Abstract
Two coxsackievirus B3 (CVB3) variants (H3 and H310A1) differ by a single amino acid mutation in the VP2 capsid protein. H3 induces severe myocarditis in BALB/c mice, but H310A1 is amyocarditic. Infection with H3, but not H310A1, preferentially activates Vγ4 Vδ4 cells, which are strongly positive for gamma interferon (IFN-γ), whereas Vγ1 Vδ4 cells are increased in both H3 and H310A1 virus-infected animals. Depletion of Vγ1+ cells using monoclonal anti-Vγ1 antibody enhanced myocarditis and CD4+-, IFN-γ+-cell responses in both H3- and H310A1-infected mice yet decreased the CD4+-, IL-4+-cell response. Depleting Vγ4+ cells suppressed myocarditis and reduced CD4+ IFN-γ+ cells but increased CD4+ IL-4+ T cells. The role of cytokine production by Vγ1+ and Vγ4+ T cells was investigated by adoptively transferring these cells isolated from H3-infected BALB/c Stat4 knockout (Stat4ko) (defective in IFN-γ expression) or BALB/c Stat6ko (defective in IL-4 expression) mice into H3 virus-infected wild-type BALB/c recipients. Vγ4 and Vγ1+ T cells from Stat4ko mice expressed IL-4 but no or minimal IFN-γ, whereas these cell populations derived from Stat6ko mice expressed IFN-γ but no IL-4. Stat4ko Vγ1+ cells (IL-4+) suppress myocarditis. Stat6ko Vγ1+ cells (IFN-γ+) were not inhibitory. Stat6ko Vγ4+ cells (IFN-γ+) significantly enhanced myocarditis. Stat4ko Vγ4+ cells (IL-4+) neither inhibited nor enhanced disease. These results show that distinct γδ-T-cell subsets control myocarditis susceptibility and bias the CD4+-Th-cell response. The cytokines produced by the Vγ subpopulation have a significant influence on the CD4+-Th-cell phenotype.
T cells expressing the γδ T-cell receptor (TcR) accumulate at inflammatory sites and can modulate disease susceptibility by either promoting or suppressing inflammation (4, 6, 9, 13, 17, 20, 27, 28, 35, 36, 38, 41, 44). In some models, γδ+ T cells have both pro- and anti-inflammatory effects at different times in the disease process. In the models of arthritis and spontaneous abortion, the γδ+ T cells that were present early in the disease in both models were proinflammatory and led to abortion while those that were present later were anti-inflammatory and inhibited abortion (5, 36). The biological effect of γδ+ T cells may be mediated by the cytokines they produce. γδ+ T cells can produce cytokines of either a Th1 (gamma interferon [IFN-γ]) or Th2 (interleukin 4 [IL-4]) phenotype, and which phenotype occurs can be dependent upon the type of antigen used for their activation or the subtype of γδ+ T cells stimulated. γδ+ T cells in arthritis (4) and in murine cytomegalovirus and Nippostrongylus brasiliosis infection (7) predominantly make IL-4 and induce Th2 differentiation in CD4+-T-cell populations. In contrast, in Listeria monocytogenes, Salmonella choleraesuis, and murine cytomegalovirus infections, γδ+ T cells produce IFN-γ and promote CD4+-Th1-cell responses (7, 30, 33). Studies by Azuara and Pereira (1) and Gerber and colleagues (10) indicate that in DBA/2 mice γδ+ T lymphocytes which express the Vγ1 Vδ6.4 TcR predominantly express IL-4. Thus, the type of γδ+ cell subset activated during an immune response may be crucial to its modulatory effect on CD4+-T-cell responses.
Previous studies from this laboratory demonstrated that γδ+ T cells control susceptibility of mice to coxsackievirus B3 (CVB3)-induced myocarditis (14, 15, 17, 18). Most recently, we demonstrated that Vγ1+ T cells cause myocarditis resistance in CVB3-infected C57BL/6 mice but that Vγ4+ T cells activated in Bl.Tg.Eα animals, i.e., C57BL/6 mice transgenically made to express major histocompatibility complex (MHC) class II IE, cause myocarditis susceptibility (14). The major problem with this earlier study was that Vγ4+ cells are more prevalent in uninfected Bl.Tg.Eα animals whereas Vγ1+ cells predominate in C57BL/6 mice. Thus, it was possible that CVB3 infection was simply activating the dominant γδ+-T-cell population in the two strains rather than selectively activating either population specifically. In the present study, we begin with the same mouse strain, BALB/c, and show that pathogenic (H3) and nonpathogenic (H310A1) virus infections selectively activate different Vγ+ cell subsets and that, again, Vγ1+ T cells cause disease resistance but Vγ4+ cells cause susceptibility. The cytokine profiles of the Vγ cell subsets suggest that these cells modulate CD4+-Th-cell responses through the cytokines they release. We tested this hypothesis by transferring Vγ subpopulations isolated from BALB/c Stat6 knockout (Stat6ko) and Stat4ko mice into H3 virus-infected recipients. Stat4 and Stat6 are transcription factors important in IL-12 and IL-4, respectively (3, 21, 29), and animals lacking Stat4 and Stat6 have defective Th1- and Th2-cell responses. Vγ1+ cells isolated from BALB/c Stat4ko mice, which stain positively for IL-4, still suppressed CD4+-Th1-cell responses and myocarditis; the same cell population derived from Stat6ko donors was not suppressive. This result implies that the cytokines made by the Vγ+ cell subpopulations are important in modulating myocarditis susceptibility.
MATERIALS AND METHODS
Mice.
Male BALB/cJ, BALB/c-Stat4tmI Gru, and BALB/c-Stat6tmI Gru mice, 5 to 6 weeks of age, were purchased from Jackson Laboratories, Bar Harbor, Maine.
Virus, virus infection, and virus titration.
Animals were infected by intraperitoneal (i.p.) injection of 0.5 ml of phosphate-buffered saline (PBS) containing 104 PFU of either the CVB3 H3 or the H310A1 variant, derived from Cos cells transfected with the infectious cDNAs of this virus (22). For virus titration, hearts were homogenized in 0.9 ml of RPMI 1640 containing 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5% fetal bovine serum. Cellular debris was removed by centrifugation at 1,045 × g for 10 min. The titer of the supernatant was determined by the plaque-forming assay on HeLa cell monolayers as described previously (16).
Antibodies.
Antibody class control (isotype control) and antigen-specific antibodies were obtained from PharMingen (San Diego, Calif.). These included phycoerythrin (PE)-conjugated anti-CD3 (clone 17A2); purified anti-TcRβ (clone H57-597); purified anti-Mac3 (clone M3/84); purified anti-IAd (clone 39-10-8); purified rat anti-mouse CD16 CD32 (Fc Block, clone 2.4G2); biotinylated or CyChrome-, fluorescein isothiocyanate (FITC)-, or PE-conjugated rat IgG1 (clone R3-34); biotinylated or FITC- or PE-conjugated rat anti-mouse IFN-γ (clone XMG 1.2); biotinylated or FITC- or PE-conjugated rat anti-mouse IL-4 (clone BVD4-1D11); FITC-conjugated hamster anti-mouse CD69 (clone H1.2F3); biotinylated or FITC-, PE-, or CyChrome-conjugated rat-anti-mouse CD4 (clone GK 1.5); purified or FITC- or PE-conjugated hamster IgG; FITC- or PE-conjugated mouse anti-hamster IgG cocktail (clones G70-204 and G94-56); and purified or FITC- or PE-conjugated hamster-anti-γδ TcR (clone GL3). Various purified, FITC- and biotin-conjugated monoclonal antibodies to Vγ1 (clone 2.11), Vγ4 (clone UC3), Vδ4 (clone GL2), and Vδ6.3 (clone 17-C) were prepared and tested in the laboratory of Rebecca O'Brien. CyChrome-, FITC-, and PE-conjugated streptavidin was purchased from PharMingen. Streptavidin-conjugated Red613 was purchased from Gibco Life Technologies, Grand Island, N.Y.
Isolation of spleen lymphocytes.
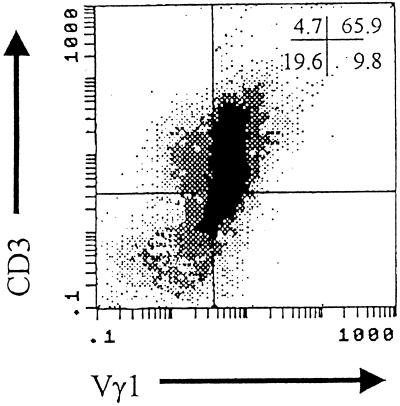
Spleens were aseptically removed from euthanized mice, pressed through fine-mesh screens to form single-cell suspensions which were layered over Histopaque-1077 (Sigma Chemical Co., St. Louis, Mo.), and centrifuged at 1,048 × g for 15 min. The lymphoid cells at the interface were retrieved and counted by trypan blue exclusion. The procedure for isolation of enriched populations of Vγ1+ or Vγ4+ cell populations has been published (14). Briefly, splenocytes obtained after Histopaque purification were incubated on nylon wool for 30 min at 37°C; the nonadherent cells were retrieved; incubated with 1:50 dilutions of Fc Block, anti-αβ TcR antibody, anti-IAd antibody, anti-Mac3 antibody, and either anti-Vγ1 or anti-Vγ4 antibody for 20 min on ice; washed twice; and incubated with a 1:50 dilution of mouse anti-hamster IgG for 20 min on ice. The cells were again washed and incubated with magnetic particles conjugated with anti-mouse IgG and anti-rat IgG (PerSeptive Biosystems, Framingham, Mass.) for 30 min at 4°C. Bound cells were removed by passing the cell suspension over a magnet. The remaining cells were washed twice, divided into two tubes, incubated in 100 μl of PBS–1% bovine serum albumin (BSA) containing a 1:50 dilution of either anti-Vγ1 or anti-Vγ4 antibody for 20 min on ice, washed, and resuspended in RPMI 1640 containing 10% fetal bovine serum (Life Technologies) with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 80 pg of recombinant IL-2 (PharMingen) per ml. Cells (5 × 105) were added to tissue culture plates coated with mouse anti-hamster IgG, centrifuged at 100 × g for 5 min, and incubated at 37°C in a humidified 5% CO2 incubator for 3 days. The cells were retrieved, centrifuged on Histopaque to remove dead cells, and counted by trypan blue exclusion, and an aliquot was surface stained with anti-CD3 and either anti-Vγ1 or anti-Vγ4 antibodies to determine purity. Figure 1 shows a representative flow diagram of Vγ1+ enriched cells obtained by this protocol. Purity of populations ranged from 65 to 78% Vγ1 CD3+ or Vγ4 CD3+ cells for all populations.
FIG. 1.
Representative purity of Vγ1+ cells isolated from the spleens of H3 virus-infected mice. BALB/c Stat4ko mice were infected with H3 virus and killed 5 days later. Spleens were removed, depleted of red blood cells, and enriched for γδ+ T cells by negative selection of αβ+, Mac3+, and IAd+ cells using monoclonal antibodies to these cells and antibody-coated magnetic particles. The remaining cells were incubated with anti-Vγ1 monoclonal antibody, washed, and added to tissue culture plates coated with anti-hamster IgG antibody for 3 days. The cells were retrieved, washed, incubated with PE-conjugated anti-CD3 antibody and biotinylated anti-Vγ1 antibody, washed, incubated with FITC-conjugated streptavidin, washed, and analyzed by flow cytometry for stained cells. Numbers in the upper right-hand corner indicate the percentages of cells in the quadrants.
Adoptive transfer of Vγ cells.
Vγ1+ or Vγ4+ cells were washed three times in PBS, resuspended to 5 × 105 cells in 0.2 ml of PBS, and injected intravenously (i.v.) through the tail vein of H3 virus-infected BALB/c recipient mice on the third day after infection. Animals were killed on day 7 after infection. Control mice received PBS alone.
Flow cytometry.
Cells were either stained directly for cell surface markers or cultured in medium containing 10 μg of brefeldin A per ml, 50 ng of phorbol myristate acetate (PMA) per ml, and 500 ng of ionomycin (Sigma Chemical Co.) per ml for 4 h at 37°C in 5% CO2 for intracellular cytokine analysis. For surface staining, cells were resuspended in PBS containing 1% BSA (Sigma), a 1:100 dilution of Fc Block, and 1:100 dilutions of either fluorochrome-conjugated or biotinylated antibodies. The cells were incubated on ice for 20 min, washed, and, where applicable (when biotinylated antibodies were used), resuspended in PBS-BSA containing a 1:50 dilution of streptavidin-fluorochrome for 20 min on ice. The cells were washed twice and resuspended in 2% paraformaldehyde. For intracellular staining, cells were washed once after the 4-h culture described above in PBS-BSA containing 10 μg of brefeldin A per ml and surface stained as described above. The amount of brefeldin A was maintained throughout the labeling procedure. After surface staining, the cells were washed and fixed in 2% paraformaldehyde for 10 min; thereafter, brefeldin A was not required. The cells were then washed once in PBS-BSA buffer, incubated for 10 min in PBS-BSA buffer containing 0.5% saponin, and stained for intracellular molecules and cytokines, as indicated in Results, by resuspending the cells in PBS-BSA-saponin containing 1:100 dilutions of Fc Block and the biotinylated or fluorochrome-conjugated antibodies and 50 μg of rat polyclonal IgG (Zymed, San Francisco, Calif.) per ml. After incubation for 30 min, the cells were washed twice in PBS-BSA-saponin and once in saponin-free PBS-BSA to close the membrane and then resuspended in PBS containing 2% paraformaldehyde. Positive controls for cytokine staining were 2.5 × 105 cells of either MIC-1 (IFN-γ)- or MIC-2 (IL-4)-fixed cells obtained from PharMingen. Negative controls were biotinylated, and fluorochrome-conjugated species- and isotype-matched Igs and streptavidin-fluorochrome combinations were as used in specific-antibody staining samples.
Stained cell populations were analyzed using a Coulter Epics Elite instrument with a single excitation wavelength (488 nm) and band filters for PE (wavelength, 575 nm), FITC (525 nm), Red613 (613 nm), and CyChrome (670 nm). Each sample population was classified for cell size (forward scatter) and complexity (side scatter) and then gated on a population of interest. At least 10,000 cells were evaluated for each sample. Criteria for positive staining were established using isotype controls. The results were expressed in one of two ways: either as the percentage of total splenocytes staining for a particular marker minus the number of splenocytes in the isotype control or as the percentage of a specific population of cells which stained positively for each marker [for example, the percentage of CD4+ cells staining for IL-4 represents CD4+ IL-4+ cells/(CD4+ IL-4− + CD4+ IL-4+ cells) × 100].
Each experiment was repeated at least two times, and the data from a representative experiment are presented.
Histology.
Hearts were removed, fixed in 10% buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Stained sections were evaluated for myocarditis as described previously (14).
Statistics.
Statistical evaluation was performed using the Wilcoxon ranked-score method.
RESULTS
Evaluation of subpopulations of γδ+ T cells in mice with myocarditis.
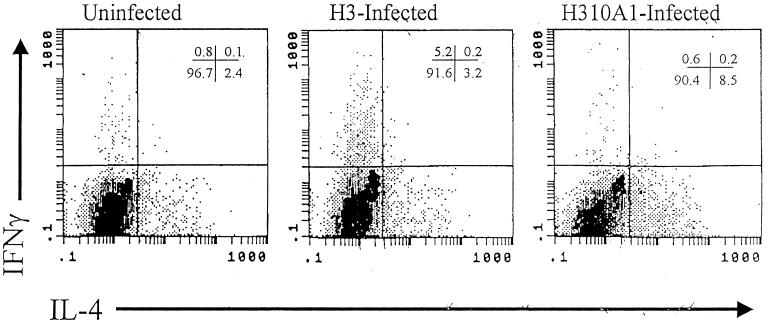
Table 1 shows that BALB/c mice infected with H3 virus develop severe myocardial inflammation (7.9% of the myocardium inflamed) but that mice given H310A1 virus have little myocarditis (1.3% of the myocardium inflamed). Cardiac virus titers were increased in animals infected with H310A1 virus, compared to those in H3 virus-infected mice, even though H310A1-infected animals had minimal myocarditis. Numbers of CD4+ IFN-γ+ cells in the spleen were increased in H3 virus-infected mice compared to numbers in H310A1 virus-infected mice, while numbers of CD4+ IL-4+ cells were reduced. Figure 2 shows representative intracellular cytokine (IFN-γ and IL-4) staining patterns in these animals after gating on the CD4+-cell population. H310A1 virus infection did not increase the number of Th1 cells relative to those found in uninfected mice but did increase the number of CD4+ IL-4+ cells in the spleen (from 2.4% of cells in normal to 8.5% of cells in H310A1 virus-infected animals).
TABLE 1.
Myocarditis in micea
| Treatment | % of myocardium inflamed | Cardiac virus titer (log 10 PFU) | Total no. of lymphocytes in spleen (106) | % of CD4+ cells in spleen | % of CD4+ cells which are:
|
|
|---|---|---|---|---|---|---|
| IFN-γ+ | IL-4+ | |||||
| None | 0 ± 0 | 16.53 ± 2.17 | 29.7 ± 3.5 | 1.3 ± 0.8 | 2.9 ± 1.5 | |
| H3 virus | 7.9 ± 0.9∗ | 5.01 ± 0.17∗ | 11.93 ± 1.02 | 24.3 ± 1.2∗ | 10.1 ± 3.1∗ | 2.3 ± 0.7∗ |
| H310A1 virus | 1.3 ± 0.5 | 6.33 ± 0.25 | 15.33 ± 1.79 | 27.7 ± 1.0 | 1.9 ± 1.0 | 9.4 ± 2.3 |
BALB/c male mice were either not infected or infected with 104 PFU of H3 or H310A1 virus in 0.5 ml of PBS by i.p. injection. Animals were killed 7 days after infection. Hearts were divided, and half were evaluated by image analysis for myocarditis and half were used to determine the titer of virus. Spleens were retrieved, processed for lymphoid cells, and counted by trypan blue exclusion. The lymphoid cells from individual animals were stained for surface expression of CD4 and for intracellular expression of IFN-γ and IL-4 using three-color flow analysis. Values are means ± SEM of results with 4 to 10 mice per group. ∗, value significantly different than that for H310A virus-infected mice at a P of ≤0.05.
FIG. 2.
Intracellular cytokine staining of CD4+ splenocytes. Single-cell suspensions from individual mice were stimulated for 4 h at 37°C with PMA, ionomycin, and brefeldin A; stained for CD4 surface marker; fixed in paraformaldehyde; permeabilized; and stained for IFN-γ and IL-4. Cells were gated in a flow cytometer on the CD4+ cell population and then analyzed for cytokine-positive subsets. Numbers in the upper right-hand corner represent the percentages of CD4+ splenocytes in the quadrants.
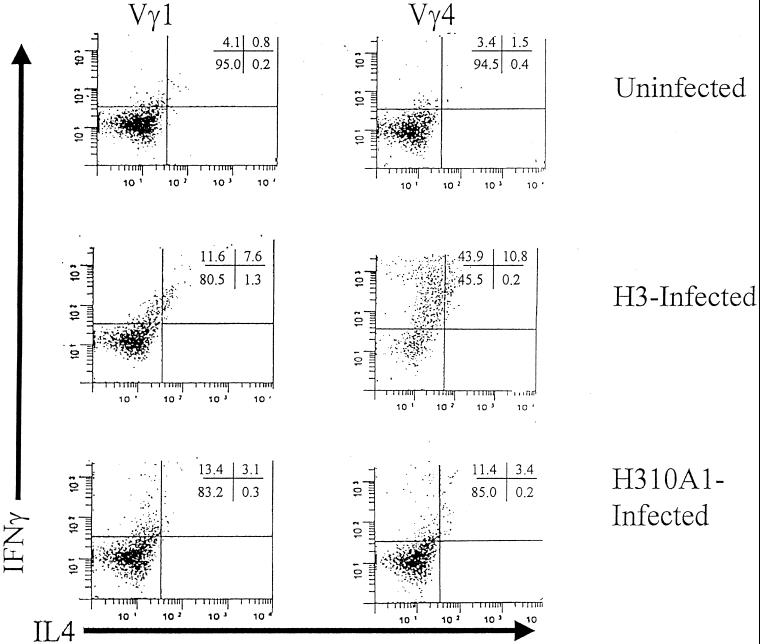
We next examined the relative proportions of Vγ1 and Vγ4 subpopulations in H3 and H310A1 virus-infected BALB/c mice (Table 2). H3 virus infection resulted in an approximately threefold increase in the percentage of splenic γδ+ T cells, whereas the percentage of these cells in H310A1 virus-infected mice stayed approximately the same as in uninfected BALB/c animals. At the same time, numbers of Vγ 4+ cells were preferentially increased in H3 virus-infected mice (by sevenfold) but remained unaltered in H310A1-infected mice compared to numbers in uninfected animals. Approximately 40% of the Vγ4+-cell population in H3 virus-infected animals was activated as assessed by CD69 expression. Both H3 and H310A1 virus-infected animals showed increases in percentages of Vγ1+ cells. Interestingly, in both H3 and H310A1 virus-infected groups, Vδ4 expression dominated. Intracellular cytokine staining of the Vγ-stained cell populations (Fig. 3) revealed that, although neither Vγ1+ nor Vγ4+ cells from uninfected mice showed much intracellular cytokine staining, cells from H3-infected mice showed substantially increased cytokine expression. About 20% of the Vγ1 cells from H3-infected animals produced low levels of IFN-γ, or both IFN-γ and IL-4, whereas over half of the Vγ4+ subpopulation showed strong IFN-γ production, again with low levels of IL-4 in some cells. H310A1 virus infection resulted in more cytokine-producing γδ+ T cells than in uninfected mice but less than in H3-infected animals. Of these, both Vγ4+ and Vγ1+ cells stained primarily for IFN-γ.
TABLE 2.
Proportions of Vγ1 and Vγ4 subpopulations in H3- and H310A1-infected BALB/c micea
| Mice | % of splenocytes which stained positively for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| γδ | γδ Vγ1 | γδ Vγ4 | γδ Vγ1 Vδ4 | γδ Vγ4 Vδ4 | γδ Vγ1 Vδ6.3 | γδ Vγ4 Vδ6.3 | γδ Vγ1 CD69 | γδ Vγ4 CD69 | |
| Uninfected | 2.6 ± 0.8 | 1.4 ± 1.0 | 0.7 ± 0.3 | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| H3 infected | 8.7 ± 2.5∗ | 3.6 ± 1.4 | 4.9 ± 1.8∗ | 1.1 ± 0.7 | 4.4 ± 1.7∗ | 0.3 ± 0.1 | 0.5 ± 0.3 | 1.0 ± 0.5 | 2.0 ± 0.4∗ |
| H310A1 infected | 3.8 ± 2.1 | 3.0 ± 1.6 | 1.2 ± 0.6 | 1.3 ± 0.7 | 0.6 ± 0.6 | 0.4 ± 0.2 | 0.6 ± 0.4 | 0.6 ± 0.2 | 1.0 ± 0.5 |
Splenocytes were stained with fluorochrome-labeled antibodies and analyzed by flow cytometry for the percentages of total splenocytes that positively stained for the markers indicated. Results are means ± SEM of values for individual mice in each group. ∗, value different than that for the uninfected group at a P of ≤0.05.
FIG. 3.
Cytokine staining of Vγ1 and Vγ4 subsets in uninfected H3- and H310A1-infected BALB/c mice. Spleen cells were stimulated for 4 h with PMA, ionomycin, and brefeldin A; surface stained for either Vγ1 or Vγ4; and then intracellularly stained for IFN-γ and IL-4. Cells were gated on the Vγ1 or Vγ4 stained population and then analyzed for cytokine-positive subpopulations. Numbers in the upper right-hand corner represent the percentages of gated cells in the quadrants.
Antibody-mediated depletion of Vγ cell subsets in vivo.
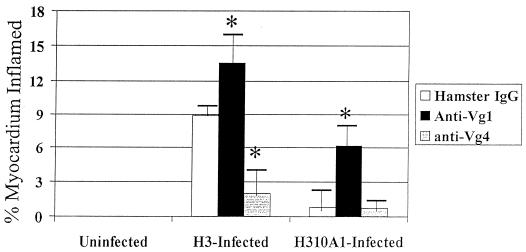
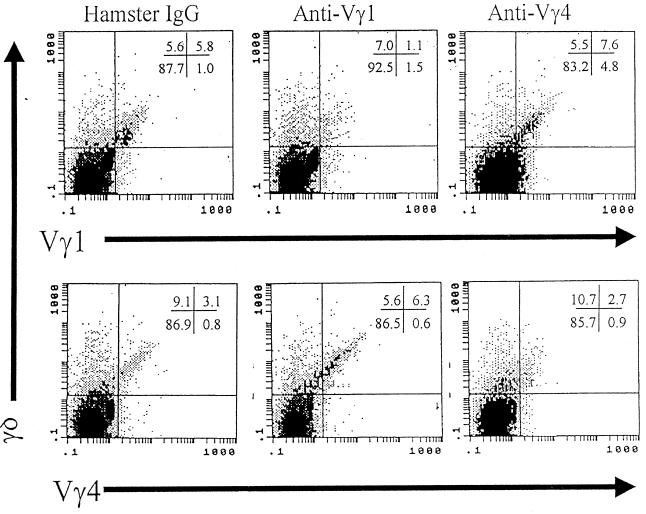
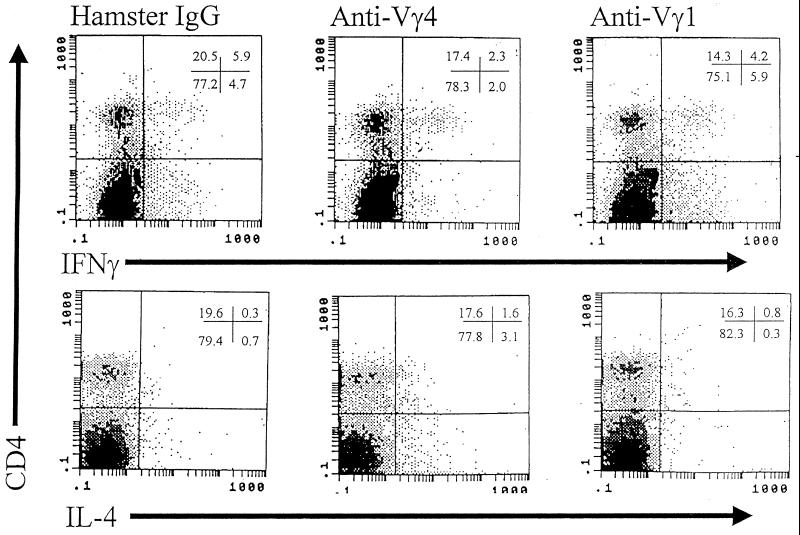
To evaluate whether Vγ populations differentially affect myocarditis susceptibility, BALB/c mice were depleted of each subset by injection of 300 μg of monoclonal hamster IgG, anti-Vγ1, or anti-Vγ4 antibodies i.v. through their tail veins. Three days later, the mice were injected i.p. with H3 or H310A1. Seven days after infection, mice were killed. Figure 4 shows myocardial inflammation in the various groups. In H3-infected mice, myocarditis was significantly inhibited by anti-Vγ4 antibody treatment. Anti-Vγ1 antibody treatment exacerbated disease in both H3- and H310A1-infected mice. Neither antibody induced cardiac lesions by itself. Hamster IgG, used as an isotype control, has little effect on Vγ1+ or Vγ4+ cell numbers (5.8% Vγ1+ and 3.1% Vγ4+ cells as shown in Fig. 5). However, anti-Vγ1 antibody reduced Vγ1+ cells by 80% and anti-Vγ4 antibody reduced Vγ4+ cells by 60 to 70% (Table 3; Fig. 5). Moreover, the cells remaining were only weakly positive for TcR expression. In the same mice, we examined splenic CD4+ cells for IFN-γ and IL-4 production by intracellular cytokine staining. Figure 6 gives representative flow diagrams from a single H3-infected mouse in each treatment group. Table 3 summarizes these data for all animals infected with either H3 or H310A1 virus. Here, we found that anti-Vγ4 treatment slightly reduced both CD4+ IFN-γ+ (Th1) and non-CD4+ IFN-γ+ cells but that it substantially increased CD4+ IL-4+ and non-CD4+ IL-4+ cells (Fig. 6). In contrast, anti-Vγ1 antibody treatment of H310A1-infected mice slightly increased IFN-γ+ and reduced IL-4+ cells.
FIG. 4.
Myocarditis in antibody-treated mice. BALB/c mice were injected i.v. with 200 μg of hamster IgG, anti-Vγ1, or anti-Vγ4 monoclonal antibodies 3 days prior to i.p. infection with either H3 or H310A1 virus. All animals were killed 7 days after infection (10 days after antibody administration), and hearts were evaluated for myocarditis. Results represent means ± standard errors of the means (SEM) of results with at least four mice per group. ∗, P ≤ 0.05 compared to results for hamster IgG-treated animals.
FIG. 5.
Flow analysis of splenocytes expressing the γδ, Vγ1, and Vγ4 TcRs. BALB/c mice were treated with 300 μg of monoclonal antibody to Vγ1 or Vγ4 i.v. through the tail vein 3 days before i.p. injection of 104 PFU of CVB3. Control mice were given 300 μg of purified hamster IgG. Animals were killed 7 days after infection. Spleens were removed, and the lymphoid cell population was isolated. Aliquots of the spleen cells were stained with FITC-conjugated anti-γδ and either biotinylated hamster anti-Vγ1 or anti-Vγ4 and PE-conjugated streptavidin to indicate Vγ1+ and Vγ4+ cell subpopulations. Numbers in upper right-hand corner indicate percentages of cells in the quadrants.
TABLE 3.
Antibody-mediated depletion of Vγ-cell subsets in vivoa
| Virus | Treatment | % of total splenotypes staining positively for both:
|
|||
|---|---|---|---|---|---|
| γδ and Vγ1 | γδ and Vγ4 | CD4 and IFN-γ | CD4 and IL-4 | ||
| H3 | Hamster IgG (200 μg) | 3.5 ± 1.6 | 3.8 ± 1.2 | 5.1 ± 1.1 | 0.2 ± 0.2 |
| Anti-Vγ1 (200 μg) | 0.8 ± 0.3∗ | 4.5 ± 1.4 | 5.0 ± 1.8 | 1.5 ± 0.3∗ | |
| Anti-Vγ4 (200 μg) | 8.3 ± 1.6∗ | 1.4 ± 0.9∗ | 2.2 ± 0.6∗ | 0.5 ± 0.5 | |
| H310A1 | Hamster IgG | 4.3 ± 1.5 | 2.1 ± 0.5 | 3.0 ± 1.2 | 1.4 ± 0.5 |
| Anti-Vγ1 | 0.5 ± 0.4∗ | 5.9 ± 1.5∗ | 9.1 ± 1.6∗ | 0.4 ± 0.1∗ | |
| Anti-Vγ4 | 4.7 ± 0.8 | 0.6 ± 0.3∗ | 2.5 ± 1.3 | 2.2 ± 0.9 | |
∗, value different than that for mice treated with hamster IgG at a P of ≤0.05.
FIG. 6.
Flow analysis of IFN-γ and IL-4 intracellular staining in the CD4+ cell population of antibody-treated H3 virus-infected mice. Splenocytes from control and antibody-treated mice described in the legend to Fig. 4 were stimulated with PMA, ionomycin, and brefeldin A for 4 h; stained for CD4 surface marker; fixed; permeablized; stained intracellularly for IFN-γ and IL-4; and evaluated by flow cytometry. Numbers in the upper right-hand corner indicate percentages of cells in the quadrants.
Adoptive transfer of Vγ1+ and Vγ4+ cells from BALB/c Stat4 and BALB/c Stat6ko mice.
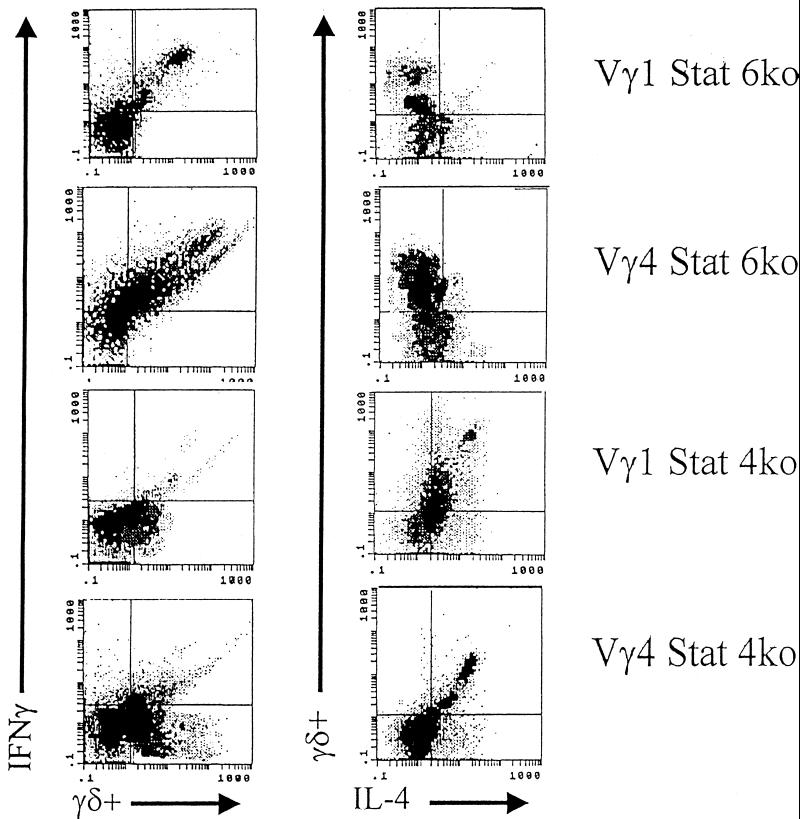
To determine whether the cytokine made by the γδ+-T-cell subset is primarily responsible for its effect on CD4+-cell responses and myocarditis susceptibility, BALB/c Stat4ko and BALB/c Stat6 mice were infected with H3 virus and, 5 days later, enriched populations of Vγ1+ and Vγ4+ cells were isolated as described in Materials and Methods. Figure 7 shows IFN-γ and IL-4 expression in the subpopulation by intracellular cytokine staining. As expected, both Vγ1+ and Vγ4+ cells from Stat6ko mice showed predominant IFN-γ but minimal IL-4 expression. Vγ4+ cells consistently stained more strongly for IFN-γ than did Vγ1+ cells from these animals. Similarly, while both Vγ1+ and Vγ4+ cells from Stat4ko mice stained positively for IL-4, Vγ4+ cells from Stat4ko mice also showed some residual IFN-γ expression, compared to results with the Vγ1+ subpopulation. Wild-type BALB/c mice were infected with H3 virus and 3 days later received 5 × 105 cells from the Vγ subsets i.v. Table 4 shows the levels of myocarditis and percentages of CD4+ Th1 and Th2 cells in recipient animals. Vγ1+ cells from Stat4ko (IFN-γ-deficient) mice were strongly suppressive for both myocarditis and CD4+-Th1-cell responses. Somewhat surprisingly, Vγ4+ cells from Stat4ko mice were not suppressive, even though these cells express IL-4. Similarly, Vγ1+ cells from Stat6ko (IL-4 deficient) mice had no significant effect on either myocarditis or CD4+-cell responses, yet Vγ4+ cells from these donors substantially enhanced both disease and Th1 cell bias.
FIG. 7.
Flow analysis of intracellular cytokine staining of enriched Vγ1+ and Vγ4+ cells from BALB/c Stat6ko and BALB/c Stat4ko mice. Mice were infected with H3 virus and killed 5 days later. Spleens were removed, and enriched populations of Vγ subpopulations were isolated as described both in Materials and Methods and in the legend to Fig. 1. Aliquots of the cell populations were stimulated with PMA, ionomycin, and brefeldin A; surface stained for the FITC-conjugated γδ TcR; and intracellularly stained with PE-conjugated anti-IL-4 and biotinylated anti-IFN-γ and then with Red 613 streptavidin.
TABLE 4.
Effect of cytokine production by γδ+ T-cell subsets on CVB3-induced myocarditisa
| Subset transferred | % of myocardium inflamed | % of CD4 IFN-γ+ cells | % of CD4 IL-4+ cells |
|---|---|---|---|
| None | 9.3 ± 1.6 | 12.6 ± 2.7 | 3.3 ± 0.9 |
| Vγ1 Stat6ko | 7.2 ± 2.4 | 9.9 ± 2.5 | 2.5 ± 0.7 |
| Vγ1 Stat4ko | 2.0 ± 0.8∗ | 4.3 ± 1.5∗ | 7.0 ± 1.6∗ |
| Vγ4 Stat6ko | 15.6 ± 1.2∗ | 22.4 ± 4.1∗ | 1.9 ± 1.5 |
| Vγ4 Stat4ko | 10.4 ± 2.8 | 10.2 ± 1.9 | 4.8 ± 2.1 |
Donor mice were infected with 104 PFU of H3 in 0.5 ml of PBS. Spleens were removed 5 days later. αβ+, MAC3+, and IAd+ cells were depleted using antibody and magnetic particles. The residual cells were cultured on anti-Vγ1- or anti-Vγ4-coated plates for 3 days. The cells were retrieved, centrifuged on Histopaque, resuspended in PBS to 2.5 × 106 cells/ml, and injected i.v. through the tail vein (0.2 ml or 5 × 105 cells) into BALB/c recipients infected 3 days earlier with H3 virus. Recipients were killed 4 days after receiving Vγ-enriched populations. Hearts of recipients were evaluated for myocardial inflammation. Spleen lymphocytes were evaluated as described in Materials and Methods for percentages of CD4+ cells staining intracellularly for IFN-γ or IL-4. Results are means ± SEM of results with four or more mice per group. ∗, value significantly different than that for H3-infected BALB/c mice not given Vγ cells.
DISCUSSION
We had previously found that H3 virus infection of C57BL/6 mice activates Vγ1+ cells, which suppress myocarditis, but that infection of Bl.Tg.Eα mice activates Vγ4+ cells, which promote myocarditis (14). Since Vγ4+ cells are 4- to 10-fold more abundant in Bl.Tg.Eα than in C57BL/6 animals, possibly because the expression of MHC class II IE antigen in Bl.Tg.Eα mice influences γδ+ cell subset generation during T-cell ontogeny, activation of distinct Vγ+ cell subsets in the C57BL/6 and Bl.Tg.Eα animals might simply reflect virus activation of whatever γδ+ cells are present. In contrast, the results presented in this paper clearly demonstrate that myocarditic and nonmyocarditic CVB3s selectively activate different γδ+ subsets associated with modulating the CD4+-Th-cell phenotype.
The Th1-cell–Th2-cell dichotomy in disease susceptibility and resistance is well recognized (8, 11, 19, 24, 32, 37). In those diseases which are dependent on proinflammatory cytokines such as IL-1 and tumor necrosis factor alpha, Th1-cell responses are often pathogenic while Th2-cell responses are protective. In contrast, in diseases requiring T-cell-dependent B-cell responses, such as IgE-dependent allergies, Th2 cells correlate with disease whereas Th1 cells can be protective. In CVB3-induced myocarditis, tumor necrosis factor alpha and IL-1β are crucial to myocarditis susceptibility (25) but T-cell-dependent responses are not required for virus clearance (43). Thus, Th1-cell responses are predicted to be pathogenic in this disease. γδ+ cells clearly can modulate Th1 and Th2 responses either directly or indirectly, as has been shown by various published reports. The assumed mechanism for this modulation is through cytokine production by the γδ+ cells, which provide the appropriate environment to influence CD4+-cell differentiation (7, 8, 26, 44). In this case, γδ+ cells should be quickly activated in peripheral lymphoid tissues and rapidly accumulate at local inflammatory sites in order to maximally influence the developing antigen-specific immune response. γδ+ T cells are excellent candidates for the early cytokine response because these cells can respond to broadly distributed antigens in damaged tissues and microbes, such as heat shock proteins (2), and do not require classical antigen processing and MHC-restricted presentation. Moreover, γδ+ T cells have a memory phenotype in the periphery, suggestive of prior antigen activation, and the potential for rapid expansion (40, 42). In the present communication, we demonstrate that few γδ+ T cells from uninfected mice express cytokines by intracellular cytokine staining but that many γδ+ T cells from H3-infected animals express high levels of IFN-γ. In these animals, the stronger IFN-γ expression in Vγ4+ cells than that in Vγ1+ cells conforms to a predicted superiority of the Vγ4+ population in Th1-cell modulation. Thus, based on the cytokine patterns, depletion of Vγ4+ cells in H3-infected animals is expected to reduce Th1-cell responses, as was seen in this study. There are certain discrepancies in the data, however. Anti-Vγ1 antibody treatment of H3 virus-infected mice results in an increase in CD4+ IL-4+ cells from 0.2 to 1.5% (Table 3). If Vγ1+ cells uniformly promote CD4+-Th2-cell responses, this result is perplexing. We do not have an explanation for this finding, but as discussed below, antibody treatment might stimulate as well as eliminate specific cell populations. Activating Vγ1+ cells prior to their elimination might simultaneously cause increases in CD4+ Th1 cells (through elimination of Vγ1+ cells) and increases in CD4+ Th2+ cells (through activation of Vγ1+ cells prior to their elimination and the release of Th2-promoting factors). The results are also unclear for H310A1-infected mice. Here, anti-Vγ1 antibody treatment enhanced myocarditis but the IFN-γ staining of the Vγ4+ cells in these animals was modest. Thus, it is not clear why depletion of the Vγ1+ cells promoted CD4+-Th1-cell responses based solely on the cytokine data. One possibility is that other cytokines differ in their levels of Vγ1+ and Vγ4+ cells, which may be potential regulators of the CD4+ response, either through direct action on the CD4+-cell population or by influencing other cell types, such as macrophages, which subsequently alter Th-cell development (23). γδ+ T cells have been reported to make IL-13, a cytokine known to promote Th2-dependent responses such as IgE production (12, 30). This would enable Vγ1+ T cells to promote Th2-cell responses even if they make little if any IL-4.
Experiments using antibody-induced depletion of γδ+ cells in vivo have potential problems, even though it has been used by various investigators for Vγ-subset depletion (31, 34). Antibody binding to the TcR might activate cells to release cytokines and immunomodulate αβ+-T-cell responses, prior to their elimination. Thus, the antibody depletion experiments might indicate that either the lack of or the activation of specific subpopulations resulted in the observed effects. Studies by R. O'Brien and her colleagues indicate that treatment of mice with the monoclonal antibodies causes depletion of cells within 24 h and remains effective for 2 weeks. Since the monoclonal antibodies in this study were given 3 days prior to infection, presumably any cytokines released due to antibody-mediated TcR cross-linking should have been cleared by the time of infection. Another potential problem is that the antibodies did not totally eliminate the relevant cell populations, and although it is quite clear that most Vγ1+ or Vγ4+ cells had been eliminated, some dull-staining cells remained. Whether these cells can affect CD4+-cell responses is not known. It is also not clear whether the residual IFN-γ+ cells in anti-Vγ4 antibody-treated mice reflect cells resistant to γδ+-T-cell influence or whether complete elimination of the Vγ4+ cells would have more effectively reduced the number of IFN-γ-producing cells.
Adoptive-transfer experiments using enriched populations of Vγ1+ and Vγ4+ cells were done to complement the antibody depletion experiments. In this case, we know that the γδ+ subpopulations are strongly activated prior to injection into recipient mice, and effects observed in the recipient should reflect the direct or indirect consequences of these specific Vγ subpopulations. To clarify whether cytokines made by γδ+ cells exclusively control myocarditis susceptibility, we isolated Vγ1+ and Vγ4+ cells from BALB/c transgenic mice lacking either the Stat4 or Stat6 transcription factor required for expression of IL-12 or IL-4, respectively (3, 29). Stat4ko mice are strongly biased toward a Th2-cell phenotype because of the lack of IL-12 needed to stimulate IFN-γ responses. Both Vγ1+ and Vγ4+ cells from Stat4ko mice showed substantial IL-4 expression by intracellular cytokine staining, yet only the Vγ1+ subpopulation suppressed myocarditis. The lack of suppression by the Vγ4+ cells might result from residual IFN-γ expression in this population. A study by Cai et al. (3) also found residual IFN-γ expression in Toxoplasma gondii-infected animals, indicating an IL-12-independent pathway for IFN-γ expression. The modest IFN-γ expression in Vγ4+ cells from Stat4ko mice might be sufficient to negate any suppressive activity of IL-4 on CD4+-cell responses. However, this study might also indicate that the cytokines made by the Vγ subpopulations only partially explain their effects on CD4+ responses. Vγ1+ cells from Stat6ko mice express IFN-γ but negligible IL-4. These cells lose their ability to suppress myocarditis but fail to aggravate the disease, as is seen with Vγ4+ cells from Stat6ko animals. Again, the intensity of the IFN-γ responses is greater in the Vγ4+ than in the Vγ1+ cell population. The relative concentrations of IFN-γ and IL-4 may be the determining factor in the intensity of the Th1- or Th2-cell response.
As shown in Fig. 6, CD4+ cells are not the only splenocytes making IFN-γ or IL-4. One or more populations of non-CD4+ cells also stain intracellularly for these cytokines. To date, the presence of the non-CD4+ cytokine-positive cell population(s) correlates with the CD4+-cell response. That is, treatment of H3-infected mice with anti-Vγ4 antibody reduces not only the percentage of CD4+ IFN-γ+ cells but also that of these non-CD4+ IFN-γ+ cells as well. Many of these cells can be accounted for by Vγ4+ cells themselves. CD8+ T cells cannot be shown to stain for cytokines. The remaining cells besides CD4+ and γδ+ cells might be natural killer (NK) cells which are often cytokine positive (39). If NK cells are present in the cytokine-positive population, then γδ+ cells might be able to affect cytokine responses in this population as well.
ACKNOWLEDGMENTS
This work was supported by the following grants and institutional support: grant R01 HL58583 (S. A. Huber); grant R01 AI44920 (from the Rocky Mountain Chapter of the Arthritis Foundation) and EPA project grant R825793 (R. L. O'Brien); and NIH grant R01 AI40611 and EPA project grant R825793 (W. K. Born).
We gratefully acknowledge the expert secretarial assistance of Debbie Perrotte. We are grateful for the expert flow cytometric analyses performed by Colette Charland and Julie Wolfe.
REFERENCES
- 1.Azuara V, Pereira P. Genetic mapping of two murine loci that influence the development of IL-4-producing Thy-1 dull gamma delta thymocytes. J Immunol. 2000;165:42–48. doi: 10.4049/jimmunol.165.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Born W, Happ M, Dallas A, Reardon C, Kubo R, Shinnick T, Brennan P, O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990;11:40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- 3.Cai G, Radzanowski T, Villegas E, Kastelein R, Hunter C. Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii. J Immunol. 2000;165:2619–2627. doi: 10.4049/jimmunol.165.5.2619. [DOI] [PubMed] [Google Scholar]
- 4.Chomarat P, Kjeldsen-Kragh J, Quayle A, Natvig J, Miossec P. Different cytokine production profiles of gamma delta T cell clones: relation to inflammatory arthritis. Eur J Immunol. 1994;24:2087–2091. doi: 10.1002/eji.1830240923. [DOI] [PubMed] [Google Scholar]
- 5.Clark D, Chaouat G, Arck P, Mittruccker H, Levy G. Cutting edge: cytokine-dependent abortion in CBA × DBA/2 mice is mediated by the procoagulant fg12 prothrombinase. J Immunol. 1998;160:545. [PubMed] [Google Scholar]
- 6.D'Souza C, Cooper A, Frank A, Mazzaccro R, Bloom B, Orme I. An anti-inflammatory role for gamma-delta T lymphocytes in acquired immunity to mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 7.Ferrick D, Schrenzel M, Mulvania T, Hsieh B, Ferlin W, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 8.Fitch F, McKisic M, Lancki D, Gajewski T. Differential regulation of T lymphocyte subsets. Annu Rev Immunol. 1993;11:29–48. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- 9.Fu Y-X, Roark C, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma-delta T cells. J Immunol. 1994;153:3101. [PubMed] [Google Scholar]
- 10.Gerber D, Azuara V, Levraud J, Huang S, Lembezat M, Pereira P. IL-4-producing gamma delta T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 11.Hoag K, Lipscomb M, Izzo A. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino T, Yagita H, Ortaldo J, Wiltrout R, Young H. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30:1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh J, Schrenzel M, Mulvania T, Lepper H, DiMolfetto-Landon L, Ferrick D. In vitro cytokine production in murine listeriosis. Evidence for immunoregulation by gamma delta+ T cells. J Immunol. 1996;156:232–237. [PubMed] [Google Scholar]
- 14.Huber S, Graveline D, Newell M, Born W, O'Brien R. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 15.Huber S, Kupperman J, Newell M. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber S, Lodge P. Coxsackievirus B3 myocarditis in Balb/c mice: evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984;116:21. [PMC free article] [PubMed] [Google Scholar]
- 17.Huber S, Moraska A, Choate M. T cells expressing the γδ T-cell receptor potentiate coxsackievirus B3-induced myocarditis. J Virol. 1992;66:6541–6546. doi: 10.1128/jvi.66.11.6541-6546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber S, Mortensen A, Moulton G. Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the γδ+ T cell receptor. J Virol. 1996;70:3039–3045. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber S, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones-Carson J, Vazquel-Torres A, van der Heyde H, Warner T, Wagner R, Balish E. Gamma-delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto K, Dong V, Issazadeh S, Fedoseyeva E, Waaga A, Yamada A, Sho M, Benichou G, Auchincloss H J, Grusby M, Khoury S, Sayegh M. The role of CD154-CD40 versus CD28–B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Investig. 2000;106:63–72. doi: 10.1172/JCI9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowlton K, Jeon E, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodukula P, Liu T, Rooijen N, Jager M, Hendricks R. Macrophage control of herpes simplex virus type 1 replication in peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 24.Krenger W, Ferrara J. Graft-versus-host disease and the Th1/Th2 paradigm. Immunol Res. 1996;15:50–73. doi: 10.1007/BF02918284. [DOI] [PubMed] [Google Scholar]
- 25.Lane J, Neumann D, Lanfond-Walker A, Herskowitz A, Rose N. Role of IL-1 and tumor necrosis factor in coxsackievirus-induced autoimmune myocarditis. J Immunol. 1993;151:1682–1690. [PubMed] [Google Scholar]
- 26.McMenamin C, Pimm C, McKersey M, Holt P. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma-delta+ T cells. Science. 1994;265:1869–1873. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S. Different roles of alpha-beta and gamma-delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 28.Mukasa A, Yoshida H, Kobayashi N, Matsuzaki G, Nomoto K. Gamma delta T cells in infection-induced and autoimmune-induced testicular inflammation. Immunology. 1998;95:395–401. doi: 10.1046/j.1365-2567.1998.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy K, Ouyang W, Farrar J, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy T. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 30.Naiki Y, Nishimura H, Itohara S, Yoshikai Y. Gamma-delta T cells may dichotomously modulate infection with avirulent Salmonella choleraesuis via IFN-gamma and IL-13 in mice. Cell Immunol. 2000;202:61–69. doi: 10.1006/cimm.2000.1659. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Matsuzaki G, Nomoto K. The protective role of T-cell receptor Vγ1+ T cells in primary infection with Listeria monocytogenes. Immunology. 1999;96:29–34. doi: 10.1046/j.1365-2567.1999.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson L, Kuchroo V. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1997;8:837–842. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya T, Takimoto H, Matsuzaki G, Hamano S, Yoshida H, Yoshikai Y, Kimura G, Nomoto K. Vγ1+ γδ T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon gamma. Immunology. 2000;99:187–194. doi: 10.1046/j.1365-2567.2000.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien R L, Yin X, Huber S A, Ikuta K, Born W. Depletion of a gamma-delta T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 35.Pelegri C, Kuhlein P, Buchner E, Schmidt C B, Franch A, Castell M, Hunig T, Emmrich F, Kinne R W. Depletion of gamma delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 1996;38:204–215. doi: 10.1002/art.1780390206. [DOI] [PubMed] [Google Scholar]
- 36.Peterman G M, Spencer C, Sperling A I, Bluestone J A. Role of gamma delta T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 37.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 38.Rossman M, Carding S. Gamma delta T cells in asthma. Ann Intern Med. 1996;124:266–267. doi: 10.7326/0003-4819-124-2-199601150-00013. [DOI] [PubMed] [Google Scholar]
- 39.Scharton T, Scott P. Natural killer cells or a source of interferon gamma drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tough D, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent M, Roessner K, Lynch D, Wilson D, Cooper S, Tschopp J, Sigal L, Budd R. Apoptosis of Fashigh CD4+ synovial T cells by borrelia-reactive Fas-ligand(high) gamma delta T cells in Lyme arthritis. J Exp Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weintraub B C, Jackson M R, Hedrick S M. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994;153:3051–3058. [PubMed] [Google Scholar]
- 43.Woodruff J, Woodruff J. Involvement of T lymphocytes in the pathogenesis of coxsackievirus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]
- 44.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig B, Pereira P, Pretolani M. Requirement for gamma delta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]