, but not IFN-γ (Figure 6B).
Figure 6: Adar1 D1113H mutant ER-Hoxb8 macrophages persistently drive brain ISG expression.
(A) In vitro ER-Hoxb8 cell counts over time (p-values calculated via one-way ANOVA with multiple comparisons) (B) Multiplex bead array data for interferons, cytokines, and chemokines produced via ER-Hoxb8 macrophages (p-values calculated via one-way ANOVA with multiple comparisons) (C) Sagittal sections of non-transplanted (tamoxifen (tam) sham control) Cx3cr1CreERT; Csf1rfl/fl brains (left) and Adar1 D1113H mutant brains (right) at age P15 and P28–31; nuclei (blue, DAPI), Isg15 (white via RNA in situ hybridization (ISH)); scale bar = 1000um; red arrow depicts location of corresponding closeup images below, showing IBA1 (red, protein stain), Cre (green, ISH), Isg15 (purple, ISH), and nuclei (teal, DAPI); scale bar = 20um; see Supplemental Figure 7A for further corresponding closeup images (D) Sagittal sections of Cx3cr1CreERT; Csf1rfl/fl brains intracranially transplanted with WT ER-Hoxb8s (left) and Adar1 D1113H ER-Hoxb8s (right) at 13 and 27 days post-injection (dpi); nuclei (blue, DAPI), Isg15 (white, ISH); scale bar = 1000um; red arrow depicts location of corresponding closeup images below, showing IBA1 (red, protein stain), Cre (green, ISH), Isg15 (purple, ISH), and nuclei (teal, DAPI); scale bar = 20um; see Supplemental Figure 7A for further corresponding closeup images
We next characterized D1113H ER-Hoxb8 cells after transplantation into the Cx3cr1CreERT; Csf1rfl/fl brain. As D1113H cells are not genetically tagged, we performed RNA in situ hybridization (ISH), using Cre expression (Supplemental Figure 6A/B) to distinguish endogenous microglia (Cre+) from donor cells (Cre-). Unlike Adar KO cells, D1113H mutant ER-Hoxb8s engrafted the parenchyma (Figures 6C/D, n = 5 biological replicates). Notably, both D1113H homozygous mutant brains (Figure 6C) and mice engrafted with D1113H ER-Hoxb8 microglia-like cells exhibited similar and persistent upregulation in brain Isg15, with pockets of robust expression around engrafted Iba1+ MLCs, clustering around Iba1− nuclei that morphologically resemble neurons (Figure 6D, Supplemental Figure 7A). To get a clearer idea of their overall engraftment potential, we transplanted D1113H mutant ER-Hoxb8s into Csf1r−/− mice, which are unconfounded by repopulating endogenous microglia. Similar to brains transplanted with Adar1/Ifih1 dKO cells, we found dispersed cell engraftment that did not reach quantifiable levels (Supplemental Figure 7B, n = 3 biological replicates). Consistent with results from Cx3cr1CreERT; Csf1rfl/fl host mice, we saw upregulation in brain Isg15 clustering around Iba1− nuclei (Supplemental Figure 7C, n = 3 biological replicates).
In sum, D1113H ER-Hoxb8 macrophages produce type I interferon and ISGs and induce Isg15 production by neighboring cells after brain engraftment. These results demonstrate that Adar1 mutation in microglia-like cells is sufficient to drive brain ISG production.
Discussion:
Realizing the potential of microglia replacement requires new tools for research and therapy development. Here, we show the power of estrogen-regulated (ER) homeobox B8 (Hoxb8) conditionally immortalized myeloid cells to robustly model microglia for rapid assessment of gene-targeted perturbations on their identity and function. We find that ER-Hoxb8 macrophages are similar to bone marrow-derived (BMD) macrophages both in vitro and in vivo, where they robustly engraft the microglia-deficient brain and upregulate microglial identity genes. We then use this model to demonstrate an approach to untangle macrophage contributions to a neurological disease, establishing the impact of macrophage-specific mutations to clinically relevant phenotypes both in vitro and in vivo.
Microglia are difficult to target and manipulate for preclinical study, necessitating the use of robust microglial surrogates for discovery, such as the ER-Hoxb8 system described here. Powerfully programmed by the brain environment, microglia lose their unique transcriptional identity in vitro (Bohlen et al., 2017; Gosselin et al., 2017) and are difficult to engineer (O’Brien et al., 2022). Common surrogates include macrophages from isolated bone marrow or immortalized cell lines derived from macrophages or microglia (Stanley and Heard, 1977; Raschke et al., 1978; Tsuchiya et al., 1980; Righi et al., 1989; Blasi et al., 1990; Chanput et al., 2014; Muffat et al., 2016; Timmerman et al., 2018).
BMD cells are finite in number and exist as a heterogeneous population. Cell lines such as induced pluripotent stem cell-derived microglia-like cells can be expensive to generate and time-intensive to maintain (Timmerman et al., 2018). ER-Hoxb8 cells are expandable, readily transducible, and brain-engraftable, making them a robust microglia surrogate. Building on the work of others (Roberts et al., 2019; Accarias et al., 2020; Xu et al., 2022), we demonstrated straightforward gene editing using CRISPR-Cas9, generated stable lines using standard cell culture reagents, and found that ER-Hoxb8s are transcriptionally similar to primary BMD cells in vitro and in vivo. We used these strengths (and spared months of generating transgenic mouse lines) to readily test hypotheses about Adar1 function in microglia to demonstrate the potential of this system. We generated Adar1 knockout (KO) ER-Hoxb8 lines, as well as ER-Hoxb8 lines derived from mice harboring Adar1 patient mutations, to study the effect of microglia-specific Adar1 mutation on macrophage generation and interferon production. The ease with which ER-Hoxb8 cells can be modified, sorted, clonally expanded, banked, and transplanted was critical to these discoveries and will enable further innovation for the study of central nervous system macrophages.
In the future, the ER-Hoxb8 system will enable additional study of Adar1 and microglia in Aicardi-Goutières Syndrome (AGS). We found that both complete Adar1 KO, as well as an RNA-editing catalytic domain mutation in microglia-like cells, leads to increased interferon responses cell-autonomously in vitro, as well as non-cell-autonomously as demonstrated by direct transplant. This effect is blocked by Ifih1/MDA5 deletion, which is thought to sense abnormal RNA species formed by Adar1 mutation and rescues Adar1-catalytic domain point mutation phenotypes (Liddicoat et al., 2015; Guo et al., 2022). In contrast, brain engraftment is impacted by Adar1 KO regardless of Ifih1 genotype and not by Adar1 catalytic domain mutation, suggesting that the full KO phenotype may be independent of RNA editing and interferon production. Mitochondrial antiviral signaling (MAVS) adaptor protein rescues the embryonic lethality of Adar1 null mice (Mannion et al., 2014), suggesting that manipulation of other Adar1 pathway components could reveal Adar1’s role in brain engraftment and macrophage maintenance. This system can allow for interrogation of alternative Adar1 functions, as well as other AGS mutations, and enables testing of AGS-targeted therapies.
All cell models have limitations, and we highlight two of particular relevance to the study of microglia. First, like primary BMD macrophages, ER-Hoxb8 macrophages derive from definitive as opposed to primitive hematopoiesis - the authentic origin of microglia. Though very similar to microglia, ER-Hoxb8s cannot attain full microglial identity, conspicuously lacking Sall1 expression, an important transcription factor in regulating homeostatic microglia (Buttgereit et al., 2016). Second, we observe that ER-Hoxb8 macrophages express slightly higher levels of inflammation-associated genes than primary BMD macrophages after brain engraftment. Though insufficient to elicit GFAP upregulation, a sensitive biomarker for inflammation, it remains an important consideration for experimental design. Interestingly, a recent study suggests it may be possible to address both limitations by treating yolk sac progenitors with the ER-Hoxb8 virus to generate macrophages with reduced inflammatory reactivity that may serve as more authentic microglia surrogates (Elhag et al., 2021). It also raises the exciting potential of an analogous system for study of human, patient-derived macrophages.
More broadly, the future of engineered microglia surrogates, including ER-Hoxb8 microglia-like cells, is bright. Leveraging the advantages of ER-Hoxb8 cells, one could create controllable gene editing via an inducible Cas9 or sgRNA and perform large, pooled screens in vitro and in vivo simultaneously, using progressively advanced methods for gene targeting. We envision microglia as potent neurotherapeutics (Bennett and Bennett, 2020), and toward that goal, the ER-Hoxb8 model provides an ideal milieu for the testing of therapeutic cell engineering strategies, such as payload delivery, synthetic receptors, and customizable gene circuits. Here we applied two engraftment models, utilizing both Csf1r−/− and inducible Cx3cr1CreERT; Csf1rfl/fl host mice. Pairing the ER-Hoxb8 system with our CSF1R inhibitor-resistant receptor transplantation models will allow even more widespread adoption of these techniques (Chadarevian, Lombroso, et al., 2023). Together, the combination of accessible and modifiable microglial surrogates alongside increasingly effective transplantation models holds great potential for research and therapy development.
Materials/Methods:
Mouse Models
All animal studies were performed with approval from the Children’s Hospital of Pennsylvania IACUC panel in accordance with institutional and national regulations. All animals were housed in a non-barrier facility with 12-hour light/dark cycles at 23+/−2 degrees C in ventilated cages with no more than five animals per cage. Animals were provided water and standard chow ad libitum. Cages and bedding were changed weekly.
Csf1r−/− (FVB.129×1-Csf1rtm1Ers) and Csf1r+/+ littermate animals on the FVB background were a generous gift from Dr. Richard Stanley (Albert Einstein College of Medicine). Cx3cr1CreERT; Csf1rfl/fl mice were generated by intercrossing JAX 021212 and 021160 strains. Adar D1113H mice (p.Asp1113His) were a generous gift from Dr. Qingde Wang (University of Pittsburgh). For experiments using GFP-expressing donor cells, we backcrossed Osb-GFP (C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ (JAX 006567)) onto FVB WT mice (JAX 001800) for 22 generations. For experiments using constitutively-expressed Cas9 donor cells, we used FVB.129(B6)-Gt(ROSA)26Sortm1.1(CAG-cas9*,-EGFP)Fezh/J mice (JAX 026558).
In Vitro Experiments
ER-Hoxb8 Conditionally Immortalized Cell Production
Immortalization of murine myeloid progenitor cells was completed as outlined in Wang et al. (2006). Briefly, bone marrow progenitors were isolated from the femurs and tibias of wildtype or Cas9-expressing FVB/NJ mice using a Percoll density separation (Cytiva, 17544501). Cells were cultured for three days in RPMI media (Invitrogen, 7240047) supplemented with 10 ng/mL IL3 (Peprotech, 213–13), IL6 (Peprotech, 216–16), and SCF (Peprotech, 250–03) before transduction with MSCVneo-HA-ER-Hoxb8 virus. Originally created by Dr. David Sykes (Massachusetts General Hospital), the MSCVneo-HA-ER-Hoxb8 plasmid was a generous gift from Dr. Igor Brodsky (University of Pennsylvania School of Veterinary Medicine). 24 hours after transduction, the cells were recovered and grown for 48 hours in RPMI media (Invitrogen, 7240047) supplemented with 1mM Na-Pyruvate (Invitrogen, 11360070), 0.0005 mM beta-estradiol (Sigma Aldrich, E2758), and 10 ng/mL GM-CSF (Peprotech, 315–03). Cells were then selected for transduction with the addition of 1mg/mL Geneticin (Thermofisher, 10131035) for 48 hours. Cells were then expanded and passaged every 48–72 hours at 25–50,000 cells/mL for two weeks, or until all non-immortalized cells were terminally differentiated or killed.
Bone Marrow Isolations
Bone marrow harvests were completed as previously described (Bennett et al., 2016). Briefly, femurs and tibias were dissected and flushed with 1x PBS to collect whole bone marrow. Red blood cells were then lysed with ACK Lysis Buffer (Quality Biological, 118–156-101). For experiments requiring bone marrow progenitors, whole bone marrow samples were subsequently enriched for lineage-negative cells using the Miltenyi Direct Lineage Cell Depletion Kit (130–110-470) via MACS Column separation with LS Columns (Miltenyi, 130–042-401). For experiments requiring bone marrow monocytes, whole bone marrow samples were subsequently enriched for monocytes using the Miltenyi Monocyte Isolation Kit (130–100-629) via MACS Column separation with LS Columns.
Macrophage Differentiation
Bone marrow-derived macrophages were differentiated in vitro by plating isolated bone marrow-derived progenitors in petri dishes at a density of five million cells per dish in differentiation media - DMEM media (ThermoFisher, 10569010), 10% FBS (VWR, 89510–186), 1% Penn/Strep (Invitrogen, 15140122), supplemented with 30 ng/mL murine M-CSF (Peprotech, 315–02). Cells were differentiated for seven to nine days in a 37 degree C incubator with 5% CO2, with media changes every two to three days.
ER-Hoxb8-derived macrophages were differentiated in vitro using methods adapted from Wang, et al. (2006). Briefly, ER-Hoxb8 progenitors were plated in petri dishes at a density of two million cells per dish, and then differentiated in the same media and timetable as noted above.
Viral Production and Transduction
To create retroviral supernatants, Lenti-X 293T cells (Takara Bio, 632180) were transfected using 850 ng/mL pCL-Eco (Addgene, 12371) and 850 ng/mL desired retroviral plasmid, supplemented with 5 ul/mL LipoD293 (SignaGen, 504782) in media containing DMEM (ThermoFisher, 10569010), 25mM HEPES (Invitrogen, 15630080), 10% FBS (VWR, 89510–186), and 1% Penn/Strep (Invitrogen, 15140122). Six hours post-transfection, media was replaced and viral supernatants were collected 48 and 72 hours later. Viral collections were combined and concentrated 10x using Retroviral Precipitation Solution (Alstem, VC200) according to manufacturer’s instructions.
Transduction of ER-Hoxb8 progenitors was completed in 12-well plates coated with 1 ug/mL fibronectin (Sigma-Aldrich, F1141). Cells were plated at 200,000 cells/mL and desired titer volume of virus was added. Plates were then spun for 90 minutes at 1000×g. Post-spinfection, cells were gently mixed and resuspended in an additional 2–3 mL of fresh media. 24 hours post-transduction, cells were recovered and expanded at normal growing conditions (25–50,000 cells/mL at 37 degrees C, 5% CO2).
CRISPR Editing
Target gene guide sequences were created using the Broad Institute’s CRISPick website (Mouse GRCm38 reference genome, CRISPRko mechanism, SpyoCas9 enzyme, Hsu (2013) tracrRNA) and adapted to ligate into our plasmid backbone. This backbone, which was a generous gift from Dr. Will Bailis (University of Pennsylvania; Bailis et al., 2019) was adapted from the MSCV-MigR1-GFP/BFP plasmid (Addgene, 27490) to include a U6-driven gRNA scaffold for the insertion of guide sequences. The BbsI-HF restriction enzyme (NEB, R3539) was used to linearize the plasmid backbone at the gRNA scaffold site, and annealed guide oligos were ligated in. The ligated plasmids were then transformed using NEB Stable Cells (C3040), plated onto LB plates supplemented with 50ug/mL ampicillin, and grown for 24 hours at 30 degrees C. Correct insertion of guide sequences were confirmed using bacterial colony sanger sequencing via GeneWiz/Azenta, and full plasmid sequences were confirmed using Plasmidsaurus. Viral supernatants were then created as described above and Cas9-expressing ER-Hoxb8 macrophage progenitor cells were transduced as described above. Cells were then double-FACS sorted for transduction via fluorophore expression and expanded as normal. Successful editing was confirmed using TIDE as described in Brinkman et al. (2014). Briefly, gDNA was collected from both control and experimental macrophages and the cut site was amplified via PCR and sent for Sanger sequencing. Indel creation was then confirmed via the TIDE application (Brinkman et al., 2014; http://shinyapps.datacurators.nl/tide/). When an antibody was commercially available, such as with TLR4 (Biolegend, 145403, PE), loss of protein was also confirmed via flow cytometry.
TNF-α ELISA
ER-Hoxb8 progenitor cells were plated and differentiated as described in the above “Macrophage Differentiation” methodology. After seven days in vitro, cells were gently removed from the petri dishes using ice cold 1x PBS + 2mM EDTA (ThermoFisher, 15575020). Cells were then re-plated at 50,000 cells/well in a TC-treated 96-well plate in 100ul fresh differentiation media. 24 hours later, LPS (Sigma, L2880–25MG) and R848 (Invivogen, TLRL-R848) were added at 100 ng/mL to respective wells. After 9.5h, supernatants were collected and diluted to 25% initial concentration to stay within kit ranges. TNF-α concentrations were measured using the TNF alpha Mouse Uncoated ELISA Kit with Plates (ThermoFisher, 88–7324-22) according to manufacturer’s instructions.
Adar1 Immunofluorescence
ER-Hoxb8 progenitor cells were plated and differentiated as described in the above “Macrophage Differentiation” methodology. NTC and Adar1-edited cells were plated at 5,000 cells per well in a glass-bottom 96-well plate. Cells were fixed on day eight of differentiation in 4% PFA ((EMS, 15714) for 10 minutes. Cells were washed, blocked in 0.1% Tween20 (Bio-Rad, 1706531) + 5% donkey serum (Sigma, S39–100ML) for one hour, and then stained overnight with CD11B (BioLegend, 101208) and Hoescht 33342 Solution (ThermoFisher, 66249) at 4 degrees C. The following morning, cells were washed and imaged at 40x magnification Z-stacks using a BZ-X800 (Keyence) microscope.
Adar1 Cell Quantification
ER-Hoxb8 progenitor cells were plated and differentiated as described in the above “Macrophage Differentiation” methodology. NTC and Adar1-edited cells were plated at 5,000 cells per well in glass-bottom 96-well plates (n = 3 independently differentiated replicates per condition). Cells were plated in fresh differentiation media supplemented with nothing, vehicle (DMSO), or 10uM Baricitinib (Selleck Chemicals, S2851). Cells were plated with 125uL media on day 0, 62.5uL media was added on day 3, media was removed and replaced with 100uL media on day 6. On differentiation days 0, 3, 6, and 9, corresponding subsets of cells were stained with 1:10000 Hoescht for 30 minutes at 37 degrees C and imaged with the ImageXpress Micro Confocal System. Images were taken at 10x magnification at nine sites per well, with exposure settings consistent between wells. Cell number quantification was performed using the “Count Nuclei” pipeline in the ImageXpress software. Signal thresholds were set, masks were created over the Hoescht+ signal, and nuclei were counted (3–16 pixel width). Cell counts were averaged across the nine imaging sites and plotted as average nuclei per field per day of differentiation.
Adar1 Multiplex Arrays
ER-Hoxb8 progenitor cells were plated and differentiated as described in the above “Macrophage Differentiation” methodology. NTC and Adar1-edited cells (Adar1 KO #1, Adar1 KO #3, Adar1/Ifih1 dKO) were plated at 100,000 cells per well in an untreated 24-well plate (n=4 independently differentiated replicates per condition), whereas WT and D1113H cells were plated at 25,000 cells per well in an untreated 24-well plate (n=4 independently differentiated replicates per condition). Media was changed every two days, with the baricitinib groups receiving 0uM, 0.00064uM, 0.16uM, 0.4uM, or 10uM Baricitinib (Selleck Chemicals, S2851) with each media change. On day seven of differentiation (24h post final media change), cell supernatant was collected, spun down, and immediately frozen. Multiplexing analysis was performed using the LuminexTM 200 system by Eve Technologies Corp. (Calgary, Alberta). Assays used include the Mouse Cytokine/Chemokine 44-Plex Discovery Assay® Array (MD44), the Mouse Cytokine/Chemokine 32-Plex Discovery Assay® Array (MD32), as well as the Mouse IFN-α + IFN-β Assay (MDIFNAB). For the baricitinib groups, RNA was immediately collected from adherent cells for subsequent sequencing analyses.
In Vivo Experiments
Neonatal Transplantation
For intracranial transplantation into Csf1r−/− mice, p0-p5 pups were injected as previously described (Bohlen et al., 2017) by hand using a pulled glass capillary tube (World Precision Instruments, 1B100F-4) in an electrode holder connected by silicon tubing to a syringe. One microliter containing a single-cell suspension (300,000 cells/ul unless otherwise noted) of donor cells in 1x PBS was slowly injected bilaterally into cortex, 1–2mm anterior and 2–3mm lateral to lambda at a depth of 0.5–1mm. Host animals were harvested 12–16 days after injection unless otherwise noted.
Microglia/MLC Isolations
Single-cell suspensions of microglia/engrafted brain macrophages were isolated as previously described (Bennett et al., 2016). Briefly, mice were anesthetized using a cocktail of ketamine (100mg/kg) plus xylazine (10mg/kg) in 1x PBS and perfused with 10mL cold PBS. Brains were dounce homogenized in 10mL cold Medium A Buffer - 10% 10x HBSS (Fisher, 14185052), 1.5% 1M HEPES (Invitrogen, 15630080), 1.67% 30% glucose (Sigma, G7021–1KG), 86.8% ddH2O supplemented with 2% DNase (Worthington, LS002007). Homogenate was filtered through a 70um strainer and pelleted. Microglia were collected following a spin with isotonic percoll - 10% 10x HBSS, 90% Percoll PLUS density gradient media (GE Healthcare, 17544501). Cells were washed with Medium A Buffer and resuspended in desired media.
In Vivo Baricitinib Treatment
Baricitinib (Selleckchem, S2851) was reconstituted in DMSO (Sigma-Aldrich, D26250) and added to a diluent of 40% PEG300 (Sigma-Aldrich, 90878), 10% Tween80 (Sigma-Aldrich, P1754), and 50% water. Mice were administered 20uL daily by intraperitoneal injection at doses of 10mg/kg, 5mg/kg, or 1mg/kg.
Staining Experiments
Immunofluorescence
Mice were perfused with 15mL 1x PBS followed by 15mL 4% PFA (EMS, 15714) and organs were then drop-fixed in 4% PFA for 16–24 hours at 4 degrees C. Organs were then cryo-protected in 30% sucrose (Neta Scientific, SIAL-S5391–25KG), embedded in OCT (Fisher, 23–730-571), cryo-sectioned at 16um, mounted on Superfrost Plus slides (Fisher, 1255015), and frozen at −80 degrees C. Slides were then thawed at 60 degrees C, rehydrated in 1x PBS, and blocked for one hour at RT in blocking buffer - 90% 1x PBS, 9.5% donkey serum (Sigma, S39–100ML), 0.5% Triton X-100 (Sigma, T8787–50ML). After blocking, slides were incubated with primary antibodies (described below) in staining buffer (98.5% 1x PBS, 1% donkey serum, 0.5% Triton X-100) overnight at 4 degrees C. In the morning, slides were washed with 1x PBS and incubated with secondary antibodies (described below) for one hour at RT in staining buffer. Slides were washed once more and coverslipped with DAPI mounting media (EMS, 17989–60) before imaging.
Antibodies used: Rabbit Anti-Iba1 (Fujifilm, 019–19741, 1:500); Mouse Anti-GFAP (Agilent, MAB360, 1:500); Donkey anti-rabbit IgG 594 (ThermoFisher, A-21207, 1:500); Donkey anti-mouse IgG 647 (ThermoFisher, A32787, 1:500)
RNA In Situ Hybridization
In situ hybridization (ISH) was performed on mounted fixed frozen tissues using the RNAscope system per the manufacturer’s protocol (Advanced Cell Diagnostics). Fluorescent detection was achieved using the RNAscope Multiplex Fluorescent Detection kit V2 (cat # 323110). Samples were probed for mouse Isg15 (cat # 559271-C2) and Cre (cat # 312281-C3) and visualized with TSA Vivid dyes (cat #s 7534 and 7536). Following ISH, slides were immunostained with rabbit anti-Iba1 (cat # 019–19741, Fujifilm WAKO) and detected using fluorescently labeled donkey anti-rabbit secondary antibody (A21207, Invitrogen). Slides were coverslipped and nuclei were stained with Prolong Gold Antifade Mounting Medium with DAPI (cat # P36931, Invitrogen)
Imaging Acquisition and Processing
Slides were imaged using a BZ-X800 (Keyence) microscope. Whole-organ tile images plus z-stack images were captured and stitched/compressed using Keyence Analyzer software prior to image export. All images within experiment panels were taken with equivalent exposure settings. All images were then analyzed with FIJI (https://imagej.net/Fiji), equivalently adjusting only for brightness and black values (raw images available upon request).
Engraftment renderings were created using GFP (488) or Iba1 (594) and DAPI channels of 10x stitches. Background was equivalently subtracted using the “subtract background” function before manually thresholding via the “otsu” setting. Rendered dot masks were created by analyzing particles (size = 2–144 px; circularity = 0.5–1.0) and overlaying them on the corresponding DAPI channel. The boundaries of the brains were then outlined using the “polygon selections” tool, and everything outside this boundary was cleared and excluded from the final image.
16 um tissue sections used for RNA scope were imaged using an Andor BC43 spinning disc confocal microscope (Oxford Instruments). Sections that were directly compared were acquired using the same exposure, laser power, gain, and resolution settings. Subsequently, image analysis on raw unmanipulated images was performed using Imaris software. Representative images displayed for publication were processed equally in Imaris across all conditions to best display the data. Comprehensive notes and raw images are available upon request.
Percent Area Quantifications
For each brain sample, average total engrafted area was calculated from three matched sagittal sections (200uM apart starting at the medial-most point of the tissue), each using a 4x tile-scan image collected via Keyence microscope. Calculations were made in FIJI using the Iba1 and/or GFP channel tiled images. FIJI’s “polygon selections” tool and “measure” function were used to draw and calculate a total brain ROI, as well as engrafted area ROI(s). The latter were defined as regions with Iba1+ and/or GFP+ cell groups with no fewer than 50 total cells per “group” nor 50 cells/mm2. Edge limits of engraftment areas were defined by the nucleus of the edge-most cell being no more than 200uM from nearest-neighbor cells. Percent area engrafted was then calculated by dividing total engrafted area(s) by total brain area. Olfactory bulb was omitted for consistency as it was not present in all samples.
For each brain sample, average total area of GFAP+ cells was calculated from three matched sagittal sections (250uM apart starting at the medial-most point of the tissue), each using a 10x tile-scan image collected via Keyence microscope. Calculations were made in FIJI using the GFAP channel tiled images. Background was equivalently subtracted from all images using the “subtract background” function. Images were then converted into 8-bit format and thresholded at 40/255 with the “intermodes” setting. ROIs were manually drawn for cortex, hippocampus, midbrain, and thalamus using FIJI’s “polygon selections” tool. Percent area covered was then calculated using the “measure” function to quantify positive GFAP signal over total ROI area.
Cell Density Quantification
For each brain sample, average cortical cell density was calculated using the same three matched sagittal sections used above to calculate area of engraftment. Here, three 20x representative images were included that spanned the cortical region of engraftment. If the region of engraftment was small, fewer images were included such that no cells overlapped and were counted more than once. Cells were counted manually using FIJI’s “Cell Counter” tool and were defined as cells that were Iba1+ and/or GFP+ and directly overlaid with a DAPI+ nucleus. Cell counts per image were averaged and divided by total area per image to obtain densities at cells/mm2.
Flow Cytometry and FACS
Once isolated into single cell suspensions, cells were washed 2x in FACS Buffer - 1x PBS, 0.5% BSA (Sigma, 126609–10GM), 0.5mM EDTA (ThermoFisher, 15575020) - and blocked with FC Block - 0.2% CD16/CD32 (BD Biosciences, 553142) in FACS Buffer - for 10m at RT. Cells were then stained using antibodies (described below) for 30–60m at 4c. Cells were washed 2x more in FACS Buffer before being analyzed or sorted as described below.
Antibodies used: LIVE/DEAD fixable far red dead cell stain kit (Invitrogen, L10120, 1:1600); Anti-mouse CD45 (PE/Cyanine7, Clone 30-F11, Biolegend, 103113, 1:400); Anti-mouse/human CD11b (PerCP/Cyanine5.5, Clone M1/70, Biolegend, 101227, 1:400); Anti-mouse monoclonal TMEM119 (PE, Clone V3RT1GOsz, Invitrogen, 12–6119-82, 1:400)
For flow cytometry experiments, stained cells were analyzed on a CytoFLEX LX (6 Laser) Flow Cytometer and visualized via FlowJo (10.8.1). For sorting experiments, stained cells were sorted with a 100uM nozzle on a FACSAria Fusion, FACSJazz, Aurora, or MoFlo Astrios depending on Core availability (no effects on data were seen between FACS machines). Cells were deposited into media for cell expansion, or TRIzol LS Reagent (Fisher, 10296028) for subsequent RNA extraction.
Bulk RNA-Sequencing and Analysis
RNA Extraction & Quantification
For in vitro populations, RNA was extracted using the Qiagen RNeasy Mini Kit (74104) according to manufacturer’s instructions. For suspended populations, cells were collected and spun down at 200×g for 5m, supernatant was removed, and 350ul Buffer RLT was added directly to the cell pellet. For adherent populations, supernatant was removed, plates were washed with 1x PBS, and 650ul of Buffer RLT was added directly to the dish to create cell lysates.
For in vivo populations, cells were isolated as described in the above “Microglia/MLC Isolations” methodology and FACS sorted into TRIZol LS reagent as described in the above “Flow Cytometry and FACS” methodology. Cells were briefly vortexed and 0.2 volumes of chloroform (Sigma, C2432–500ML) were added. Cells were again vortexed, incubated for 3m at RT, and then centrifuged at 12,000×g for 15m at 4c. The upper aqueous phase was then collected and 1 volume of fresh 70% EtOH was added. Cells were again vortexed and then run through the Qiagen RNeasy Micro Kit (74004) according to manufacturer’s instructions. Isolated RNA was stored at −80.
When able, RNA Integrity Numbers (RINs) were calculated using an Agilent TapeStation with High Sensitivity RNA ScreenTape (5067–5579) and Sample Buffer (5067–5580) according to manufacturer’s instructions. Cells with a RIN >= 7.5 were used for subsequent library preparations. For in vitro datasets, the average RIN score was 9.03. Quantification was often not possible for brain-isolated cell populations due to low yield. In this case, all samples were used for subsequent library preparations, and quality was assessed later during preparation.
Library Preparation & Sequencing
Sequencing libraries were prepared in-house using either 40ng or 8ul RNA per sample, depending on if RIN scores and concentrations were able to be calculated as discussed above, using the NEBNext Single Cell/Low Input RNA Library Prep Kit for Illumina (NEB, E6420) according to manufacturer’s instructions. Quality and quantity steps were performed using an Agilent TapeStation using High Sensitivity D5000 ScreenTape (5067–5592), and High Sensitivity D5000 Reagents (5067–5593).
Bulk RNA-sequencing of completed libraries was performed by the Children’s Hospital of Philadelphia’s Center for Applied Genomics (CAG) Core Facility using a NovaSeq 6000 (Illumina) system with the SP Reagent Kit v1.5 (2×100bp). Data was de-multiplexed and sent to us as FastQ files via BaseSpace (Illumina).
For sequencing of NTC and Adar1-edited cells with varying doses of Baricitinib, cDNA libraries were sent to Novogene for sequencing using a NovaSeq 6000 (Illumina) system with the S4 Reagent Kit (2×150bp). Data was de-multiplexed and sent to us as FastQ files for manual download. Data sequenced by Novogene was never combined with data sequenced by CAG.
Sequencing Analysis
All pre-processing steps were run using Terminal. FastQ files were downloaded from BaseSpace (Illumina) and concatenated across lanes using the “cat” function. All files were processed using FastP (Chen et al., 2018; Chen, 2023) to filter reads and trim Illumina adaptors, as well as FastQC (Andrews, 2014) to assess quality. All files were determined to be of sufficient quality for subsequent downstream processing. Reads were pseudoaligned to the mouse GRCm39 cDNA transcriptome (Ensembl release 99; Schneider et al., 2017; Zerbino et al., 2018) using Kallisto (version 0.46.0; Bray et al., 2016) and run through MultiQC (Ewels et al., 2016) for a final quality check after mapping.
All post-processing steps were run using RStudio (R Core Team, 2022). Transcripts were summarized to the gene level through tximport (version 1.14.2; Soneson et al., 2015) with abundance counts calculated via TPM and then normalized using TMM via edgeR (Robinson et al., 2010; McCarthy et al., 2012; Chen et al., 2016). Hierarchical cluster dendrograms were created using the “dist” function (method = euclidean) and “hclust” function (method = complete) and were visualized with the plot function. PCA plots were created using the “prcomp” function. Differential gene expression analysis was performed using linear modeling via limma (Ritchie et al., 2015), and differentially expressed genes (DEGs) were decided by applying an FDR cutoff of 0.05, a counts per million (CPM) cutoff of +1, and a Log2(FC) cutoff of +/− 2. Linear models and R2 values were created using the “lm” function. All output plots were visualized with ggplot2 (Wickham, 2016).
Statistical overrepresentation tests and related GO Terms were calculated using the Panther Classification System (Version 18.0). Respective lists of DEGs were manually imported into the Gene List Analysis tab, selected for mus musculus, and run through the statistical overrepresentation test option using the PANTHER GO-Slim options for biological process, molecular function, and cellular component. Tests were run using the Fisher’s Exact option while calculating the false discovery rate. FDR was set to a threshold of 0.05, and terms were ranked via the hierarchy option.
Statistical Calculations
GraphPad Prism (Version 10.0.0) was used to perform statistical tests and generate p values with standard designations (ns = not significant or p >= 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). All values are shown as mean +/− standard error of the mean (SEM). Details regarding replicate numbers and individual statistical test used are provided in the respective figure legends.
Supplementary Material
Acknowledgements & Funding:
We thank Dr. Richard Stanley (Albert Einstein College of Medicine, New York, NY, USA) for the Csf1r−/− (FVB.129X1-Csf1rtm1Ers) and Csf1r+/+ littermate animals on the FVB background; Dr. Qingde Wang (University of Pittsburgh, Pittsburgh, PA, USA) for the Adar1 D1113H mice (p.Asp1113His); Dr. David Sykes (Mass General Hospital, Boston, MA, USA) and Dr. Igor Brodsky (University of Pennsylvania, Philadelphia, PA, USA) for the MSCVneo-HA-ER-HoxB8 plasmid; Dr. Will Bailis (University of Pennsylvania, Philadelphia, PA, USA) for the MG-Guide CRISPR vector; Dr. Dan Beiting for the bulk RNA sequencing DIY Transcriptomics scripts; the Children’s Hospital of Philadelphia Center for Applied Genomics for bulk RNA sequencing assistance; the Children’s Hospital of Philadelphia Flow Cytometry core for flow cytometry and sorting assistance; BioRender.com for graphics; and Kayla Peelman (Emory University, Atlanta, GA, USA) for assistance with Illustrator. This work was supported by National Science Foundation Graduate Research Fellowship Program DGE-1845298 (KMN, SIL); NIH Training in Age Related Neurodegenerative Diseases T32 2-T32-AG-000255-26 (VSC); NIH Medical Scientist Training Program T32 GM007170 (VSC); NIH T32MH019112 (WHA); NIH T32 GM008076 (SIL); Blavatnik Family Fellowship, Blavatnik Foundation (SIL); R01AI139544 (QW); R01NS134651 (QW); NIH DP5OD036159 (MLB); NIH R01-NS-120960-01 (FCB); Klingenstein-Simons Fellowship in Neuroscience (FCB); and The Paul Allen Frontiers Group GRT-00000774 (FCB).
Funding Statement
We thank Dr. Richard Stanley (Albert Einstein College of Medicine, New York, NY, USA) for the Csf1r−/− (FVB.129X1-Csf1rtm1Ers) and Csf1r+/+ littermate animals on the FVB background; Dr. Qingde Wang (University of Pittsburgh, Pittsburgh, PA, USA) for the Adar1 D1113H mice (p.Asp1113His); Dr. David Sykes (Mass General Hospital, Boston, MA, USA) and Dr. Igor Brodsky (University of Pennsylvania, Philadelphia, PA, USA) for the MSCVneo-HA-ER-HoxB8 plasmid; Dr. Will Bailis (University of Pennsylvania, Philadelphia, PA, USA) for the MG-Guide CRISPR vector; Dr. Dan Beiting for the bulk RNA sequencing DIY Transcriptomics scripts; the Children’s Hospital of Philadelphia Center for Applied Genomics for bulk RNA sequencing assistance; the Children’s Hospital of Philadelphia Flow Cytometry core for flow cytometry and sorting assistance; BioRender.com for graphics; and Kayla Peelman (Emory University, Atlanta, GA, USA) for assistance with Illustrator. This work was supported by National Science Foundation Graduate Research Fellowship Program DGE-1845298 (KMN, SIL); NIH Training in Age Related Neurodegenerative Diseases T32 2-T32-AG-000255-26 (VSC); NIH Medical Scientist Training Program T32 GM007170 (VSC); NIH T32MH019112 (WHA); NIH T32 GM008076 (SIL); Blavatnik Family Fellowship, Blavatnik Foundation (SIL); R01AI139544 (QW); R01NS134651 (QW); NIH DP5OD036159 (MLB); NIH R01-NS-120960-01 (FCB); Klingenstein-Simons Fellowship in Neuroscience (FCB); and The Paul Allen Frontiers Group GRT-00000774 (FCB).
References:
- Abud Edsel M., Ramirez Ricardo N., Martinez Eric S., Healy Luke M., Nguyen Cecilia H. H., Newman Sean A., Yeromin Andriy V., et al. 2017. “iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases.” Neuron 94 (2): 278–93.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accarias Solene, Sanchez Thibaut, Labrousse Arnaud, Myriam Ben-Neji Aurélien Boyance, Poincloux Renaud, Maridonneau-Parini Isabelle, and Le Cabec Véronique. 2020. “Genetic Engineering of Hoxb8-Immortalized Hematopoietic Progenitors - a Potent Tool to Study Macrophage Tissue Migration.” Journal of Cell Science 133 (5). 10.1242/jcs.236703. [DOI] [PubMed] [Google Scholar]
- Andrews S. 2014. “FastQC a Quality-Control Tool for High-Throughput Sequence Data.” 2014. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Bailis Will, Shyer Justin A., Zhao Jun, Garcia Canaveras Juan Carlos, Al Khazal Fatimah J., Qu Rihao, Steach Holly R., et al. 2019. “Distinct Modes of Mitochondrial Metabolism Uncouple T Cell Differentiation and Function.” Nature 571 (7765): 403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcaitis Stephanie, Weinstein Jonathan R., Li Sheng, Chamberlain Jeffrey S., and Thomas Möller. 2005. “Lentiviral Transduction of Microglial Cells.” Glia 50 (1): 48–55. [DOI] [PubMed] [Google Scholar]
- Behrendt Rayk, Schumann Tina, Gerbaulet Alexander, Nguyen Laura A., Schubert Nadja, Alexopoulou Dimitra, Berka Ursula, et al. 2013. “Mouse SAMHD1 Has Antiretroviral Activity and Suppresses a Spontaneous Cell-Intrinsic Antiviral Response.” Cell Reports 4 (4): 689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett F. Chris, Bennett Mariko L., Yaqoob Fazeela, Mulinyawe Sara B., Grant Gerald A., Gephart Melanie Hayden, Plowey Edward D., and Barres Ben A.. 2018. “A Combination of Ontogeny and CNS Environment Establishes Microglial Identity.” Neuron 98 (6): 1170–83.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett Mariko L., and Bennett F. Chris. 2020. “The Influence of Environment and Origin on Brain Resident Macrophages and Implications for Therapy.” Nature Neuroscience 23 (2): 157–66. [DOI] [PubMed] [Google Scholar]
- Bennett Mariko L., Bennett F. Chris, Liddelow Shane A., Ajami Bahareh, Zamanian Jennifer L., Fernhoff Nathaniel B., Mulinyawe Sara B., et al. 2016. “New Tools for Studying Microglia in the Mouse and Human CNS.” Proceedings of the National Academy of Sciences of the United States of America 113 (12): E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Barluzzi R., Bocchini V., Mazzolla R., and Bistoni F.. 1990. “Immortalization of Murine Microglial Cells by a v-Raf/v-Myc Carrying Retrovirus.” Journal of Neuroimmunology 27 (2–3): 229–37. [DOI] [PubMed] [Google Scholar]
- Bohlen Christopher J., Bennett F. Chris, Tucker Andrew F., Collins Hannah Y., Mulinyawe Sara B., and Barres Ben A.. 2017. “Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures.” Neuron 94 (4): 759–73.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray Nicolas L., Pimentel Harold, Melsted Páll, and Pachter Lior. 2016. “Near-Optimal Probabilistic RNA-Seq Quantification.” Nature Biotechnology 34 (5): 525–27. [DOI] [PubMed] [Google Scholar]
- Brinkman Eva K., Chen Tao, Amendola Mario, and van Steensel Bas. 2014. “Easy Quantitative Assessment of Genome Editing by Sequence Trace Decomposition.” Nucleic Acids Research 42 (22): e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger Thomas, Klapproth Sarah, Sperandio Markus, and Moser Markus. 2022. “Humanized β2 Integrin-Expressing Hoxb8 Cells Serve as Model to Study Integrin Activation.” Cells 11 (9). 10.3390/cells11091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit Anne, Lelios Iva, Yu Xueyang, Vrohlings Melissa, Krakoski Natalie R., Gautier Emmanuel L., Nishinakamura Ryuichi, Becher Burkhard, and Greter Melanie. 2016. “Sall1 Is a Transcriptional Regulator Defining Microglia Identity and Function.” Nature Immunology 17 (12): 1397–1406. [DOI] [PubMed] [Google Scholar]
- Chadarevian Jean Paul, Lombroso Sonia I., Peet Graham C., Hasselmann Jonathan, Tu Christina, Marzan Dave E., Capocchi Joia, et al. 2023. “Engineering an Inhibitor-Resistant Human CSF1R Variant for Microglia Replacement.” The Journal of Experimental Medicine 220 (3). 10.1084/jem.20220857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanput Wasaporn, Mes Jurriaan J., and Wichers Harry J.. 2014. “THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach.” International Immunopharmacology 23 (1): 37–45. [DOI] [PubMed] [Google Scholar]
- Chen Shifu. 2023. “Ultrafast One-Pass FASTQ Data Preprocessing, Quality Control, and Deduplication Using Fastp.” iMeta 2 (2): e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Shifu, Zhou Yanqing, Chen Yaru, and Gu Jia. 2018. “Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor.” Bioinformatics 34 (17): i884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Yunshun, Lun Aaron T. L., and Smyth Gordon K.. 2016. “From Reads to Genes to Pathways: Differential Expression Analysis of RNA-Seq Experiments Using Rsubread and the edgeR Quasi-Likelihood Pipeline.” F1000Research 5 (June):1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk James C., Filiano Anthony J., Louveau Antoine, Marin Ioana, Marsh Rachel, Ji Emily, Goldman Dylan H., et al. 2018. “Peripherally Derived Macrophages Can Engraft the Brain Independent of Irradiation and Maintain an Identity Distinct from Microglia.” The Journal of Experimental Medicine 215 (6): 1627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Xu-Ming, Ryan Gregory R., Hapel Andrew J., Dominguez Melissa G., Russell Robert G., Kapp Sara, Sylvestre Vonetta, and Stanley E. Richard. 2002. “Targeted Disruption of the Mouse Colony-Stimulating Factor 1 Receptor Gene Results in Osteopetrosis, Mononuclear Phagocyte Deficiency, Increased Primitive Progenitor Cell Frequencies, and Reproductive Defects.” Blood 99 (1): 111–20. [DOI] [PubMed] [Google Scholar]
- Demerdash Yasmin, Kain Bailee, Essers Marieke A. G., and King Katherine Y.. 2021. “Yin and Yang: The Dual Effects of Interferons on Hematopoiesis.” Experimental Hematology 96 (April):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras Panagiotis, Sun Bruce, Wang Minghui, Kruglikov Ilya, Lallos Gregory, Zimmer Matthew, Terrenoire Cecile, et al. 2017. “Directed Differentiation of Human Pluripotent Stem Cells to Microglia.” Stem Cell Reports 8 (6): 1516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhag Sara, Stremmel Christopher, Zehrer Annette, Plocke Josefine, Hennel Roman, Keuper Michaela, Knabe Clarissa, et al. 2021. “Differences in Cell-Intrinsic Inflammatory Programs of Yolk Sac and Bone Marrow Macrophages.” Cells 10 (12). 10.3390/cells10123564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas Caroline C., Dorman Leah C., Nguyen Phi T., Christian Lagares-Linares Haruna Nakajo, Anderson Sarah R., Barron Jerika J., et al. 2024. “Type-I-Interferon-Responsive Microglia Shape Cortical Development and Behavior.” Cell 187 (8): 1936–54.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels Philip, Magnusson Måns, Lundin Sverker, and Max Käller. 2016. “MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report.” Bioinformatics 32 (19): 3047–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Fites, J., Gui Michael, Kernien John F., Negoro Paige, Dagher Zeina, Sykes David B., Nett Jeniel E., Mansour Michael K., and Klein Bruce S.. 2018. “An Unappreciated Role for Neutrophil-DC Hybrids in Immunity to Invasive Fungal Infections.” PLoS Pathogens 14 (5): e1007073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi Francesco, Carlos Dominguez Gonzalez Kaley Arnold, Swantkowski Meghan, Charlton Lauren, Modesti Nicholson, Dar Asif A., Vanderver Adeline, Bennett Mariko, and Adang Laura A.. 2024. “Nucleotide Metabolism, Leukodystrophies, and CNS Pathology.” Journal of Inherited Metabolic Disease, February. 10.1002/jimd.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin David, Skola Dylan, Coufal Nicole G., Holtman Inge R., Schlachetzki Johannes C. M., Sajti Eniko, Jaeger Baptiste N., et al. 2017. “An Environment-Dependent Transcriptional Network Specifies Human Microglia Identity.” Science 356 (6344). 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran Sandra, Honold Lisa, Fehler Olesja, Zenker Stefanie, Eligehausen Sarah, Kuhlmann Michael T., Geven Edwin, et al. 2018. “Imaging, Myeloid Precursor Immortalization, and Genome Editing for Defining Mechanisms of Leukocyte Recruitment in Vivo.” Theranostics 8 (9): 2407–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Xinfeng, Steinman Richard A., Sheng Yi, Cao Guodong, Wiley Clayton A., and Wang Qingde. 2022. “An AGS-Associated Mutation in ADAR1 Catalytic Domain Results in Early-Onset and MDA5-Dependent Encephalopathy with IFN Pathway Activation in the Brain.” Journal of Neuroinflammation 19 (1): 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurzeler U., Rabachini T., Dahinden C. A., Salmanidis M., Brumatti G., Ekert P. G., Echeverry N., Bachmann D., Simon H. U., and Kaufmann T.. 2013. “In Vitro Differentiation of near-Unlimited Numbers of Functional Mouse Basophils Using Conditional Hoxb8.” Allergy 68 (5): 604–13. [DOI] [PubMed] [Google Scholar]
- Haenseler Walther, Sansom Stephen N., Buchrieser Julian, Newey Sarah E., Moore Craig S., Nicholls Francesca J., Chintawar Satyan, et al. 2017. “A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-Culture-Specific Expression Profile and Inflammatory Response.” Stem Cell Reports 8 (6): 1727–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Velda X., Mohammad Shekeeb S., Jones Hannah F., Bandodkar Sushil, Crow Yanick J., Dale Russell C., and AGS-JAKi Study Group. 2022. “Cerebrospinal Fluid Neopterin as a Biomarker of Treatment Response to Janus Kinase Inhibition in Aicardi-Goutières Syndrome.” Developmental Medicine and Child Neurology 64 (2): 266–71. [DOI] [PubMed] [Google Scholar]
- Hartner Jochen C., Schmittwolf Carolin, Kispert Andreas, Müller Albrecht M., Higuchi Miyoko, and Seeburg Peter H.. 2004. “Liver Disintegration in the Mouse Embryo Caused by Deficiency in the RNA-Editing Enzyme ADAR1.” The Journal of Biological Chemistry 279 (6): 4894–4902. [DOI] [PubMed] [Google Scholar]
- Hasselmann Jonathan, Coburn Morgan A., England Whitney, Figueroa Velez Dario X., Shabestari Sepideh Kiani, Tu Christina H., McQuade Amanda, et al. 2019. “Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo.” Neuron 103 (6): 1016–33.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller Bjoern, Achleitner Martin, Glage Silke, Naumann Ronald, Behrendt Rayk, and Roers Axel. 2012. “Mammalian RNase H2 Removes Ribonucleotides from DNA to Maintain Genome Integrity.” The Journal of Experimental Medicine 209 (8): 1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Xiao-Shuang, Ni Ying-Qin, Liu Tian-Jin, Zhang Meng, Ren Hui, Jiang Rui, Huang Xin, and Xu Ge-Zhi. 2012. “CCR2 Overexpression Promotes the Efficient Recruitment of Retinal Microglia in Vitro.” Molecular Vision 18 (December):2982–92. [PMC free article] [PubMed] [Google Scholar]
- Kanazawa Nobuo, Ishii Taeko, Takita Yasushi, Nishikawa Atsushi, and Nishikomori Ryuta. 2023. “Efficacy and Safety of Baricitinib in Japanese Patients with Autoinflammatory Type I Interferonopathies (NNS/CANDLE, SAVI, And AGS).” Pediatric Rheumatology Online Journal 21 (1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Qingyun, and Barres Ben A.. 2018. “Microglia and Macrophages in Brain Homeostasis and Disease.” Nature Reviews. Immunology 18 (4): 225–42. [DOI] [PubMed] [Google Scholar]
- Liddicoat Brian J., Piskol Robert, Chalk Alistair M., Ramaswami Gokul, Higuchi Miyoko, Hartner Jochen C., Jin Billy Li Peter H. Seeburg, and Walkley Carl R.. 2015. “RNA Editing by ADAR1 Prevents MDA5 Sensing of Endogenous dsRNA as Nonself.” Science 349 (6252): 1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Guangxin, Gezer Deniz, Herrmann Oliver, Feldberg Kristina, Schemionek Mirle, Jawhar Mohamad, Reiter Andreas, Brümmendorf Tim H., Koschmieder Steffen, and Chatain Nicolas. 2020. “LCP1 Triggers mTORC2/AKT Activity and Is Pharmacologically Targeted by Enzastaurin in Hypereosinophilia.” Molecular Carcinogenesis 59 (1): 87–103. [DOI] [PubMed] [Google Scholar]
- Mannion Niamh M., Greenwood Sam M., Young Robert, Cox Sarah, Brindle James, Read David, Nellåker Christoffer, et al. 2014. “The RNA-Editing Enzyme ADAR1 Controls Innate Immune Responses to RNA.” Cell Reports 9 (4): 1482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Takahiro, Tsuda Makoto, Tozaki-Saitoh Hidetoshi, and Inoue Kazuhide. 2013. “Lentiviral Transduction of Cultured Microglia.” Methods in Molecular Biology 1041:63–67. [DOI] [PubMed] [Google Scholar]
- McCarthy Davis J., Chen Yunshun, and Smyth Gordon K.. 2012. “Differential Expression Analysis of Multifactor RNA-Seq Experiments with Respect to Biological Variation.” Nucleic Acids Research 40 (10): 4288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller Alexander, Saskia-Larissa Jauch-Speer Shrey Gandhi, Vogl Thomas, Roth Johannes, and Fehler Olesja. 2023. “The Roles of Toll-like Receptor 4, CD33, CD68, CD69, or CD147/EMMPRIN for Monocyte Activation by the DAMP S100A8/S100A9.” Frontiers in Immunology 14 (March):1110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Masashi, Stamp Gordon, Robins Peter, Dulic Anna, Rosewell Ian, Hrivnak Geza, Daly Graham, Lindahl Tomas, and Barnes Deborah E.. 2004. “Gene-Targeted Mice Lacking the Trex1 (DNase III) 3’-->5’ DNA Exonuclease Develop Inflammatory Myocarditis.” Molecular and Cellular Biology 24 (15): 6719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat Julien, Li Yun, Yuan Bingbing, Mitalipova Maisam, Omer Attya, Corcoran Sean, Bakiasi Grisilda, et al. 2016. “Efficient Derivation of Microglia-like Cells from Human Pluripotent Stem Cells.” Nature Medicine 22 (11): 1358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray Peter J., Allen Judith E., Biswas Subhra K., Fisher Edward A., Gilroy Derek W., Goerdt Sergij, Gordon Siamon, et al. 2014. “Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines.” Immunity 41 (1): 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien Carleigh A., Bennett F. Chris, and Bennett Mariko L.. 2022. “Microglia in Antiviral Immunity of the Brain and Spinal Cord.” Seminars in Immunology 60 (March):101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto Taisuke, Ahmed Abu Tayeh Ryuta Nishikomori, Abe Hiroto, Hashimoto Kyota, Baba Shiro, Arias-Loza Anahi-Paula, et al. 2022. “Intracellular Virus Sensor MDA5 Mutation Develops Autoimmune Myocarditis and Nephritis.” Journal of Autoimmunity 127 (February):102794. [DOI] [PubMed] [Google Scholar]
- Pandya Hetal, Shen Michael J., Ichikawa David M., Sedlock Andrea B., Choi Yong, Johnson Kory R., Kim Gloria, et al. 2017. “Differentiation of Human and Murine Induced Pluripotent Stem Cells to Microglia-like Cells.” Nature Neuroscience 20 (5): 753–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Lopes Selma, Celhar Teja, Gloria Sans-Fons Maria Serra, Fairhurst Anna-Marie, Lloberas Jorge, and Celada Antonio. 2013. “The Exonuclease Trex1 Restrains Macrophage Proinflammatory Activation.” Journal of Immunology 191 (12): 6128–35. [DOI] [PubMed] [Google Scholar]
- Priller J., Flügel A., Wehner T., Boentert M., Haas C. A., Prinz M., Fernández-Klett F., et al. 2001. “Targeting Gene-Modified Hematopoietic Cells to the Central Nervous System: Use of Green Fluorescent Protein Uncovers Microglial Engraftment.” Nature Medicine 7 (12): 1356–61. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2022. “R: A Language and Environment for Statistical Computing.” Foundation for Statistical Computing, Vienna, Austria. 2022. https://www.R-project.org/. [Google Scholar]
- Raschke W. C., Baird S., Ralph P., and Nakoinz I.. 1978. “Functional Macrophage Cell Lines Transformed by Abelson Leukemia Virus.” Cell 15 (1): 261–67. [DOI] [PubMed] [Google Scholar]
- Redecke Vanessa, Wu Ruiqiong, Zhou Jingran, Finkelstein David, Chaturvedi Vandana, High Anthony A., and Hans Häcker. 2013. “Hematopoietic Progenitor Cell Lines with Myeloid and Lymphoid Potential.” Nature Methods 10 (8): 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel Jan, Maelfait Jonathan, Bridgeman Anne, Rigby Rachel, Hayward Bruce, Liberatore Rachel A., Bieniasz Paul D., et al. 2013. “SAMHD1-Dependent Retroviral Control and Escape in Mice.” The EMBO Journal 32 (18): 2454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice Gillian I., Del Toro Duany Yoandris, Jenkinson Emma M., Forte Gabriella Ma, Anderson Beverley H., Ariaudo Giada, Bader-Meunier Brigitte, et al. 2014. “Gain-of-Function Mutations in IFIH1 Cause a Spectrum of Human Disease Phenotypes Associated with Upregulated Type I Interferon Signaling.” Nature Genetics 46 (5): 503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M., Mori L., De Libero G., Sironi M., Biondi A., Mantovani A., Donini S. D., and Ricciardi-Castagnoli P.. 1989. “Monokine Production by Microglial Cell Clones.” European Journal of Immunology 19 (8): 1443–48. [DOI] [PubMed] [Google Scholar]
- Ritchie Matthew E., Phipson Belinda, Wu Di, Hu Yifang, Law Charity W., Shi Wei, and Smyth Gordon K.. 2015. “Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies.” Nucleic Acids Research 43 (7): e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Allison W., Popov Lauren M., Mitchell Gabriel, Ching Krystal L., Licht Daniel J., Golovkine Guillaume, Barton Gregory M., and Cox Jeffery S.. 2019. “Cas9+ Conditionally-Immortalized Macrophages as a Tool for Bacterial Pathogenesis and beyond.” eLife 8 (June):e45957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson Mark D., McCarthy Davis J., and Smyth Gordon K.. 2010. “edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data.” Bioinformatics 26 (1): 139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas Marcela, Osorio Fabiola, Robinson Matthew J., Davies Luke C., Dierkes Nicola, Jones Simon A., Reis e Sousa Caetano, and Taylor Philip R.. 2011. “Hoxb8 Conditionally Immortalised Macrophage Lines Model Inflammatory Monocytic Cells with Important Similarity to Dendritic Cells.” European Journal of Immunology 41 (2): 356–65. [DOI] [PubMed] [Google Scholar]
- Salter Michael W., and Stevens Beth. 2017. “Microglia Emerge as Central Players in Brain Disease.” Nature Medicine 23 (9): 1018–27. [DOI] [PubMed] [Google Scholar]
- Schneider Valerie A., Tina Graves-Lindsay Kerstin Howe, Bouk Nathan, Chen Hsiu-Chuan, Kitts Paul A., Murphy Terence D., et al. 2017. “Evaluation of GRCh38 and de Novo Haploid Genome Assemblies Demonstrates the Enduring Quality of the Reference Assembly.” Genome Research 27 (5): 849–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer Anat, Grozovski Jonathan, Tuan Leng Tay Jenhan Tao, Volaski Alon, Patrick Süß Alberto Ardura-Fabregat, et al. 2018. “Engrafted Parenchymal Brain Macrophages Differ from Microglia in Transcriptome, Chromatin Landscape and Response to Challenge.” Nature Communications 9 (1): 5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Zeyang, Li Rick Z., Prohaska Thomas A., Hoeksema Marten A., Spann Nathan J., Tao Jenhan, Fonseca Gregory J., et al. 2022. “Systematic Analysis of Naturally Occurring Insertions and Deletions That Alter Transcription Factor Spacing Identifies Tolerant and Sensitive Transcription Factor Pairs.” eLife 11 (January):e70878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson Charlotte, Love Michael I., and Robinson Mark D.. 2015. “Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences.” F1000Research 4 (December):1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., and Heard P. M.. 1977. “Factors Regulating Macrophage Production and Growth. Purification and Some Properties of the Colony Stimulating Factor from Medium Conditioned by Mouse L Cells.” The Journal of Biological Chemistry 252 (12): 4305–12. [PubMed] [Google Scholar]
- Su Wei, Kang John, Sopher Bryce, Gillespie James, Aloi Macarena S., Odom Guy L., Hopkins Stephanie, et al. 2016. “Recombinant Adeno-Associated Viral (rAAV) Vectors Mediate Efficient Gene Transduction in Cultured Neonatal and Adult Microglia.” Journal of Neurochemistry 136 Suppl 1 (0 1): 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Kazuyuki, Kozaki Tatsuya, Lee Christopher Zhe Wei, Thion Morgane Sonia, Otsuka Masayuki, Lim Shawn, Utami Kagistia Hana, et al. 2020. “Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function.” Immunity 52 (2): 417–18. [DOI] [PubMed] [Google Scholar]
- Timmerman Raissa, Burm Saskia M., and Bajramovic Jeffrey J.. 2018. “An Overview of in Vitro Methods to Study Microglia.” Frontiers in Cellular Neuroscience 12 (August):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., and Tada K.. 1980. “Establishment and Characterization of a Human Acute Monocytic Leukemia Cell Line (THP-1).” International Journal of Cancer. Journal International Du Cancer 26 (2): 171–76. [DOI] [PubMed] [Google Scholar]
- Vanderver Adeline, Adang Laura, Gavazzi Francesco, Katherine McDonald Guy Helman, Frank David B., Jaffe Nicole, et al. 2020. “Janus Kinase Inhibition in the Aicardi-Goutières Syndrome.” The New England Journal of Medicine 383 (10): 986–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Gang G., Calvo Katherine R., Pasillas Martina P., Sykes David B., Häcker Hans, and Kamps Mark P.. 2006. “Quantitative Production of Macrophages or Neutrophils Ex Vivo Using Conditional Hoxb8.” Nature Methods 3 (4): 287–93. [DOI] [PubMed] [Google Scholar]
- Ward Simone V., George Cyril X., Welch Megan J., Liou Li-Ying, Hahm Bumsuk, Lewicki Hanna, de la Torre Juan C., Samuel Charles E., and Oldstone Michael B.. 2011. “RNA Editing Enzyme Adenosine Deaminase Is a Restriction Factor for Controlling Measles Virus Replication That Also Is Required for Embryogenesis.” Proceedings of the National Academy of Sciences 108 (1): 331–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Jane Jialu, Chalk Alistair M., Nikolic Iva, Simpson Kaylene J., Smeets Monique F., and Walkley Carl R.. 2022. “Genome-Wide Screening Identifies Cell-Cycle Control as a Synthetic Lethal Pathway with SRSF2P95H Mutation.” Blood Advances 6 (7): 2092–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XuFeng Richard, Boyer Matthew J., Shen Hongmei, Li Yanxin, Yu Hui, Gao Yindai, Yang Qiong, Wang Qingde, and Cheng Tao. 2009. “ADAR1 Is Required for Hematopoietic Progenitor Cell Survival via RNA Editing.” Proceedings of the National Academy of Sciences of the United States of America 106 (42): 17763–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zach Frank, Mueller Alexandra, and Gessner André. 2015. “Production and Functional Characterization of Murine Osteoclasts Differentiated from ER-Hoxb8-Immortalized Myeloid Progenitor Cells.” PloS One 10 (11): e0142211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino Daniel R., Achuthan Premanand, Akanni Wasiu, Amode M. Ridwan, Barrell Daniel, Bhai Jyothish, Billis Konstantinos, et al. 2018. “Ensembl 2018.” Nucleic Acids Research 46 (D1): D754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Lian-Shun, Kaneko Naoko, and Sawamoto Kazunobu. 2015. “Minocycline Treatment Ameliorates Interferon-Alpha- Induced Neurogenic Defects and Depression-like Behaviors in Mice.” Frontiers in Cellular Neuroscience 9 (January):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.