Abstract
Intercellular adhesion molecule 1 (ICAM-1/CD54), a transmembrane glycoprotein, has been considered as one of the most important adhesion molecules during leukocyte recruitment. It is encoded by the ICAM1 gene and plays a central role in inflammation. Its crucial role in many inflammatory diseases such as ulcerative colitis and rheumatoid arthritis are well established. Given that neuroinflammation, underscored by microglial activation, is a key element in neurodegenerative diseases such as Parkinson’s disease (PD), we investigated whether ICAM-1 has a role in this progressive neurological condition and, if so, to elucidate the underpinning mechanisms. Specifically, we were interested in the potential interaction between ICAM-1, glial cells, and ferroptosis, an iron-dependent form of cell death that has recently been implicated in PD. We conclude that there exist direct and indirect (via glial cells and T cells) influences of ICAM-1 on ferroptosis and that further elucidation of these interactions can suggest novel intervention for this devastating disease.
Keywords: ICAM-1, Parkinson’s disease, ferroptosis, glial cells, T cells, neuroinflammation
1. Introduction
Intercellular adhesion molecule 1 (ICAM-1/CD54) is a transmembrane glycoprotein that was discovered in the 1980s and was identified as a ligand of the β2 integrin lymphocyte function-associated antigen (LFA)-1 (CD11a/CD18), and as an important switch to initiate a key adhesion pathway [1,2]. Since then, its critical role in inflammatory responses and a plethora of inflammatory diseases has been verified [3,4,5,6,7,8,9].
Although neurodegenerative diseases in general, and Parkinson’s disease (PD) in particular, are triggered and/or exacerbated by neuroinflammatory mediators, an association between ICAM-1 and PD has not been adequately studied. Justification for such pursuit is supported by several premises. First, there is a well-established involvement of neuroinflammation in PD [10,11,12,13]. Second, the co-morbid presentation of depression with PD is extensively documented [14,15,16], and the involvement of ICAM-1 in late-life depression has been verified [17]. Moreover, the presence of ICAM-1 in reactive astrocytes was identified in patients with PD as well as in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys, a nonhuman primate model of PD [18]. Therefore, the aim of the current review is to provide the mechanistic implications of ICAM-1 in PD, specifically in relation to glial cell-mediated neuroinflammation underscored by ferroptosis, a recently implicated pathway in PD pathology, as well as T cell reactivity. Thus, following brief descriptions of ICAM-1, PD, glial cells, T cells, and ferroptosis, we seek to provide convincing evidence on a causal relationship between them, with the hope of identifying novel targets for the treatment of PD.
1.1. ICAM-1
The well-known function of ICAM-1 involves leukocyte extravasation and has been described as one of the most important adhesion molecules during leukocyte recruitment [19,20,21]. Specifically, the expression of ICAM1, located on chromosome 19, is induced in endothelial cells by a variety of cytokines and inflammatory mediators, including tumor-necrosis factor alpha (TNF-α), nuclear factor kappa B (Nf-kB), interferon gamma (IFN-γ), interleukin-1 beta (IL-1β), IL-6, as well as hydrogen peroxide (H2O2) and NADPH oxidase (NOX) enzyme activity [1,22,23,24,25,26,27]. ICAM-1 is expressed in the plasma membrane and binds to the β2 integrins LFA-1 and macrophage antigen 1 (MAC-1/CD11b/CD18) expressed by leukocytes [28,29,30]. ICAM-1-LFA-1/MAC-1 binding mediates leukocyte rolling, crawling, adhesion, and the passage of blood cells through the intact walls of the capillaries (diapedesis), often accompanied by inflammation during extravasation, the process by which leukocytes move out of the circulatory system to the site of tissue damage or infection [30]. Upon binding to LFA-1/MAC-1, ICAM-1 induces the dissociation of junction proteins with adjacent endothelial cells, cytoskeletal rearrangement, and endothelial nitric oxide synthase (eNOS) activity, enabling transendothelial leukocyte migration [9,30,31,32]. However, as discussed below, the implications of ICAM-1 extend beyond the transmigration of leukocytes.
ICAM-1 is expressed in neurons and immune, endothelial, and epithelial cells, among others, albeit at low levels of expression during basal conditions [33,34,35]. ICAM-1 has several notable ligands, including fibrinogen, mucin 1 (MUC1), cluster of differentiation 43 (CD43), hyaluronan, rhinoviruses, and plasmodium falciparum [36,37,38,39,40,41]. Moreover, ICAM-1 is involved in a myriad of physiological processes, such as T cell regulation (discussed below), macrophage polarization, cellular migration, reactive oxygen species (ROS) production, and cancer development and metastasis [7,26,42,43,44]. Aldosterone and angiotensin II have been found to induce atherosclerosis and hypertension, respectively, via ICAM-1-dependent mechanisms in experimental models [45,46,47]. The infusion of angiotensin II also increases ICAM-1 in human subjects [45]. The role of ICAM-1 in atherosclerosis and cardiovascular disorders has been extensively evaluated and verified [8,46,47,48]. The adhesion molecule appears to have a fundamental role in intestinal and blood–brain barrier (BBB) permeability, and neuroinflammation [49,50,51,52,53]. In addition to inflammation remediation, ICAM-1 also plays a role in wound healing and efferocytosis or the clearance of apoptotic cells [7,54,55].
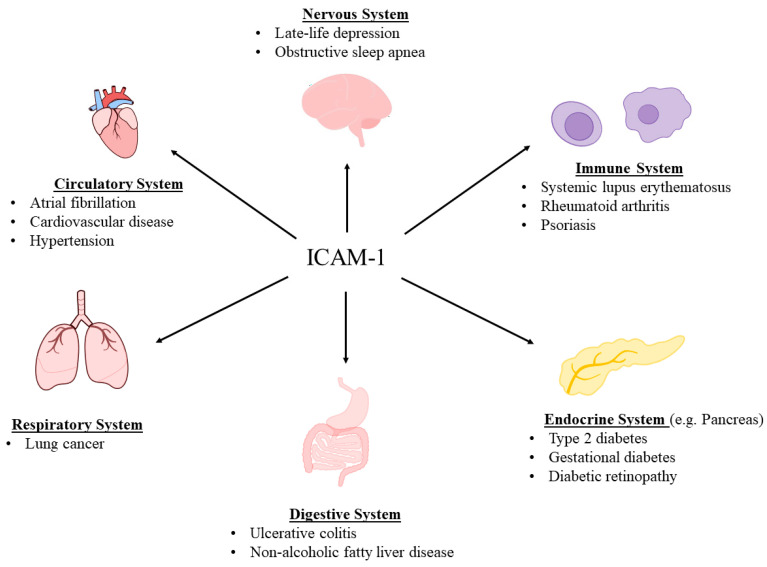
As a transmembrane glycoprotein, ICAM-1 is expressed in the plasma membrane and extends into the cytoplasm and onto the cell surface, enabling the participation in signal transduction, interactions with cytoskeletal structures, and ligand binding [7,30,56]. Nevertheless, ICAM-1 can be enzymatically cleaved from the cell surface to circulate freely as a form of the protein known as soluble ICAM-1 (sICAM-1) [57,58]. Specifically, a disintegrin and metalloproteinase 10 (ADAM10), ADAM17, matrix metalloproteinase 2 (MMP-2), and MMP-9 have been found to cleave membrane-bound ICAM-1 [59,60,61,62]. The role of leukocyte elastase and cathepsin G may be particularly relevant regarding cleaving ICAM-1 isoforms that arise due to alternative splicing [63,64]. Furthermore, sICAM-1 can be generated because of alternative splicing, in which case it lacks transmembrane and cytoplasmic domains [30,65]. In vitro sICAM-1 concentrations have been found to quantitatively relate to cell surface ICAM-1 expression [58,66]. Conversely, alternative splicing, protease activity, and ICAM-1 ectodomain shedding would have the potential to impact this relationship and should be considered during in vivo analyses [30,65,67]. sICAM-1 concentrations have been reported to range from 100 to 450 ng/mL in the serum of the general population [4,68]. Increased sICAM-1 levels have been associated with an array of conditions, notably, endometriosis, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, obstructive sleep apnea, non-alcoholic fatty liver disease, lung cancer, atrial fibrillation, obesity, type 2 diabetes, diabetic retinopathy, gestational diabetes mellitus, and late-life depression [17,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. More recently, its crucial role in ulcerative colitis (an inflammatory bowel disease), where higher levels of ICAM-1 were associated with worse prognosis, was revealed [83] (Figure 1).
Figure 1.
ICAM-1 has been implicated in and associated with an array of diseases.
Thus, ICAM-1 has central yet diverse roles in inflammation and has an important role in the initiation of inflammatory responses. Nonetheless, the entirety of these implications has yet to be elucidated in specific phenotypes and/or disease states. Of particular importance and unexplored implication is to what extent and through what mechanisms ICAM-1 may be involved in PD pathology.
1.2. Parkinson’s Disease (PD)
PD is a progressive neurodegenerative disease marked by the gradual deterioration of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNpc), the accumulation of misfolded α-synuclein proteins as Lewy bodies, and the dysregulation of glial cells (discussed below). Although both genetic and environmental influences are known risk factors, most PD cases are sporadic or what is commonly referred to as idiopathic, meaning no known cause. PD is characterized by motor and non-motor symptoms. The motor symptoms include resting tremor, bradykinesia, rigidity or inflexibility, dystonia, and postural and walking abnormalities [11,84]. Freezing of gait is also a common feature. Non-motor symptoms, which often precede the motor symptoms, include partial or total loss of smell (anosmia), mood disorders (e.g., depression), excessive sweating, hypotension, fatigue, cognitive impairment, inability to produce facial expressions or recognize other’s verbal and nonverbal cues, sleep perturbations (e.g., insomnia/hypersomnia), gastrointestinal problems (e.g., difficulty in swallowing, constipation, and nausea), and urinary and sexual dysfunction [11,85].
Since DA loss is the primary underlying cause, therapeutic interventions are focused on replacing this neurotransmitter or its function. This is primarily achieved by the administration of L-dopa, considered the gold standard, plus drugs that interfere with DA breakdown, such as monoamine oxidase or catechol-o-methyltransferase inhibitors (e.g., selegiline/rasagiline and tolcapone/entacapone, respectively) and/or newer non-ergot DA agonists, such as pramipexole, ropinirole, rotigotine, and apomorphine [10,86]. Carbidopa is given in conjunction with L-dopa to prevent its peripheral breakdown. All of these drugs may provide remarkable symptomatic relief, yet none of them address the progression of neurodegeneration. Moreover, L-dopa, the most efficacious drug, not only loses its efficacy over months or years but may induce severe dyskinesia, which may be worse than the initial tremors [10,87]. Hence, more efficacious interventions without such adverse effects are urgently needed [11,88].
Neuroinflammation and oxidative stress have been extensively implicated in PD [11,89,90,91,92]. Oxidative stress (OS) occurs due to an imbalance of oxidants and antioxidant capacity, which can lead to oxdiant-induced damage to DNA, proteins, and lipids [11]. Within the context of PD, oxidants may originate from a variety of sources, which includes, but is not limited to, mitochondrial dysfunction, DA metabolism, and glial cells [11,89,90,91,92]. Oxidative stress and ROS can induce the activation of immune cells and immune responses, and ultimately inflammation. However, inflammation and OS have a bidirectional relationship, which can contribute to a vicious and reciprical cycle in PD [11,89,90,91,92]. Mitochdondrial damage and NOX also have roles in this process [90,91,92]. An emphasis of this review is the elucidation of how ICAM-1 interacts with the intermediaries of OS or inflammation and how it might provide a therapeutic target in PD.
1.3. Glial Cells
Glial cells were first identified in the mid-19th century and were referred to as neuroglia (neuro-glue), since they were thought to provide merely structural support for the neurons. However, it is now known that glial cells carry a variety of crucial functions, not only as structural support for neurons [93,94,95], but also in myelination [96,97], the control of energetics and metabolism [95,98,99], the formation of the BBB [100,101], the development and remodeling of synapses [102,103], the control of the fluid/electrolyte homeostasis [104], the regulation of neurotransmitters [105,106], neuroendocrine function [107], detoxification [108,109], and innate immunity response [110,111]. It is not surprising, therefore, that their disruption or dysregulation may lead to neuropsychiatric and neurodegenerative diseases [13,97,112,113,114,115,116]. By the same token, they may present novel targets in neurological diseases [117]. Indeed, it has been suggested that the manipulation of the nicotinic cholinergic receptors (nAChRs) in these cells may be a viable target for intervention in PD [13], mood disorders, and even drug addiction [118].
Four main types of glial cells include microglia, astrocytes, oligodendrocytes, and synantocytes or NG2 cells. Below, following a brief description of each, we specifically concentrate on their interactions with ICAM-1 vis-a-vis neuroinflammatory response.
1.3.1. Microglia
Microglia, representing 10–15% of all of the central nervous system (CNS) cells, cover a significant volume of the adult brain parenchyma. These cells, through rapid movements of their fine filopodia, constantly survey the environment and react quickly to any kind of insult. They share the same origin as peripheral macrophages but are considered the resident immune cells of the CNS [119,120]. By regulating neurogenesis, the formation and elimination of neuronal synapses, mediating T cell infiltration into the brain, and, most importantly, eliminating pathogens and cell residues, they play a vital role in maintaining brain homeostasis [121]. On the other hand, if overactivated, microglia can cause neuroinflammation, leading to neuronal damage or death, and neuropsychiatric and/or neurodegenerative diseases, including PD [122,123,124,125]. A major culprit in microglial overactivation is persistent stress causing the release of proinflammatory mediators such as IL-1β and IL-6 [126,127,128].
It is of relevance to note that, based on their activation state, different microglial subtypes were described previously. Hence, the M1 microglia was associated with a proinflammatory state and the M2 with an opposite or anti-inflammatory state [121,129]. However, emerging evidence suggests that differences in microglia functions are due to their inherent properties, and that the subtypes should be categorized based on their function and avoid the use of the M1 or M2 state as such [117,130,131].
Microglia express various receptors, including the calcium-sensing receptor (CASR), low-density lipoprotein receptor-related protein 1 (LRP1), triggering receptor expressed on myeloid cells-2 (TREM2), nAChRs, and Toll-like receptors such as TLR2 and TLR4 [13]. TLRs are a well-characterized family of pattern-recognition receptors (PRRs) that initiate the innate immune response by sensing the endogenous debris or pathogens. Because of their significant role in neurodegenerative diseases, TLRs are investigated intensely as potential therapeutic targets in such diseases [121,132,133,134].
1.3.2. Astroglia
Due to their star-like shape, these cells were named astroglia or astrocytes [135] and may constitute anywhere between 17 and 61% of the total brain cells, depending on the area. Astrocytes also play a crucial role in maintaining neuronal integrity and function, as they provide nutrients, monitor and regulate pH homeostasis, remove waste, and are a key constituent of the BBB [135,136].
Astrocytes contain both the glial-derived neurotrophic factor (GDNF), that provides trophic support to neuronal cells including DAergic neurons [137], and glial fibrillary astrocytic protein (GFAP), which is a key protein responsible for maintaining astrocyte strength and the BBB. The GFAP is commonly used as a marker for astrocyte identification [138] and may serve as a biomarker for brain and spinal cord disorders [139,140,141,142]. These glial cells also express brain-derived neurotrophic factor (BDNF) and the highest amount of taurine, a free amino acid with antioxidant and anti-inflammatory properties that is required for optimal postnatal brain development [143]. More recently it was reported that astrocytes are the necessary source of TNF-α for the mediation of homeostatic synaptic plasticity [144].
Astrocytes in conjunction with microglia provide the first line of defense against insults. Here, also, the overstimulation of the proinflammatory signals may synergistically contribute to neuronal dysregulation and ensuing neuropsychiatric/neurodegenerative diseases [145,146,147]. Moreover, the elucidation of the intimate interaction between astrocytes and microglia, as well as astrocytes and neurons, referred to as crosstalk, could provide novel intervention in such diseases [137,148,149,150].
1.3.3. Oligodendrocytes
Oligodendrocytes (OLs), representing 75% of all glial cells, are the major source of myelination in the CNS [151]. In addition to axonal myelination, OLs have other crucial functions, such as providing the metabolic and trophic supply by the secretion of GDNF and BDNF, controlling the extracellular potassium concentration, and modulating axonal growth [151,152]. They also express TLRs, which are important in myelin formation [96,153,154]. It is not surprising, therefore, that the dysregulation of these glial cells could lead to a variety of neurological diseases, including PD (discussed in more detail below).
1.3.4. Synantocytes (NG2 Cells)
The fourth subset of major glial cells in the CNS are synantocytes, which are also referred to as neuron glial 2 or nerve/glial antigen 2 (NG2) cells, and oligodendrocyte precursor cells (OPCs). NG2 cells are expressed in both white and gray matter areas, and can keep proliferating in the adult brain [151,155,156]. In addition to being OL progenitors, NG2 cells can also transform to astrocytes and neurons [151,155,156]. They have been implicated in a variety of neurological disorders, including multiple sclerosis, Alzheimer’s disease (AD), epilepsy, traumatic brain injury, acute ischemic stroke, neurovascular unit formation during development, glioma, and experimental autoimmune encephalomyelitis (EAE), a disease associated with increased BBB permeability and neuroinflammation [157,158,159,160,161]. Moreover, their communication and influence on neurons renders them a potential therapeutic target in many diseases, including PD [162,163], as discussed in more detail below.
1.4. ICAM-1—Glial Cells
1.4.1. ICAM-1—Microglia
Microglia express ICAM-1 and constitutively express LFA-1 and Mac-1, which enables various direct interactions between ICAM-1 and microglia within multiple contexts [164,165]. Notably, ICAM-1 has a role in the activation of microglia [166,167,168]. Activated microglia, in turn, secrete TNF-α, which induces the expression of ICAM-1 in vascular endothelial cells and facilitates leukocyte infiltration [23,169,170]. ICAM-1 may also indirectly activate microglia. This occurs due to the vascular endothelial expression of ICAM-1, which promotes the transendothelial migration of leukocytes and their infiltration into the CNS, resulting in microglial activation [171,172,173,174]. Interestingly, leukocytes that have infiltrated into the CNS may adhere to microglia [171,175]. Thus, there is a positive feedback loop between ICAM-1 and microglia [166,167,168,171,172,173,174].
1.4.2. ICAM-1—Astroglia
Astrocytes also contain ICAM-1, the expression of which is increased by TNF-α, IL-1β, and IFN-γ [176,177,178,179,180]. ICAM-1, in turn, may cause the release of inflammatory cytokines, including TNF-α in astrocytes [181,182]. The ICAM-1 activation of astrocytes may also be brought indirectly via fibrinogen, which is induced in various neuroinflammatory states and binds to ICAM-1 [183,184]. Fibrinogen-activated astrocytes further enhance ICAM-1 expression and promote the production of NO and ROS, leading to neuronal death [183,184]. Curiously, ROS may induce astrocytic ICAM-1 production in an Nf-kB-dependent mechanism [185,186]. Therefore, here, also, there appears to exist a positive feedback loop between astrocytes and ICAM-1 [179,181,183,184,185,186].
1.4.3. ICAM-1—Oligodendrocytes
The enhanced expression of ICAM-1 in OLs during inflammatory conditions is postulated to be a defense mechanism in response to immunogenic insult [177,187]. The direct contact of OLs with T cells has been suggested to induce OL damage, and anti-ICAM-1 antibodies were found to inhibit Th1 cell contact with OLs [187]. Due to the role of OLs in myelination, T cell-induced damage in these cells may contribute to neurodegeneration, as seen in EAE [187]. The inhibitory action of anti-ICAM-1 antibodies on EAE in vivo has been shown in animal models, including marmoset monkeys [188,189]. As discussed for microglia, ICAM-1 has a role in T cell infiltration into the CNS, which again provides an indirect mechanism for ICAM-1 to influence OL homeostasis [173,177,187].
1.4.4. ICAM-1—NG2 Cells
The maturation of NG2 cells is inhibited by proinflammatory cytokines [190,191,192]. Moreover, microglia can influence NG2 cell proliferation, differentiation, migration, and apoptosis, while NG2 cells can regulate microglia homeostasis and activation [193]. This suggests an indirect interaction between ICAM-1 and NG2 cells. While recent findings implicate NG2 cells in the initiation of neuroinflammation via the activation of immunogenic cells, the NG2 protein appears to be a negative regulator of ICAM-1 expression in pericytes and two different glioblastoma cell lines [194,195,196].
1.5. ICAM-1—Glial Cells—PD
Neuroinflammation has been extensively implicated in the pathophysiology of PD, with significant contributions from glial cells [197,198]. The role of glial cells in neuroinflammation and PD is supported by a recent meta-analysis reporting increased cerebral spinal fluid (CSF) concentrations of TNF-α, IL-6, IL-1β, nitric oxide (NO), chemokine ligand 2 (CCL2), and c-reactive protein (CRP) in individuals with this disease [199]. Despite the protective roles of transiently activated microglia, the chronic activation of microglia has been widely hypothesized to be involved in PD [200]. Specifically, inflammatory microglia phenotypes have been found in experimental models as well as in the SN of PD patients [200,201]. Cytokines associated with inflammatory phenotypes of microglia include TNF-α, IL-6, IL-1β, and IFN-γ, all of which induce ICAM-1 expression [1,22,23,199,202]. The role of microglia in PD is also supported by a postmortem analysis that found an increase in activated microglia, and its expression of ICAM-1, LFA-1, TNF-α, and IL-6, in the SN and various other regions of the brain in PD patients [203].
While microglia have a clear role in PD-associated neuroinflammation, they may also induce a neurotoxic and reactive phenotype of astrocytes, often referred to as the A1 phenotype, which can exacerbate PD pathology [204,205,206]. Furthermore, astrocytes have a role in the removal of dysfunctional proteins such as α-synuclein. Microglial–astrocyte interactions may also facilitate α-synuclein removal, the accumulation of which may increase ICAM-1 and IL-6 expression in astrocytes [206,207,208,209]. Moreover, increased concentrations of astrocytes and microglia, infiltration of leukocytes, and increased expression of ICAM-1 and LFA-1 were found in the SN of PD patients [18].
Abnormal and decreased myelin contents have been associated with PD symptoms [210,211]. In PD patients, 80% of connections originating from the basal ganglia displayed decreased myelin content [212]. Moreover, OLs, which are major contributors to myelination, have been found to be decreased in idiopathic PD [213]. In addition, α-synuclein transfer from neurons to OLs may exacerbate PD pathology [214,215]. Given that the OL expression of ICAM-1 is enhanced during inflammatory conditions, the modulation of this adhesion molecule may provide a novel target in PD [177,187].
Altogether, the above provides a strong connection between ICAM-1, glial cells, and PD.
1.6. ICAM-1—T Cells
Leukocytes in the circulatory system are recruited to sites of inflammation via various inflammatory signaling molecules, such as cytokines and chemokines. As mentioned earlier, once leukocytes reach the site of inflammation, they often extravasate and undergo diapedesis, that is, they pass through capillary walls, a process mediated by adhesion molecules. T cells also undergo a similar process as they express LFA-1 and interact with ICAM-1 to facilitate their endothelial transmigration [216,217]. ICAM-1 not only facilitates T cell transmigration [216,218], but also facilitates their activation [219,220]. In addition, it plays an important role in enabling T cell interactions with other leukocytes [219,220,221].
1.7. ICAM-1—T Cells—PD
T cells have diverse roles in PD and appear to be influenced by DA [222]. Moreover, peripheral concentrations of T cell subpopulations are generally heterogenous and are dependent on a variety of patient characteristics, such as sex, age, and disease severity and duration [223,224]. Specifically, PD patients present with increased Th1 and Th17 cells and decreased Th2 and regulatory T cells (Tregs) [224], and some PD patients possess α-synuclein-specific T cells [225,226,227].
Under inflammatory conditions, endothelial cells in the CNS express various proteins and adhesion molecules, including ICAM-1, which facilitate the migration and infiltration of immune cells and antibodies [225,228]. T cell infiltration into the CNS of PD patients is supported by numerous animal studies, including nonhuman primates, as well as postmortem human studies [229,230,231,232,233,234]. For example, it was shown that ICAM-1 and LFA-1 expression were increased in endothelial and T cells, respectively, and that the administration of ICAM-1 or LFA-1 antibodies reduced immunological and behavioral changes in MPTP-treated mice [231]. Furthermore, contact between CD8 T cells and dopaminergic cells was observed in postmortem PD patients [231,233]. The ICAM-1/LFA-1 axis has also been shown to mediate the Th17-induced death of dopaminergic neurons [35]. Thus, it can be asserted that ICAM-1 interaction with T cells is part of PD pathology. As discussed, increased ICAM-1 expression in PD may be most relevant in endothelial cells and the BBB, and in glial cells [18,203,235,236,237,238]. Regarding circulating sICAM-1 concentrations, although decreased ICAM1 gene expression has been detected in PD patients [239], increased sICAM-1 levels have also been noted in the sera, plasma, and CSF of such patients [231,240,241,242]. Therefore, it remains to be determined how the gene expression of ICAM1 may translate into protein production in PD.
2. Fe—Ferroptosis
Ferroptosis is an iron-dependent form of regulated cell death that is unique relative to other mechanisms of cell death [243,244]. While the term and concept of ferroptosis was introduced in 2012, the central role of iron in non-apoptotic cell death emerged several years prior, in 2008 [244,245]. Ferroptosis has since been implicated in an array of conditions, including diseases of the liver, kidney, intestines, lungs, heart, blood cells, and nervous systems, among others [246,247,248]. Iron, as Fe2+, reacts with hydrogen peroxide (H2O2) and generates a hydroxyl radical (•OH), which induces lipid peroxidation, known as the Fenton Reaction (Fe2+ + H2O2 → Fe3+ + •OH + OH−) [244]. This reaction and the hydroxyl radical ultimately lead to the peroxidation of cellular membrane lipids and cell death (ferroptosis) [244,246,249]. Iron can also lead to the generation of alkoxyl radicals via the reaction with lipid hydroperoxides and the activation of arachidonate lipoxygenase (ALOX) enzymes [246,249]. ALOX enzymes oxygenate polyunsaturated fatty acids (PUFAs), leading to the generation of lipid hydroperoxides, and subsequently malondialdehyde (MDA) and 4-hydroxynonenal. Due to the role of PUFAs in ferroptosis, the PUFA-synthesizing enzymes ACSL4 and LPCAT3 have also been implicated in this process [249].
H2O2 can originate from a variety of sources, including the reduction of superoxide (O2−) via superoxide dismutase (SOD) enzymes [250]. The metabolism of DA via monoamine oxidase B also produces H2O2 [251]. The mitochondria are a major source of superoxide, as electrons can escape from the electron transport chain and react with oxygen [250]. NOX enzymes utilize NADPH as an electron donor to generate superoxide and are also a major source of this free radical [249,250]. Following the reduction of superoxide via SOD, the catalase enzyme can catalyze the reduction of two hydrogen peroxide molecules to water and diatomic oxygen [250]. However, as alluded to earlier, in the presence of iron, hydrogen peroxide can participate in the Fenton Reaction and contribute to the synthesis of the hydroxyl radical, which is a highly potent oxidant [244].
Ferroptotic cells have several key features, including abnormal mitochondria. While iron has a central role, the lipid metabolism and glutathione homeostasis are also regulators of ferroptosis. Likewise, glutathione peroxidase 4 (GPX4) is a major regulator of ferroptosis [244]. GPX4 utilizes glutathione (GSH) to reduce peroxidized phospholipids and cholesterol [246]. Due to the importance of glutathione, the cystine–glutamate antiporter xCT, also known as SLC7A11, has a role in mediating ferroptosis. Additionally, SLC7A11 transports cystine into the cytoplasm while transporting glutamate into the extracellular space. In an NADPH-dependent mechanism, cystine is then converted into cysteine, which is a rate-limiting amino acid in the synthesis of glutathione. Notably, erastin, a small-molecule compound, can induce ferroptosis by inhibiting SLC7A11 [252]. GPX4 and SLC11A7 expression is regulated by the transcription factor known as nuclear factor erythroid 2-related factor 2 (Nrf2), which is sequestered and controlled by KEAP1, and binds to the antioxidant response element (ARE) [252,253]. As a result, Nrf2 is an important mediator of ferroptosis and regulates the expression of additional ferroptosis-related genes, such as glutathione synthetase, ferroportin 1 (FPN1), heme oxygenase 1 (HO-1), transferrin receptors (TFRC), and ferritin heavy chain 1 (FTH1) [253]. Conversely, the transcription factor Bach1, also known as the BTB domain and CNC homolog 1, represses the expression of several Nrf2-regulated genes, and thus can induce ferroptosis by reducing the expression of glutathione and iron metabolism-related genes [252,253,254,255]. It has also recently been revealed that a specific form of autophagy, known as ferritinophagy, has a notable role in ferroptosis, as it regulates the degradation of ferritin, an intracellular protein that stores and releases iron in a controlled fashion. Hence, ferritinophagy may be considered as a new player in maintaining iron homeostasis [250,253].
2.1. Ferroptosis—PD
The role of ferroptosis in the pathophysiology of PD has been extensively discussed and is supported by a wide range of data [85]. Iron can accumulate in the SN of PD patients, leading to the death of dopaminergic neurons [85,256]. PD has also been associated with lipid peroxidation, aberrant iron metabolism, decreased GSH, and ROS production, all of which are reflected in differences in gene expression in the SN of PD patients [85,251,257,258,259]. Specifically, differences in the expression of ferroptosis-related genes have been observed in dopaminergic and non-dopaminergic neurons, microglia, astrocytes, OLs, NG2 cells, and endothelial cells/pericytes of PD patients [259].
Interestingly, α-synuclein also has a role in the iron metabolism and PUFA synthesis, as it induces lipid peroxidation and increases the risk of ferroptosis in dopaminergic neurons [85,246,260,261,262]. Iron may also enhance the oxidation of DA, an unstable neurotransmitter, leading to the formation of 6-hydroxydopamine (6-OHDA) and DA quinone (DAQ) [263,264]. DAQ, in turn, may enhance neuron susceptibility to ferroptosis by facilitating the degradation of GPX4 [264,265]. It has also been suggested that Fe3+ may be reduced by lipid hydroperoxides, creating an iron–DA complex that produces 6-OHDA and hydroxyl radicals [263,266]. 6-OHDA has been found to increase the concentrations of free iron via releasing it from ferritin, and ultimately may create a vicious cycle of free radical production. This toxic consequence is further enhanced by the metabolism of 6-OHDA leading to H2O2 generation [263].
Ferroptosis may also have a role in BBB disruption and dysfunction [85,267]. Specifically, increased iron, lipid peroxidation, and decreased antioxidant concentrations have been found in the BBB of PD patients [267]. BBB impairment, which may also involve α-synuclein, has been observed in PD patients [268,269,270,271] and involves disrupted tight junction proteins and adhesion molecules, contributing to the pathophysiology of the disease [267,270].
2.2. Glial Cells—Ferroptosis
Glial cells have complex and multifaceted interactions with the iron metabolism and ferroptosis. They can be a direct source of iron in the CNS, as they contain ferritin, the concentration of which is increased during aging and pathological conditions [263]. Glial cells can also indirectly facilitate the influx of iron and inhibit its efflux across the BBB via secreting ceruloplasmin and hepcidin, respectively [263,272]. Hepcidin, a peptide hormone produced in the liver, plays a crucial role in iron homeostasis [85]. Glial cells may also facilitate iron accumulation in the CNS via the cytokine-induced regulation of iron transporters [273].
Since glial cells can regulate iron homeostasis in the CNS and are involved in the induction of ferroptosis, they may have critical roles in neurodegenerative processes [274]. Specifically, activated astrocytes may induce neuronal ferroptosis via secreting the CXCL3R ligand CXCL10 and decreasing the expression of SLC7A11 [275]. On the other hand, in a BDNF- and Nrf2-dependent mechanism, astrocytes may protect dopaminergic neurons from ferroptosis [276,277]. A similar scenario exists for microglia, in which lipopolysaccharide (LPS)-activated microglia may protect neurons against glutamate-induced ferroptosis [278]. The complexity of these interactions is further underscored by the findings that glial cells themselves can undergo ferroptosis, and hence contribute to neurodegeneration [279]. While NG2 cells are particularly prone to ferroptosis, OLs, with their greatest concentrations of iron in the CNS, may protect themselves against ferroptosis by secreting ferritin heavy chain [246,277,280,281].
Additionally, ferroptosis may activate glial cells by releasing damage-associated molecular patterns (DAMPs) [253,282,283,284]. DAMPs are molecules that are released from damaged or dying cells and are considered a component of the innate immune response [253,282]. Glial cells also express PRRs, such as TLRs, that can recognize and activate DAMPs, and hence contribute to neurodegeneration [283,284]. It is, however, important to note that the DAMP-mediated activation of microglia may have neuroprotective effects in some instances [284]. This is consistent with the concept that the acute activation of microglia can have neuroprotective properties, whereas chronic activation can lead to neurodegeneration [200,201].
2.3. T Cells—Ferroptosis
In tumor cells, T cells can induce ferroptosis via IFN-γ-mediated SLC7A11 inhibition and ACSL4 activation [285,286,287,288]. This has emerged as an important innate antitumor immune response [285,289]. T cells have also the potential to contribute to neuronal ferroptosis by increasing the expression of transferrin receptor 1 (TfR1) in neurons [290]. Moreover, the interaction between T cells and ferroptosis is reciprocal, as neuronal ferroptosis can activate T cells [291,292], and T cells themselves can undergo ferroptosis [289]. However, it appears that ferroptosis may be less immunogenic than other forms of cell death [244,246].
2.4. ICAM-1—Ferroptosis
To our knowledge, the interactions between ICAM-1 and ferroptosis in various contexts have not been adequately addressed. Direct bidirectional interactions between ferroptosis and ICAM-1 is suggested by multiple experimental analyses. For example, in an in vivo contusion spinal cord injury model, the ferroptosis inhibitor SRS 16-86 decreased ICAM-1 expression, among other changes in protein and cytokine expression [293]. Similar results were found in a diabetic neuropathy rat model, in which SRS 16-86 reduced ICAM-1 as well as IL-1β and TNF-α [294]. Likewise, ferrostatin-1 (Fer-1), a ferroptosis inhibitor, inhibited oxidized low-density-lipoprotein-induced ICAM-1 expression in endothelial cells [295,296,297]. Moreover, erastin, a ferroptosis inducer, has been found to increase ICAM-1 expression and activate endothelial transmigration [298]. Nonetheless, further verifications of the direct interactions between ICAM-1 and ferroptosis in other models and contexts are required.
Hydrogen peroxide, lipid peroxides, and ROS, which have a central role in ferroptosis, also appear to have a role in ICAM-1 expression [23,26,236,299,300,301,302]. Specifically, hydrogen peroxide has been found to increase ICAM-1 expression in endothelial cells [23,299,300]. It is important, however, to note that some analyses have failed to find the H2O2-induced expression of ICAM-1 in endothelial cells [169,303], likely due to methodological differences [23,169,299,300,303]. Nevertheless, H2O2 appears to increase ICAM-1 expression via the AP-1 and Ets cis-regulatory elements in the ICAM1 gene promoter [23,299]. H2O2 has also been shown to have a role in the post-translational modification of ICAM-1 [304,305,306]. Additionally, plasma with elevated lipid peroxides obtained from women with severe pre-eclampsia increased ICAM-1 expression in human umbilical cord endothelial cells [302].
ICAM-1 was suggested to have a regulatory role in ferroptosis in a recent study in which the administration of recombinant ICAM-1 (rICAM-1) increased intracellular ROS and Fe2+, and decreased GPX4 and SLC7A11 expression in LPS-stimulated macrophages and human umbilical cord endothelial cells, likely mediated by PTGS2. Moreover, the inhibition of PTGS2 inhibited the impact of rICAM-1 on ferroptosis-related parameters, suggesting that PTGS2 has a mechanistic role in this interaction [307]. However, further elucidation of the mechanism of action of ICAM-1 in its various roles is warranted.
2.5. ICAM-1—Glial Cells—T Cells—Ferroptosis—PD
In PD, dopamine oxidation and mitochondrial dysfunction are widely considered to be underlying characteristics of the disease. Dopamine oxidation appears to have a role in the induction of mitochondrial dysfunction, including in sporadic PD cases [308,309]. Mitochondrial dysfunction in both neurons and microglia themselves can induce microglia activation, resulting in the release of inflammatory cytokines, such as TNF-α and IL-1β, ultimately leading to neuroinflammation and neurodegeneration [310,311]. This self-sustaining cascade of events was postulated two decades ago, although the details of the steps were not evident [312]. Now, it is known that inflammatory cytokines released by microglia disrupt the BBB and induce the expression of adhesion molecules, such as ICAM-1, which facilitate the infiltration of leukocytes, including T cells [310]. Once infiltrated, T cell differentiation is stimulated by different glial cells, but particularly microglia via the release of cytokines. Thus, naïve T cells differentiate into Th1 and Th17 cells, whereas their differentiation into regulatory T cells is suppressed [310,313]. In turn, CD8+ T, Th1, and Th17 cells release inflammatory cytokines, which further promote microglia into an inflammatory and neurotoxic phenotype [310]. IL-17 has been found to increase adhesion molecule expression in microglia [310,314]. Thus, this vicious cycle of glia and T cell reciprocal activation is believed to contribute to the self-sustaining activation of neuroinflammation and neurodegeneration in PD [198,230,310].
Although α-synuclein may contribute to the disruption of the BBB [271,315], T cell infiltration appears to precede α-synuclein accumulation in the brain [233]. Interestingly, increased CSF concentrations of ICAM-1 have been associated with increased CSF concentrations of α-synuclein in PD patients [242]. Once α-synuclein begins to accumulate in the SN, the susceptibility to neuronal ferroptosis increases [85,246,260,261,262]. This coincides with the presence of activated glia and T cells and the potentiation of ferroptosis [170,260,261,267,273,310]. Neuronal ferroptosis, in turn, activates T and glial cells, further propagating the inflammatory and degenerative cycle [283,291,292]. In this scenario, ICAM-1 abundance in the SN may facilitate T cell-induced dopaminergic neuronal death and further facilitate interactions amongst glia and T cells [18,35,166,167,168,203].
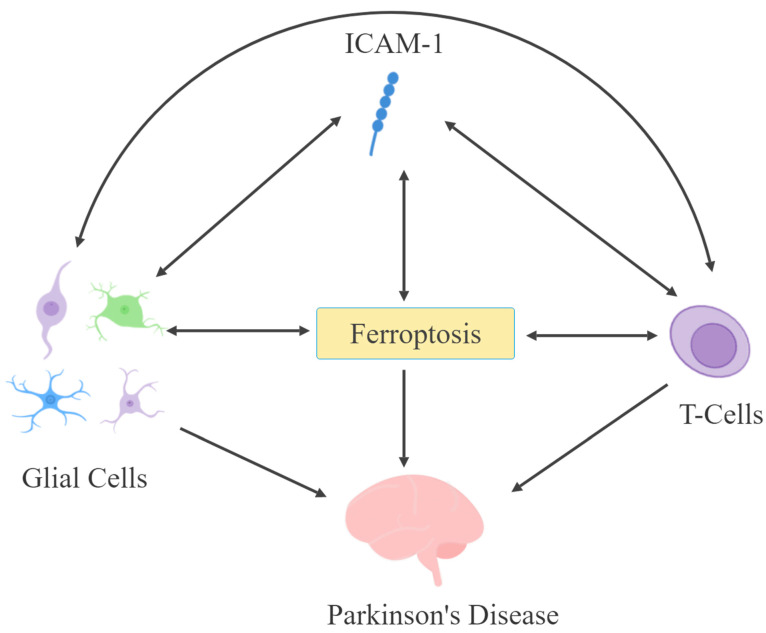
ICAM-1 expression in astrocytes may be enhanced by α-synuclein [207]. Moreover, ICAM-1-expressing astrocytes are present in the SN and may promote their own expression of ICAM-1 in an ROS- and NF-kB-dependent mechanism [18,183,184,185,186]. However, by far, inflammatory conditions, via the release of cytokines, enhance ICAM-1 expression and can lead to ferroptosis [1,22,23,177,316]. Hence, a vicious cycle may be generated in which ferroptosis would lead to an increase in ICAM-1 expression in endothelial cells, causing the disruption of the BBB and the facilitation of T cell infiltration, leading to further cytokine release, neuroinflammation, and neurodegeneration [85,267,293,298,310]. These direct and indirect interactions between ICAM-1, glia, T cells, and ferroptosis, while elucidating potential mechanisms leading to PD pathophysiology (Figure 2), may also offer novel interventions, as discussed below.
Figure 2.
ICAM-1, glial cells, T cells, and ferroptosis may have bidirectional interactions to influence the pathophysiology of Parkinson’s disease.
The associations of increased iron accumulation, lipid peroxidation, ROS, ICAM-1, and decreased GSH in the SN during PD further supports that a potential direct ICAM-1–ferroptosis axis exists in this disease [18,85,203,251,257,258,310]. Although circumstantial, various forms of exercise, which was recently advocated as a potential mediator of ferroptosis, has been associated with the decreases in lipid peroxidation, H2O2, iron accumulation, and sICAM-1 concentrations in patients with PD [317,318,319,320].
3. Novel Interventions
The critical need for novel treatment options for PD is well recognized [11,88]. In this regard, ICAM-1 and the ICAM-1–ferroptosis axis could be promising novel targets in PD. The internalization of ICAM-1 in endothelial cells following ICAM-1 antibody binding, and the subsequent recycling of ICAM-1 back into the plasma membrane, has been documented [321]. ICAM-1 antibodies have anti-inflammatory potential by inhibiting leukocyte interactions [321], and have been shown to mitigate PD pathology and symptoms in vivo [231,322]. For example, ICAM-1 antibodies were found to reduce dopaminergic cell death, glial cell activation, gut dysbosis, and behavioral changes in MPTP-treated mice [322]. Likewise, in a previously discussed analysis, LFA-1 and ICAM-1 antibodies were found to decrease immunological and behavioral changes in MPTP-treated mice [231]. Moreover, inhibiting ICAM-1 or LFA-1 has also been found to decrease Treg concentrations in the SN of MPTP-treated mice [323]. Furthermore, catalase-bound ICAM-1 antibodies have been found to inhibit H2O2 toxicity in endothelial cells during multiple analyses [321,324,325]. The ability for antioxidant enzyme-bound ICAM-1 antibodies to mitigate various neurological conditions, including glial activation, in experimentally induced traumatic brain injury has also been demonstrated in vivo [326,327]. Thus, a number of preclinical studies confirm the utility of ICAM-1 antibodies in mitigating the toxic or neurodegenerative processes.
Moreover, the F(ab’)2 fragment from a murine ICAM-1 antibody was shown to inhibit EAE, and, unlike the murine IgG2a ICAM-1 monoclonal antibody, the F(ab’)2 fragment did not result in the activation of human neutrophils in vitro [188,328]. Although extracellular adherence protein (Eap) of staphylococcus aureus interacts with multiple ligands, it binds to ICAM-1, inhibits ICAM-1/LFA-1 interactions, and has been shown to inhibit EAE [329]. The modulation of NG2 protein expression may also represent a viable target for regulating ICAM-1 expression [194]. ICAM-1 is also highly expressed by various cancer cells, and ICAM-1 antibodies conjugated with anticancer drugs have recently been evaluated in vivo as novel approaches to cancer treatment [330,331]. Although these novel approaches in targeting ICAM-1 have yet to be considered within the context of PD, with the execption of ICAM-1 antibodies, the available data suggest potential exploitations of such targets.
The role of L-dopa in oxidative stress has been debated [332,333,334,335]. Under physiologically relevant conditions, it appears to have antioxidant activity [333,335]. However, elevated concentrations of plasma sICAM-1 were found in stage 1 and 2 in idiopathic PD patients receiving L-dopa, suggesting the particular relevance of ICAM-1 during the early stages of L-dopa treatment [240]. L-dopa-induced dyskinesia has been found to occur concomitantly with an increase in inflammatory cytokines and ROS, and is enhanced in the presence of systemic inflammation in vivo [336,337]. Therefore, the combination of dopamine-enhancing treatments with anti-ICAM-1 treatments would not only address multiple key pathophysiological mechanisms in PD but may also have a synergistic effect with current approaches via mitigating side effects. Together, innovative methods of targeting ICAM-1 and/or the ICAM-1–ferroptosis axis may be a promising option for the treatment and/or mitigation of PD.
4. Conclusions
Recent discoveries indicate a central role for ICAM-1 in PD pathology manifested via its activation of glial cells, as well as the activation and migration of the T cells. Since both glial and T cells are directly linked to ferroptosis, this suggests an indirect connection between ICAM-1 and ferroptosis. ICAM-1 may also have a direct interaction with ferroptosis, which is likely to occur within the context of PD. Although further confirmation of the latter link is needed, collectively, the present knowledge advocates ICAM-1 as a promising target in PD.
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AD | Alzheimer’s disease |
| ADAM10 | a disintegrin and metalloproteinase 10 |
| ADAM17 | a disintegrin and metalloproteinase 17 |
| ALOX | arachidonate lipoxygenase |
| ARE | antioxidant response element |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CASR | calcium-sensing receptor |
| CCL2 | chemokine ligand 2 |
| CD43 | cluster of differentiation 43 |
| CNS | central nervous system |
| CRP | c-reactive protein |
| CSF | cerebral spinal fluid |
| DA | dopamine |
| DAergic | dopaminergic |
| DAMPs | damage-associated molecular patterns |
| DAQ | dopamine quinone |
| EAE | experimental autoimmune encephalomyelitis |
| eNOS | endothelial nitric oxide synthase |
| Fer-1 | ferrostatin-1 |
| FPN1 | ferroportin 1 |
| FTH1 | ferritin heavy chain 1 |
| GDNF | glial-derived neurotrophic factor |
| GFAP | glial fibrillary astrocytic protein |
| GPX4 | glutathione peroxidase 4 |
| GSH | glutathione |
| H2O2 | hydrogen peroxide |
| HO-1 | heme oxygenase 1 |
| ICAM1 | intercellular adhesion molecule 1 |
| ICAM-1 | intercellular adhesion molecule 1 |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IFN-γ | interferon gamma |
| LFA-1 | lymphocyte function-associated antigen 1 |
| LPS | lipopolysaccharide |
| LRP1 | low-density lipoprotein receptor-related protein 1 |
| MAC-1 | macrophage antigen 1 |
| MDA | malondialdehyde |
| MMP-2 | matrix metalloproteinase 2 |
| MMP-9 | matrix metalloproteinase 9 |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MUC1 | mucin 1 |
| nAChRs | nicotinic cholinergic receptors |
| Nf-kB | nuclear factor kappa B |
| NG2 | nerve/glial antigen 2 (NG2) |
| NO | nitric oxide |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OLs | oligodendrocytes |
| OPCs | oligodendrocyte precursor cells |
| OS | oxidative stress |
| PD | Parkinson’s disease |
| PRRs | pattern-recognition receptors |
| PUFAs | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| sICAM-1 | soluble intercellular adhesion molecule 1 |
| SN | substantia nigra |
| SNpc | substantia nigra pars compacta |
| SOD | superoxide dismutase |
| TfR1 | transferrin receptor 1 |
| TFRCs | transferrin receptors |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| TLRs | Toll-like receptors |
| TNF-α | tumor-necrosis factor alpha |
| TREM2 | triggering receptor expressed on myeloid cells-2 |
| VCAM-1 | vascular cell adhesion molecule 1 |
Data Availability Statement
No new data was created. All citations are availale online.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was partially funded by NIH/NIGMS (2 SO6 GM08016-39) (YT).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dustin M.L., Rothlein R., Bhan A.K., Dinarello C.A., Springer T.A. Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J. Immunol. 1986;137:245–254. doi: 10.4049/jimmunol.137.1.245. [DOI] [PubMed] [Google Scholar]
- 2.Rothlein R., Dustin M.L., Marlin S.D., Springer T.A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 1986;137:1270–1274. doi: 10.4049/jimmunol.137.4.1270. [DOI] [PubMed] [Google Scholar]
- 3.Dunne J.L., Collins R.G., Beaudet A.L., Ballantyne C.M., Ley K. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J. Immunol. 2003;171:6105–6111. doi: 10.4049/jimmunol.171.11.6105. [DOI] [PubMed] [Google Scholar]
- 4.Lawson C., Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009;61:22–32. doi: 10.1016/S1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 5.Long E.O. ICAM-1: Getting a grip on leukocyte adhesion. J. Immunol. 2011;186:5021–5023. doi: 10.4049/jimmunol.1100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyun Y.M., Choe Y.H., Park S.A., Kim M. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) distinctly regulate neutrophil extravasation through hotspots I and II. Exp. Mol. Med. 2019;51:1–13. doi: 10.1038/s12276-019-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bui T.M., Wiesolek H.L., Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020;108:787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V., Kaur R., Kumari P., Pasricha C., Singh R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta. 2023;548:117487. doi: 10.1016/j.cca.2023.117487. [DOI] [PubMed] [Google Scholar]
- 9.Guerra-Espinosa C., Jiménez-Fernández M., Sánchez-Madrid F., Serrador J.M. ICAMs in Immunity, Intercellular Adhesion and Communication. Cells. 2024;13:339. doi: 10.3390/cells13040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tizabi Y., Getachew B., Aschner M. Novel Pharmacotherapies in Parkinson’s Disease. Neurotox. Res. 2021;39:1381–1390. doi: 10.1007/s12640-021-00375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bej E., Cesare P., Volpe A.R., d’Angelo M., Castelli V. Oxidative Stress and Neurodegeneration: Insights and Therapeutic Strategies for Parkinson’s Disease. Neurol. Int. 2024;16:502–517. doi: 10.3390/neurolint16030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mochizuki H. Pathological mechanisms and treatment of sporadic Parkinson’s disease: Past, present, and future. J. Neural Transm. 2024;131:597–607. doi: 10.1007/s00702-024-02788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares É.N., Costa A.C.D.S., Ferrolho G.J., Ureshino R.P., Getachew B., Costa S.L., da Silva V.D.A., Tizabi Y. Nicotinic Acetylcholine Receptors in Glial Cells as Molecular Target for Parkinson’s Disease. Cells. 2024;13:474. doi: 10.3390/cells13060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tizabi Y. Duality of Antidepressants and Neuroprotectants. Neurotox. Res. 2016;30:1–13. doi: 10.1007/s12640-015-9577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tizabi Y., Getachew B., Csoka A.B., Manaye K.F., Copeland R.L. Novel targets for parkinsonism-depression comorbidity. Prog. Mol. Biol. Transl. Sci. 2019;167:1–24. doi: 10.1016/bs.pmbts.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P., Jin W., Lyu Z., Lyu X., Li L. Study on the mechanism of gut microbiota in the pathogenetic interaction between depression and Parkinson’s disease. Brain Res. Bull. 2024;215:111001. doi: 10.1016/j.brainresbull.2024.111001. [DOI] [PubMed] [Google Scholar]
- 17.Thomas A.J., Ferrier I.N., Kalaria R.N., Woodward S.A., Ballard C., Oakley A., Perry R.H., O’Brien J.T. Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am. J. Psychiatry. 2000;157:1682–1684. doi: 10.1176/appi.ajp.157.10.1682. [DOI] [PubMed] [Google Scholar]
- 18.Miklossy J., Doudet D.D., Schwab C., Yu S., McGeer E.G., McGeer P.L. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Patarroyo M., Makgoba M.W. Leucocyte adhesion to cells in immune and inflammatory responses. Lancet. 1989;334:1139–1142. doi: 10.1016/S0140-6736(89)91498-0. [DOI] [PubMed] [Google Scholar]
- 20.Koning G.A., Schiffelers R.M., Storm G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium. 2002;9:161–171. doi: 10.1080/10623320213631. [DOI] [PubMed] [Google Scholar]
- 21.Hua S. Targeting sites of inflammation: Intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front. Pharmacol. 2013;4:127. doi: 10.3389/fphar.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldenhoven E., Coffer P., Yuan J., Van de Stolpe A., Horn F., Kruijer W., Van der Saag P.T. Stimulation of the human intercellular adhesion molecule-1 promoter by interleukin-6 and interferon-gamma involves binding of distinct factors to a palindromic response element. J. Biol. Chem. 1994;269:21146–21154. doi: 10.1016/S0021-9258(17)31942-7. [DOI] [PubMed] [Google Scholar]
- 23.Roebuck K.A., Rahman A., Lakshminarayanan V., Janakidevi K., Malik A.B. H2O2 and tumor necrosis factor-alpha activate intercellular adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J. Biol. Chem. 1995;270:18966–18974. doi: 10.1074/jbc.270.32.18966. [DOI] [PubMed] [Google Scholar]
- 24.Fan J., Frey R.S., Rahman A., Malik A.B. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J. Biol. Chem. 2002;277:3404–3411. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- 25.Frey R.S., Ushio-Fukai M., Malik A.B. NADPH oxidase-dependent signaling in endothelial cells: Role in physiology and pathophysiology. Antioxid. Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman A., Fazal F. Hug tightly and say goodbye: Role of endothelial ICAM-1 in leukocyte transmigration. Antioxid. Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habas K., Shang L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell. 2018;54:139–143. doi: 10.1016/j.tice.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Diamond M.S., Staunton D.E., de Fougerolles A.R., Stacker S.A., Garcia-Aguilar J., Hibbs M.L., Springer T.A. ICAM-1 (CD54): A counter-receptor for Mac-1 (CD11b/CD18) J. Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staunton D.E., Dustin M.L., Erickson H.P., Springer T.A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–254. doi: 10.1016/0092-8674(90)90805-O. [DOI] [PubMed] [Google Scholar]
- 30.Haydinger C.D., Ashander L.M., Tan A.C.R., Smith J.R. Intercellular Adhesion Molecule 1: More than a Leukocyte Adhesion Molecule. Biology. 2023;12:743. doi: 10.3390/biology12050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinelli R., Gegg M., Longbottom R., Adamson P., Turowski P., Greenwood J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol. Biol. Cell. 2009;20:995–1005. doi: 10.1091/mbc.e08-06-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G., Place A.T., Chen Z., Brovkovych V.M., Vogel S.M., Muller W.A., Skidgel R.A., Malik A.B., Minshall R.D. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood. 2012;120:1942–1952. doi: 10.1182/blood-2011-12-397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weetman A.P., Cohen S., Makgoba M.W., Borysiewicz L.K. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J. Endocrinol. 1989;122:185–191. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]
- 34.Birdsall H.H. Induction of ICAM-1 on human neural cells and mechanisms of neutrophil-mediated injury. Am. J. Pathol. 1991;139:1341–1350. [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z., Huang Y., Cao B.B., Qiu Y.H., Peng Y.P. Th17 Cells Induce Dopaminergic Neuronal Death via LFA-1/ICAM-1 Interaction in a Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2017;54:7762–7776. doi: 10.1007/s12035-016-0249-9. [DOI] [PubMed] [Google Scholar]
- 36.Staunton D.E., Merluzzi V.J., Rothlein R., Barton R., Marlin S.D., Springer T.A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 37.Berendt A.R., Simmons D.L., Tansey J., Newbold C.I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstein Y., Park J.K., Hahn W.C., Rosen F.S., Bierer B.E., Burakoff S.J. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991;354:233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- 39.McCourt P.A., Ek B., Forsberg N., Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J. Biol. Chem. 1994;269:30081–30084. doi: 10.1016/S0021-9258(18)43775-1. [DOI] [PubMed] [Google Scholar]
- 40.Languino L.R., Plescia J., Duperray A., Brian A.A., Plow E.F., Geltosky J.E., Altieri D.C. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-Y. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi T., Takahashi T., Motoya S., Ishida T., Itoh F., Adachi M., Hinoda Y., Imai K. MUC1 mucin core protein binds to the domain 1 of ICAM-1. Digestion. 2001;63:87–92. doi: 10.1159/000051917. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein J.S., Chen T., Gubina E., Pastor R.W., Kozlowski S. ICAM-1 enhances MHC-peptide activation of CD8(+) T cells without an organized immunological synapse. Eur. J. Immunol. 2000;30:3266–3270. doi: 10.1002/1521-4141(200011)30:11<3266::AID-IMMU3266>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Zumwalde N.A., Domae E., Mescher M.F., Shimizu Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J. Immunol. 2013;191:3681–3693. doi: 10.4049/jimmunol.1201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu W., Yao L., Li L., Zhang J., Place A.T., Minshall R.D., Liu G. ICAM-1 regulates macrophage polarization by suppressing MCP-1 expression via miR-124 upregulation. Oncotarget. 2017;8:111882–111901. doi: 10.18632/oncotarget.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastore L., Tessitore A., Martinotti S., Toniato E., Alesse E., Bravi M.C., Ferri C., Desideri G., Gulino A., Santucci A. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.CIR.100.15.1646. [DOI] [PubMed] [Google Scholar]
- 46.Marzolla V., Armani A., Mammi C., Moss M.E., Pagliarini V., Pontecorvo L., Antelmi A., Fabbri A., Rosano G., Jaffe I.Z., et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int. J. Cardiol. 2017;232:233–242. doi: 10.1016/j.ijcard.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang P.P., Bai J., Zhang Y.L., Yang X.L., Xia Y.L., Lin Q.Y., Li H.H. Blockade of intercellular adhesion molecule-1 prevents angiotensin II-induced hypertension and vascular dysfunction. Lab. Investig. 2020;100:378–386. doi: 10.1038/s41374-019-0320-z. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T., Fan J. Atherosclerosis and inflammation mononuclear cell recruitment and adhesion molecules with reference to the implication of ICAM-1/LFA-1 pathway in atherogenesis. Int. J. Cardiol. 1998;66:S45–S53. doi: 10.1016/S0167-5273(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 49.Greenwood J., Etienne-Manneville S., Adamson P., Couraud P.O. Lymphocyte migration into the central nervous system: Implication of ICAM-1 signalling at the blood-brain barrier. Vasc. Pharmacol. 2002;38:315–322. doi: 10.1016/S1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich J.B. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J. Neuroimmunol. 2002;128:58–68. doi: 10.1016/S0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 51.Turowski P., Adamson P., Greenwood J. Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: Potential for treating neuroinflammation. Cell. Mol. Neurobiol. 2005;25:153–170. doi: 10.1007/s10571-004-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumagin R., Robin A.Z., Nusrat A., Parkos C.A. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunol. 2014;7:905–915. doi: 10.1038/mi.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller N. The Role of Intercellular Adhesion Molecule-1 in the Pathogenesis of Psychiatric Disorders. Front. Pharmacol. 2019;10:1251. doi: 10.3389/fphar.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalal P.J., Sumagin R. Emerging Functions of ICAM-1 in Macrophage Efferocytosis and Wound Healing. J. Cell. Immunol. 2020;2:250–253. doi: 10.33696/immunology.2.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sumagin R., Brazil J.C., Nava P., Nishio H., Alam A., Luissint A.C., Weber D.A., Neish A.S., Nusrat A., Parkos C.A. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016;9:1151–1162. doi: 10.1038/mi.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpén O., Pallai P., Staunton D.E., Springer T.A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J. Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothlein R., Mainolfi E.A., Czajkowski M., Marlin S.D. A form of circulating ICAM-1 in human serum. J. Immunol. 1991;147:3788–3793. doi: 10.4049/jimmunol.147.11.3788. [DOI] [PubMed] [Google Scholar]
- 58.Witkowska A.M., Borawska M.H. Soluble intercellular adhesion molecule-1 (sICAM-1): An overview. Eur. Cytokine Netw. 2004;15:91–98. [PubMed] [Google Scholar]
- 59.Fiore E., Fusco C., Romero P., Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–5223. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- 60.Tsakadze N.L., Sithu S.D., Sen U., English W.R., Murphy G., D’Souza S.E. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1) J. Biol. Chem. 2006;281:3157–3164. doi: 10.1074/jbc.M510797200. [DOI] [PubMed] [Google Scholar]
- 61.Manthe R.L., Muro S. ICAM-1-Targeted Nanocarriers Attenuate Endothelial Release of Soluble ICAM-1, an Inflammatory Regulator. Bioeng. Transl. Med. 2017;2:109–119. doi: 10.1002/btm2.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morsing S.K.H., Rademakers T., Brouns S.L.N., Stalborch A.D.V., Donners M., van Buul J.D. ADAM10-Mediated Cleavage of ICAM-1 Is Involved in Neutrophil Transendothelial Migration. Cells. 2021;10:232. doi: 10.3390/cells10020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robledo O., Papaioannou A., Ochietti B., Beauchemin C., Legault D., Cantin A., King P.D., Daniel C., Alakhov V.Y., Potworowski E.F., et al. ICAM-1 isoforms: Specific activity and sensitivity to cleavage by leukocyte elastase and cathepsin G. Eur. J. Immunol. 2003;33:1351–1360. doi: 10.1002/eji.200323195. [DOI] [PubMed] [Google Scholar]
- 64.Ramos T.N., Bullard D.C., Barnum S.R. ICAM-1: Isoforms and phenotypes. J. Immunol. 2014;192:4469–4474. doi: 10.4049/jimmunol.1400135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakatsuki T., Kimura K., Kimura F., Shinomiya N., Ohtsubo M., Ishizawa M., Yamamoto M. A distinct mRNA encoding a soluble form of ICAM-1 molecule expressed in human tissues. Cell Adhes. Commun. 1995;3:283–292. doi: 10.3109/15419069509081014. [DOI] [PubMed] [Google Scholar]
- 66.Leeuwenberg J.F., Smeets E.F., Neefjes J.J., Shaffer M.A., Cinek T., Jeunhomme T.M., Ahern T.J., Buurman W.A. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 67.Greenwald E., Yuki K. A translational consideration of intercellular adhesion molecule-1 biology in the perioperative setting. Transl. Perioper. Pain Med. 2016;1:17–23. [PMC free article] [PubMed] [Google Scholar]
- 68.Gearing A.J., Newman W. Circulating adhesion molecules in disease. Immunol. Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 69.Nadeem R., Molnar J., Madbouly E.M., Nida M., Aggarwal S., Sajid H., Naseem J., Loomba R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013;9:1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dowlatshahi E.A., van der Voort E.A., Arends L.R., Nijsten T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013;169:266–282. doi: 10.1111/bjd.12355. [DOI] [PubMed] [Google Scholar]
- 71.van Agtmaal M.J.M., Houben A.J.H.M., Pouwer F., Stehouwer C.D.A., Schram M.T. Association of Microvascular Dysfunction with Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li R., Qiu Y. Diagnostic value of serum ICAM-1 for endometriosis: A meta-analysis. Medicine. 2018;97:e11760. doi: 10.1097/MD.0000000000011760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nirmalkar K., Murugesan S., Pizano-Zarate M.L., Villalobos-Flores L.E., Garcia-Gonzalez C., Morales-Hernandez R.M., Nunez-Hernandez J.A., Hernandez-Quiroz F., Romero-Figueroa M.D.S., Hernandez-Guerrero C., et al. Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients. 2018;10:2009. doi: 10.3390/nu10122009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao Y., Du J., Li R., Zhao L., Luo N., Zhai J.Y., Long L. Association between ICAM-1 level and diabetic retinopathy: A review and meta-analysis. Postgrad. Med. J. 2019;95:162–168. doi: 10.1136/postgradmedj-2018-136102. [DOI] [PubMed] [Google Scholar]
- 75.Qiu S., Cai X., Liu J., Yang B., Zugel M., Steinacker J.M., Sun Z., Schumann U. Association between circulating cell adhesion molecules and risk of type 2 diabetes: A meta-analysis. Atherosclerosis. 2019;287:147–154. doi: 10.1016/j.atherosclerosis.2019.06.908. [DOI] [PubMed] [Google Scholar]
- 76.Guo Liu R.N., Cheng Q.Y., Zhou H.Y., Li B.Z., Ye D.Q. Elevated Blood and Urinary ICAM-1 is a Biomarker for Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Immunol. Investig. 2020;49:15–31. doi: 10.1080/08820139.2019.1624769. [DOI] [PubMed] [Google Scholar]
- 77.Wu M., Tong X., Wang D., Wang L., Fan H. Soluble intercellular cell adhesion molecule-1 in lung cancer: A meta-analysis. Pathol. Res. Pract. 2020;216:153029. doi: 10.1016/j.prp.2020.153029. [DOI] [PubMed] [Google Scholar]
- 78.Imani M.M., Sadeghi M., Gholamipour M.A., Bruhl A.B., Sadeghi-Bahmani D., Brand S. Evaluation of Blood Intercellular Adhesion Molecule-1 (ICAM-1) Level in Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Medicina. 2022;58:1499. doi: 10.3390/medicina58101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duan Y., Pan X., Luo J., Xiao X., Li J., Bestman P.L., Luo M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022;13:880298. doi: 10.3389/fimmu.2022.880298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahimi M., Faridi L., Nikniaz L., Daneshvar S., Naseri A., Taban-Sadeghi M., Manaflouyan H., Shahabi J., Sarrafzadegan N. Effect of Endothelial Adhesion Molecules on Atrial Fibrillation: A Systematic Review and Meta-analysis. Heart Int. 2022;16:75–84. doi: 10.17925/HI.2022.16.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roca-Rodriguez M.D.M., Ramos-Garcia P., Lopez-Tinoco C., Aguilar-Diosdado M. Significance of cell adhesion molecules profile during pregnancy in gestational diabetes mellitus. A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2023;202:110740. doi: 10.1016/j.diabres.2023.110740. [DOI] [PubMed] [Google Scholar]
- 82.Mangoni A.A., Zinellu A. A systematic review and meta-analysis of circulating adhesion molecules in rheumatoid arthritis. Inflamm. Res. 2024;73:305–327. doi: 10.1007/s00011-023-01837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He J., Ni Z., Li Z. Intercellular adhesion molecule 1 and selectin l play crucial roles in ulcerative colitis. Medicine. 2023;102:e36552. doi: 10.1097/MD.0000000000036552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anandan C., Jankovic J. Use of botulinum toxin in the management of dystonia in Parkinson’s disease. Front. Neurosci. 2024;18:1371601. doi: 10.3389/fnins.2024.1371601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carvalho F.V., Landis H.E., Getachew B., Silva V.D.A., Ribeiro P.R., Aschner M., Tizabi Y. Iron toxicity, ferroptosis and microbiota in Parkinson’s disease: Implications for novel targets. Adv. Neurotoxicol. 2024;11:105–132. doi: 10.1016/bs.ant.2024.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Regensburger M., Ip C.W., Kohl Z., Schrader C., Urban P.P., Kassubek J., Jost W.H. Clinical benefit of MAO-B and COMT inhibition in Parkinson’s disease: Practical considerations. J. Neural Transm. 2023;130:847–861. doi: 10.1007/s00702-023-02623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan J.H., Ge Y.L., Wang P.Z., Li W., Jin H., Zhang J.R., Chen J., Wang F., Li D., Mao C.J., et al. Associations between variants in levodopa metabolic pathway genes and levodopa-induced dyskinesia in Parkinson’s disease. Neurosci. Lett. 2023;801:137140. doi: 10.1016/j.neulet.2023.137140. [DOI] [PubMed] [Google Scholar]
- 88.Tizabi Y., Getachew B., Copeland R.L., Moratalla R., Patricio F., Limón I.D., Del Bel E., Aschner M. Handbook of Neurotoxicity. Springer; Cham, Switzerland: 2021. Novel Pharmacotherapies for L-DOPA-Induced Dyskinesia; pp. 1–19. [Google Scholar]
- 89.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 90.Zilberter Y., Tabuena D.R., Zilberter M. NOX-induced oxidative stress is a primary trigger of major neurodegenerative disorders. Prog. Neurobiol. 2023;231:102539. doi: 10.1016/j.pneurobio.2023.102539. [DOI] [PubMed] [Google Scholar]
- 91.Peggion C., Cali T., Brini M. Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First? Antioxidants. 2024;13:240. doi: 10.3390/antiox13020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qin P., Sun Y., Li L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review) Int. J. Mol. Med. 2024;53:47. doi: 10.3892/ijmm.2024.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Souza D.G., Almeida R.F., Souza D.O., Zimmer E.R. The astrocyte biochemistry. Semin. Cell Dev. Biol. 2019;95:142–150. doi: 10.1016/j.semcdb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Bonvento G., Bolanos J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021;33:1546–1564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Kim J., He M.J., Widmann A.K., Lee F.S. The role of neurotrophic factors in novel, rapid psychiatric treatments. Neuropsychopharmacology. 2024;49:227–245. doi: 10.1038/s41386-023-01717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanchez-Petidier M., Guerri C., Moreno-Manzano V. Toll-like receptors 2 and 4 differentially regulate the self-renewal and differentiation of spinal cord neural precursor cells. Stem Cell Res. Ther. 2022;13:117. doi: 10.1186/s13287-022-02798-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wies Mancini V.S.B., Mattera V.S., Pasquini J.M., Pasquini L.A., Correale J.D. Microglia-derived extracellular vesicles in homeostasis and demyelination/remyelination processes. J. Neurochem. 2024;168:3–25. doi: 10.1111/jnc.16011. [DOI] [PubMed] [Google Scholar]
- 98.Ebling F.J.P., Lewis J.E. Tanycytes and hypothalamic control of energy metabolism. Glia. 2018;66:1176–1184. doi: 10.1002/glia.23303. [DOI] [PubMed] [Google Scholar]
- 99.Chamberlain K.A., Huang N., Xie Y., LiCausi F., Li S., Li Y., Sheng Z.H. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron. 2021;109:3456–3472. doi: 10.1016/j.neuron.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manu D.R., Slevin M., Barcutean L., Forro T., Boghitoiu T., Balasa R. Astrocyte Involvement in Blood-Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023;24:17146. doi: 10.3390/ijms242417146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandes V.M., Auld V., Klambt C. Glia as Functional Barriers and Signaling Intermediaries. Cold Spring Harb. Perspect. Biol. 2024;16:a041423. doi: 10.1101/cshperspect.a041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lalo U., Koh W., Lee C.J., Pankratov Y. The tripartite glutamatergic synapse. Neuropharmacology. 2021;199:108758. doi: 10.1016/j.neuropharm.2021.108758. [DOI] [PubMed] [Google Scholar]
- 103.Rasia-Filho A.A., Calcagnotto M.E., von Bohlen Und Halbach O. Glial Cell Modulation of Dendritic Spine Structure and Synaptic Function. Adv. Neurobiol. 2023;34:255–310. doi: 10.1007/978-3-031-36159-3_6. [DOI] [PubMed] [Google Scholar]
- 104.Reed M.M., Blazer-Yost B. Channels and Transporters in Astrocyte Volume Regulation in Health and Disease. Cell. Physiol. Biochem. 2022;56:12–30. doi: 10.33594/000000495. [DOI] [PubMed] [Google Scholar]
- 105.Allen N.J., Eroglu C. Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017;96:697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Novikov N.I., Brazhnik E.S., Kitchigina V.F. Pathological Correlates of Cognitive Decline in Parkinson’s Disease: From Molecules to Neural Networks. Biochemistry. 2023;88:1890–1904. doi: 10.1134/S0006297923110172. [DOI] [PubMed] [Google Scholar]
- 107.Clayton R.W., Lovell-Badge R., Galichet C. The Properties and Functions of Glial Cell Types of the Hypothalamic Median Eminence. Front. Endocrinol. 2022;13:953995. doi: 10.3389/fendo.2022.953995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dringen R., Brandmann M., Hohnholt M.C., Blumrich E.M. Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res. 2015;40:2570–2582. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- 109.Ioannou M.S., Jackson J., Sheu S.H., Chang C.L., Weigel A.V., Liu H., Pasolli H.A., Xu C.S., Pang S., Matthies D., et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell. 2019;177:1522–1535. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Kofler J., Wiley C.A. Microglia: Key innate immune cells of the brain. Toxicol. Pathol. 2011;39:103–114. doi: 10.1177/0192623310387619. [DOI] [PubMed] [Google Scholar]
- 111.Chen X., Holtzman D.M. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity. 2022;55:2236–2254. doi: 10.1016/j.immuni.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rahman S., Alzarea S. Glial mechanisms underlying major depressive disorder: Potential therapeutic opportunities. Prog. Mol. Biol. Transl. Sci. 2019;167:159–178. doi: 10.1016/bs.pmbts.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Scuderi C., Verkhratsky A., Parpura V., Li B. Neuroglia in Psychiatric Disorders. Adv. Neurobiol. 2021;26:3–19. doi: 10.1007/978-3-030-77375-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanslik K.L., Marino K.M., Ulland T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021;15:718324. doi: 10.3389/fncel.2021.718324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao G. Shared and disease-specific glial gene expression changes in neurodegenerative diseases. Nat. Aging. 2023;3:246–247. doi: 10.1038/s43587-023-00378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu H., Guan A., Liu J., Peng L., Zhang Z., Wang S. Noteworthy perspectives on microglia in neuropsychiatric disorders. J. Neuroinflamm. 2023;20:223. doi: 10.1186/s12974-023-02901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Magni G., Riboldi B., Ceruti S. Human Glial Cells as Innovative Targets for the Therapy of Central Nervous System Pathologies. Cells. 2024;13:606. doi: 10.3390/cells13070606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tizabi Y., Getachew B., Hauser S.R., Tsytsarev V., Manhaes A.C., da Silva V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024;14:558. doi: 10.3390/brainsci14060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jäkel S., Dimou L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017;11:24. doi: 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nebeling F.C., Poll S., Justus L.C., Steffen J., Keppler K., Mittag M., Fuhrmann M. Microglial motility is modulated by neuronal activity and correlates with dendritic spine plasticity in the hippocampus of awake mice. Elife. 2023;12:e83176. doi: 10.7554/eLife.83176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pathak D., Sriram K. Molecular Mechanisms Underlying Neuroinflammation Elicited by Occupational Injuries and Toxicants. Int. J. Mol. Sci. 2023;24:2272. doi: 10.3390/ijms24032272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saitgareeva A.R., Bulygin K.V., Gareev I.F., Beylerli O.A., Akhmadeeva L.R. The role of microglia in the development of neurodegeneration. Neurol. Sci. 2020;41:3609–3615. doi: 10.1007/s10072-020-04468-5. [DOI] [PubMed] [Google Scholar]
- 123.Costa T., Fernandez-Villalba E., Izura V., Lucas-Ochoa A.M., Menezes-Filho N.J., Santana R.C., de Oliveira M.D., Araujo F.M., Estrada C., Silva V., et al. Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J. Neuroimmune Pharmacol. 2021;16:390–402. doi: 10.1007/s11481-020-09925-8. [DOI] [PubMed] [Google Scholar]
- 124.De Marchi F., Munitic I., Vidatic L., Papic E., Racki V., Nimac J., Jurak I., Novotni G., Rogelj B., Vuletic V., et al. Overlapping Neuroimmune Mechanisms and Therapeutic Targets in Neurodegenerative Disorders. Biomedicines. 2023;11:2793. doi: 10.3390/biomedicines11102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao C., Jiang J., Tan Y., Chen S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023;8:359. doi: 10.1038/s41392-023-01588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stein D.J., Vasconcelos M.F., Albrechet-Souza L., Cereser K.M.M., de Almeida R.M.M. Microglial Over-Activation by Social Defeat Stress Contributes to Anxiety- and Depressive-Like Behaviors. Front. Behav. Neurosci. 2017;11:207. doi: 10.3389/fnbeh.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schramm E., Waisman A. Microglia as Central Protagonists in the Chronic Stress Response. Neurol. Neuroimmunol. Neuroinflamm. 2022;9:e200023. doi: 10.1212/NXI.0000000000200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen D., Lou Q., Song X.J., Kang F., Liu A., Zheng C., Li Y., Wang D., Qun S., Zhang Z., et al. Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice. Nat. Commun. 2024;15:449. doi: 10.1038/s41467-024-44704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qin J., Ma Z., Chen X., Shu S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023;14:1103416. doi: 10.3389/fneur.2023.1103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stratoulias V., Venero J.L., Tremblay M.E., Joseph B. Microglial subtypes: Diversity within the microglial community. EMBO J. 2019;38:e101997. doi: 10.15252/embj.2019101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brandebura A.N., Paumier A., Onur T.S., Allen N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2023;24:23–39. doi: 10.1038/s41583-022-00641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fatoba O., Itokazu T., Yamashita T. Microglia as therapeutic target in central nervous system disorders. J. Pharmacol. Sci. 2020;144:102–118. doi: 10.1016/j.jphs.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 133.Heidari A., Yazdanpanah N., Rezaei N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflamm. 2022;19:135. doi: 10.1186/s12974-022-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu J., Li J.X., Wu R. Toll-Like Receptor 4: A Novel Target to Tackle Drug Addiction? Handb. Exp. Pharmacol. 2022;276:275–290. doi: 10.1007/164_2022_586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stoklund Dittlau K., Freude K. Astrocytes: The Stars in Neurodegeneration? Biomolecules. 2024;14:289. doi: 10.3390/biom14030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Verkhratsky A., Nedergaard M. Physiology of Astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kotliarova A., Sidorova Y.A. Glial Cell Line-Derived Neurotrophic Factor Family Ligands, Players at the Interface of Neuroinflammation and Neuroprotection: Focus Onto the Glia. Front. Cell. Neurosci. 2021;15:679034. doi: 10.3389/fncel.2021.679034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Z., Wang K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38:364–374. doi: 10.1016/j.tins.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abdelhak A., Foschi M., Abu-Rumeileh S., Yue J.K., D’Anna L., Huss A., Oeckl P., Ludolph A.C., Kuhle J., Petzold A., et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022;18:158–172. doi: 10.1038/s41582-021-00616-3. [DOI] [PubMed] [Google Scholar]
- 140.Boulton M., Al-Rubaie A. Neuroinflammation and neurodegeneration following traumatic brain injuries. Anat. Sci. Int. 2024 doi: 10.1007/s12565-024-00778-2. [DOI] [PubMed] [Google Scholar]
- 141.Hagbohm C., Ouellette R., Flanagan E.P., Jonsson D.I., Piehl F., Banwell B., Wickstrom R., Iacobaeus E., Granberg T., Ineichen B.V. Clinical and neuroimaging phenotypes of autoimmune glial fibrillary acidic protein astrocytopathy: A systematic review and meta-analysis. Eur. J. Neurol. 2024;31:e16284. doi: 10.1111/ene.16284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shetty D., Brahmbhatt S., Desai A., Bathla G., Mohan S., Gupta V., Soni N., Vibhute P., Agarwal A. Glial Fibrillary Acidic Protein Astrocytopathy: Review of Pathogenesis, Imaging Features, and Radiographic Mimics. AJNR Am. J. Neuroradiol. 2024. Online ahead of print . [DOI] [PMC free article] [PubMed]
- 143.Ramirez-Guerrero S., Guardo-Maya S., Medina-Rincon G.J., Orrego-Gonzalez E.E., Cabezas-Perez R., Gonzalez-Reyes R.E. Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship. Front. Mol. Neurosci. 2022;15:937789. doi: 10.3389/fnmol.2022.937789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heir R., Abbasi Z., Komal P., Altimimi H.F., Franquin M., Moschou D., Chambon J., Stellwagen D. Astrocytes Are the Source of TNF Mediating Homeostatic Synaptic Plasticity. J. Neurosci. 2024;44:e2278222024. doi: 10.1523/JNEUROSCI.2278-22.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giovannoni F., Quintana F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patani R., Hardingham G.E., Liddelow S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023;19:395–409. doi: 10.1038/s41582-023-00822-1. [DOI] [PubMed] [Google Scholar]
- 148.Vainchtein I.D., Molofsky A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020;43:144–154. doi: 10.1016/j.tins.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garland E.F., Hartnell I.J., Boche D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022;16:824888. doi: 10.3389/fnins.2022.824888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pathak D., Sriram K. Neuron-astrocyte omnidirectional signaling in neurological health and disease. Front. Mol. Neurosci. 2023;16:1169320. doi: 10.3389/fnmol.2023.1169320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Michalski J.P., Kothary R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015;9:340. doi: 10.3389/fncel.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou Y., Zhang J. Neuronal activity and remyelination: New insights into the molecular mechanisms and therapeutic advancements. Front. Cell Dev. Biol. 2023;11:1221890. doi: 10.3389/fcell.2023.1221890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bsibsi M., Nomden A., van Noort J.M., Baron W. Toll-like receptors 2 and 3 agonists differentially affect oligodendrocyte survival, differentiation, and myelin membrane formation. J. Neurosci. Res. 2012;90:388–398. doi: 10.1002/jnr.22767. [DOI] [PubMed] [Google Scholar]
- 154.Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 155.Hill R.A., Nishiyama A. NG2 cells (polydendrocytes): Listeners to the neural network with diverse properties. Glia. 2014;62:1195–1210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kirdajova D., Anderova M. NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol. 2020;50:53–60. doi: 10.1016/j.coph.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Xu J.P., Zhao J., Li S. Roles of NG2 glial cells in diseases of the central nervous system. Neurosci. Bull. 2011;27:413–421. doi: 10.1007/s12264-011-1838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dimou L., Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ferrara G., Errede M., Girolamo F., Morando S., Ivaldi F., Panini N., Bendotti C., Perris R., Furlan R., Virgintino D., et al. NG2, a common denominator for neuroinflammation, blood-brain barrier alteration, and oligodendrocyte precursor response in EAE, plays a role in dendritic cell activation. Acta Neuropathol. 2016;132:23–42. doi: 10.1007/s00401-016-1563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang S.Z., Wang Q.Q., Yang Q.Q., Gu H.Y., Yin Y.Q., Li Y.D., Hou J.C., Chen R., Sun Q.Q., Sun Y.F., et al. NG2 glia regulate brain innate immunity via TGF-beta2/TGFBR2 axis. BMC Med. 2019;17:204. doi: 10.1186/s12916-019-1439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu X., Geng P., Zhao X., Wang Q., Liu C., Guo C., Dong W., Jin X. The NG2-glia is a potential target to maintain the integrity of neurovascular unit after acute ischemic stroke. Neurobiol. Dis. 2023;180:106076. doi: 10.1016/j.nbd.2023.106076. [DOI] [PubMed] [Google Scholar]
- 162.Vélez-Fort M., Audinat E., Angulo M.C. Functional alpha 7-containing nicotinic receptors of NG2-expressing cells in the hippocampus. Glia. 2009;57:1104–1114. doi: 10.1002/glia.20834. [DOI] [PubMed] [Google Scholar]
- 163.Timmermann A., Tascio D., Jabs R., Boehlen A., Domingos C., Skubal M., Huang W., Kirchhoff F., Henneberger C., Bilkei-Gorzo A., et al. Dysfunction of NG2 glial cells affects neuronal plasticity and behavior. Glia. 2023;71:1481–1501. doi: 10.1002/glia.24352. [DOI] [PubMed] [Google Scholar]
- 164.Akiyama H., McGeer P.L. Brain microglia constitutively express beta-2 integrins. J. Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-R. [DOI] [PubMed] [Google Scholar]
- 165.Jiang H., Milo R., Swoveland P., Johnson K.P., Panitch H., Dhib-Jalbut S. Interferon beta-1b reduces interferon gamma-induced antigen-presenting capacity of human glial and B cells. J. Neuroimmunol. 1995;61:17–25. doi: 10.1016/0165-5728(95)00072-A. [DOI] [PubMed] [Google Scholar]
- 166.Kyrkanides S., O’Banion M.K., Whiteley P.E., Daeschner J.C., Olschowka J.A. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J. Neuroimmunol. 2001;119:269–277. doi: 10.1016/S0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- 167.Huber J.D., Campos C.R., Mark K.S., Davis T.P. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H732–H740. doi: 10.1152/ajpheart.00747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chu C.H., Chen J.S., Chan Y.L., Lu W.J., Huang Y.T., Mao P.C., Sze C.I., Sun H.S. TIAM2S-positive microglia enhance inflammation and neurotoxicity through soluble ICAM-1-mediated immune priming. FASEB J. 2023;37:e23242. doi: 10.1096/fj.202300462RR. [DOI] [PubMed] [Google Scholar]
- 169.True A.L., Rahman A., Malik A.B. Activation of NF-kappaB induced by H(2)O(2) and TNF-alpha and its effects on ICAM-1 expression in endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L302–L311. doi: 10.1152/ajplung.2000.279.2.L302. [DOI] [PubMed] [Google Scholar]
- 170.Zhang X., Wang R., Chen H., Jin C., Jin Z., Lu J., Xu L., Lu Y., Zhang J., Shi L. Aged microglia promote peripheral T cell infiltration by reprogramming the microenvironment of neurogenic niches. Immun. Ageing. 2022;19:34. doi: 10.1186/s12979-022-00289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Werner A., Kloss C.U., Walter J., Kreutzberg G.W., Raivich G. Intercellular adhesion molecule-1 (ICAM-1) in the mouse facial motor nucleus after axonal injury and during regeneration. J. Neurocytol. 1998;27:219–232. doi: 10.1023/A:1006928830251. [DOI] [PubMed] [Google Scholar]
- 172.Wang G.J., Deng H.Y., Maier C.M., Sun G.H., Yenari M.A. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114:1081–1090. doi: 10.1016/S0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 173.Danton G.H., Dietrich W.D. Inflammatory mechanisms after ischemia and stroke. J. Neuropathol. Exp. Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 174.Woodburn S.C., Bollinger J.L., Wohleb E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021;18:258. doi: 10.1186/s12974-021-02309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Frohman E.M., Frohman T.C., Gupta S., de Fougerolles A., van den Noort S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J. Neurol. Sci. 1991;106:105–111. doi: 10.1016/0022-510X(91)90202-I. [DOI] [PubMed] [Google Scholar]
- 176.Frohman E.M., Frohman T.C., Dustin M.L., Vayuvegula B., Choi B., Gupta A., van den Noort S., Gupta S. The induction of intercellular adhesion molecule 1 (ICAM-1) expression on human fetal astrocytes by interferon-gamma, tumor necrosis factor alpha, lymphotoxin, and interleukin-1: Relevance to intracerebral antigen presentation. J. Neuroimmunol. 1989;23:117–124. doi: 10.1016/0165-5728(89)90030-1. [DOI] [PubMed] [Google Scholar]
- 177.Satoh J., Kastrukoff L.F., Kim S.U. Cytokine-induced expression of intercellular adhesion molecule-1 (ICAM-1) in cultured human oligodendrocytes and astrocytes. J. Neuropathol. Exp. Neurol. 1991;50:215–226. doi: 10.1097/00005072-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 178.Satoh J., Paty D.W., Kim S.U. Differential effects of beta and gamma interferons on expression of major histocompatibility complex antigens and intercellular adhesion molecule-1 in cultured fetal human astrocytes. Neurology. 1995;45:367–373. doi: 10.1212/WNL.45.2.367. [DOI] [PubMed] [Google Scholar]
- 179.Shrikant P., Chung I.Y., Ballestas M.E., Benveniste E.N. Regulation of intercellular adhesion molecule-1 gene expression by tumor necrosis factor-alpha, interleukin-1 beta, and interferon-gamma in astrocytes. J. Neuroimmunol. 1994;51:209–220. doi: 10.1016/0165-5728(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 180.Lyons P.D., Benveniste E.N. Cleavage of membrane-associated ICAM-1 from astrocytes: Involvement of a metalloprotease. Glia. 1998;22:103–112. doi: 10.1002/(SICI)1098-1136(199802)22:2<103::AID-GLIA1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 181.Etienne-Manneville S., Chaverot N., Strosberg A.D., Couraud P.O. ICAM-1-coupled signaling pathways in astrocytes converge to cyclic AMP response element-binding protein phosphorylation and TNF-alpha secretion. J. Immunol. 1999;163:668–674. doi: 10.4049/jimmunol.163.2.668. [DOI] [PubMed] [Google Scholar]
- 182.Lee S.J., Drabik K., Van Wagoner N.J., Lee S., Choi C., Dong Y., Benveniste E.N. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: Involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. J. Immunol. 2000;165:4658–4666. doi: 10.4049/jimmunol.165.8.4658. [DOI] [PubMed] [Google Scholar]
- 183.Clark V.D., Layson A., Charkviani M., Muradashvili N., Lominadze D. Hyperfibrinogenemia-mediated astrocyte activation. Brain Res. 2018;1699:158–165. doi: 10.1016/j.brainres.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sulimai N., Brown J., Lominadze D. Fibrinogen Interaction with Astrocyte ICAM-1 and PrP(C) Results in the Generation of ROS and Neuronal Death. Int. J. Mol. Sci. 2021;22:2391. doi: 10.3390/ijms22052391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Song H.Y., Ryu J., Ju S.M., Park L.J., Lee J.A., Choi S.Y., Park J. Extracellular HIV-1 Tat enhances monocyte adhesion by up-regulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-kappaB activation in astrocytes. Exp. Mol. Med. 2007;39:27–37. doi: 10.1038/emm.2007.4. [DOI] [PubMed] [Google Scholar]
- 186.Song H.Y., Ju S.M., Seo W.Y., Goh A.R., Lee J.K., Bae Y.S., Choi S.Y., Park J. Nox2-based NADPH oxidase mediates HIV-1 Tat-induced up-regulation of VCAM-1/ICAM-1 and subsequent monocyte adhesion in human astrocytes. Free Radic. Biol. Med. 2011;50:576–584. doi: 10.1016/j.freeradbiomed.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 187.González-Alvarado M.N., Aprato J., Baumeister M., Lippert M., Ekici A.B., Kirchner P., Welz T., Hoffmann A., Winkler J., Wegner M., et al. Oligodendrocytes regulate the adhesion molecule ICAM-1 in neuroinflammation. Glia. 2022;70:522–535. doi: 10.1002/glia.24120. [DOI] [PubMed] [Google Scholar]
- 188.Archelos J.J., Jung S., Maurer M., Schmied M., Lassmann H., Tamatani T., Miyasaka M., Toyka K.V., Hartung H.P. Inhibition of experimental autoimmune encephalomyelitis by an antibody to the intercellular adhesion molecule ICAM-1. Ann. Neurol. 1993;34:145–154. doi: 10.1002/ana.410340209. [DOI] [PubMed] [Google Scholar]
- 189.Lee S.T., Park S.P., Park H.J., Wicks J.R., Lee J.I., Suh Y.H., Jung K.C. Attenuation of Experimental Autoimmune Encephalomyelitis in a Common Marmoset Model by Dendritic Cell-Modulating Anti-ICAM-1 Antibody, MD-3. Mol. Neurobiol. 2019;56:5136–5145. doi: 10.1007/s12035-018-1438-5. [DOI] [PubMed] [Google Scholar]
- 190.Moore C.S., Cui Q.L., Warsi N.M., Durafourt B.A., Zorko N., Owen D.R., Antel J.P., Bar-Or A. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J. Immunol. 2015;194:761–772. doi: 10.4049/jimmunol.1401156. [DOI] [PubMed] [Google Scholar]
- 191.Kirby L., Jin J., Cardona J.G., Smith M.D., Martin K.A., Wang J., Strasburger H., Herbst L., Alexis M., Karnell J., et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 2019;10:3887. doi: 10.1038/s41467-019-11638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fang L.P., Bai X. Oligodendrocyte precursor cells: The multitaskers in the brain. Pflug. Arch.-Eur. J. Physiol. 2023;475:1035–1044. doi: 10.1007/s00424-023-02837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Z., Li X., Zhou H., Zhou J. NG2-glia crosstalk with microglia in health and disease. CNS Neurosci. Ther. 2022;28:1663–1674. doi: 10.1111/cns.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schmitt B.M., Laschke M.W., Rossler O.G., Huang W., Scheller A., Menger M.D., Ampofo E. Nerve/glial antigen (NG) 2 is a crucial regulator of intercellular adhesion molecule (ICAM)-1 expression. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:57–66. doi: 10.1016/j.bbamcr.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 195.Fernandez-Castaneda A., Chappell M.S., Rosen D.A., Seki S.M., Beiter R.M., Johanson D.M., Liskey D., Farber E., Onengut-Gumuscu S., Overall C.C., et al. The active contribution of OPCs to neuroinflammation is mediated by LRP1. Acta Neuropathol. 2020;139:365–382. doi: 10.1007/s00401-019-02073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Psenicka M.W., Smith B.C., Tinkey R.A., Williams J.L. Connecting Neuroinflammation and Neurodegeneration in Multiple Sclerosis: Are Oligodendrocyte Precursor Cells a Nexus of Disease? Front. Cell. Neurosci. 2021;15:654284. doi: 10.3389/fncel.2021.654284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Q., Liu Y., Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Arena G., Sharma K., Agyeah G., Kruger R., Grunewald A., Fitzgerald J.C. Neurodegeneration and Neuroinflammation in Parkinson’s Disease: A Self-Sustained Loop. Curr. Neurol. Neurosci. Rep. 2022;22:427–440. doi: 10.1007/s11910-022-01207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Qu Y., Li J., Qin Q., Wang D., Zhao J., An K., Mao Z., Min Z., Xiong Y., Li J., et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Park. Dis. 2023;9:18. doi: 10.1038/s41531-023-00449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Isik S., Yeman Kiyak B., Akbayir R., Seyhali R., Arpaci T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells. 2023;12:1012. doi: 10.3390/cells12071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Joers V., Tansey M.G., Mulas G., Carta A.R. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 2017;155:57–75. doi: 10.1016/j.pneurobio.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu L., Su X., Li S., Zhao F., Mu D., Qu Y. Microglia and Their Promising Role in Ischemic Brain Injuries: An Update. Front. Cell. Neurosci. 2020;14:211. doi: 10.3389/fncel.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Imamura K., Hishikawa N., Sawada M., Nagatsu T., Yoshida M., Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 204.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yun S.P., Kam T.I., Panicker N., Kim S., Oh Y., Park J.S., Kwon S.H., Park Y.J., Karuppagounder S.S., Park H., et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang T., Sun Y., Dettmer U. Astrocytes in Parkinson’s Disease: From Role to Possible Intervention. Cells. 2023;12:2336. doi: 10.3390/cells12192336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Klegeris A., Giasson B.I., Zhang H., Maguire J., Pelech S., McGeer P.L. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20:2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 208.Wang C., Yang T., Liang M., Xie J., Song N. Astrocyte dysfunction in Parkinson’s disease: From the perspectives of transmitted alpha-synuclein and genetic modulation. Transl. Neurodegener. 2021;10:39. doi: 10.1186/s40035-021-00265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rostami J., Mothes T., Kolahdouzan M., Eriksson O., Moslem M., Bergstrom J., Ingelsson M., O’Callaghan P., Healy L.M., Falk A., et al. Crosstalk between astrocytes and microglia results in increased degradation of alpha-synuclein and amyloid-beta aggregates. J. Neuroinflamm. 2021;18:124. doi: 10.1186/s12974-021-02158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dean D.C., 3rd, Sojkova J., Hurley S., Kecskemeti S., Okonkwo O., Bendlin B.B., Theisen F., Johnson S.C., Alexander A.L., Gallagher C.L. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS ONE. 2016;11:e0163774. doi: 10.1371/journal.pone.0163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cai J., Kim J.L., Wang Y., Baumeister T.R., Zhu M., Liu A., Lee S., McKeown M.J. Sex, myelin, and clinical characteristics of Parkinson’s disease. Front. Neurosci. 2023;17:1235524. doi: 10.3389/fnins.2023.1235524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Boshkovski T., Cohen-Adad J., Misic B., Arnulf I., Corvol J.C., Vidailhet M., Lehericy S., Stikov N., Mancini M. The Myelin-Weighted Connectome in Parkinson’s Disease. Mov. Disord. 2022;37:724–733. doi: 10.1002/mds.28891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smajić S., Prada-Medina C.A., Landoulsi Z., Ghelfi J., Delcambre S., Dietrich C., Jarazo J., Henck J., Balachandran S., Pachchek S., et al. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain. 2022;145:964–978. doi: 10.1093/brain/awab446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Reyes J.F., Rey N.L., Bousset L., Melki R., Brundin P., Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62:387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- 215.Azevedo C., Teku G., Pomeshchik Y., Reyes J.F., Chumarina M., Russ K., Savchenko E., Hammarberg A., Lamas N.J., Collin A., et al. Parkinson’s disease and multiple system atrophy patient iPSC-derived oligodendrocytes exhibit alpha-synuclein-induced changes in maturation and immune reactive properties. Proc. Natl. Acad. Sci. USA. 2022;119:e2111405119. doi: 10.1073/pnas.2111405119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reiss Y., Hoch G., Deutsch U., Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: Essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur. J. Immunol. 1998;28:3086–3099. doi: 10.1002/(SICI)1521-4141(199810)28:10<3086::AID-IMMU3086>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 217.Verma N.K., Kelleher D. An Introduction to LFA-1/ICAM-1 Interactions in T-Cell Motility. Methods Mol. Biol. 2019;1930:1–9. doi: 10.1007/978-1-4939-9036-8_1. [DOI] [PubMed] [Google Scholar]
- 218.Teijeira A., Hunter M.C., Russo E., Proulx S.T., Frei T., Debes G.F., Coles M., Melero I., Detmar M., Rouzaut A., et al. T Cell Migration from Inflamed Skin to Draining Lymph Nodes Requires Intralymphatic Crawling Supported by ICAM-1/LFA-1 Interactions. Cell Rep. 2017;18:857–865. doi: 10.1016/j.celrep.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 219.Mekori Y.A., Metcalfe D.D. Mast cell-T cell interactions. J. Allergy Clin. Immunol. 1999;104:517–523. doi: 10.1016/S0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 220.Chirathaworn C., Kohlmeier J.E., Tibbetts S.A., Rumsey L.M., Chan M.A., Benedict S.H. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J. Immunol. 2002;168:5530–5537. doi: 10.4049/jimmunol.168.11.5530. [DOI] [PubMed] [Google Scholar]
- 221.Ma V.P., Hu Y., Kellner A.V., Brockman J.M., Velusamy A., Blanchard A.T., Evavold B.D., Alon R., Salaita K. The magnitude of LFA-1/ICAM-1 forces fine-tune TCR-triggered T cell activation. Sci. Adv. 2022;8:eabg4485. doi: 10.1126/sciadv.abg4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Saha B., Mondal A.C., Majumder J., Basu S., Dasgupta P.S. Physiological concentrations of dopamine inhibit the proliferation and cytotoxicity of human CD4+ and CD8+ T cells in vitro: A receptor-mediated mechanism. Neuroimmunomodulation. 2001;9:23–33. doi: 10.1159/000049004. [DOI] [PubMed] [Google Scholar]
- 223.Bhatia D., Grozdanov V., Ruf W.P., Kassubek J., Ludolph A.C., Weishaupt J.H., Danzer K.M. T-cell dysregulation is associated with disease severity in Parkinson’s Disease. J. Neuroinflamm. 2021;18:250. doi: 10.1186/s12974-021-02296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Contaldi E., Magistrelli L., Comi C. T Lymphocytes in Parkinson’s Disease. J. Park. Dis. 2022;12:S65–S74. doi: 10.3233/JPD-223152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Garretti F., Agalliu D., Lindestam Arlehamn C.S., Sette A., Sulzer D. Autoimmunity in Parkinson’s Disease: The Role of alpha-Synuclein-Specific T Cells. Front. Immunol. 2019;10:303. doi: 10.3389/fimmu.2019.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lindestam Arlehamn C.S., Dhanwani R., Pham J., Kuan R., Frazier A., Rezende Dutra J., Phillips E., Mallal S., Roederer M., Marder K.S., et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 2020;11:1875. doi: 10.1038/s41467-020-15626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dhanwani R., Sette A., Lindestam Arlehamn C.S. Detection of alpha-Synuclein-Specific T Cells in Parkinson’s Disease. Methods Mol. Biol. 2022;2574:123–131. doi: 10.1007/978-1-0716-2712-9_5. [DOI] [PubMed] [Google Scholar]
- 228.Garretti F., Monahan C., Sette A., Agalliu D., Sulzer D. T cells, alpha-synuclein and Parkinson disease. Handb. Clin. Neurol. 2022;184:439–455. doi: 10.1016/B978-0-12-819410-2.00023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Brochard V., Combadiere B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J.M., et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Subbarayan M.S., Hudson C., Moss L.D., Nash K.R., Bickford P.C. T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an alpha-synuclein rat model of Parkinson’s disease. J. Neuroinflamm. 2020;17:242. doi: 10.1186/s12974-020-01911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li W., Chen S., Luo Y., Xia Y., Ma Q., Yao Q., Wu J. Interaction between ICAM1 in endothelial cells and LFA1 in T cells during the pathogenesis of experimental Parkinson’s disease. Exp. Ther. Med. 2020;20:1021–1029. doi: 10.3892/etm.2020.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Seo J., Park J., Kim K., Won J., Yeo H.G., Jin Y.B., Koo B.S., Lim K.S., Jeong K.J., Kang P., et al. Chronic Infiltration of T Lymphocytes into the Brain in a Non-human Primate Model of Parkinson’s Disease. Neuroscience. 2020;431:73–85. doi: 10.1016/j.neuroscience.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 233.Galiano-Landeira J., Torra A., Vila M., Bove J. CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain. 2020;143:3717–3733. doi: 10.1093/brain/awaa269. [DOI] [PubMed] [Google Scholar]
- 234.Williams G.P., Schonhoff A.M., Jurkuvenaite A., Gallups N.J., Standaert D.G., Harms A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain. 2021;144:2047–2059. doi: 10.1093/brain/awab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kurkowska-Jastrzebska I., Wrońska A., Kohutnicka M., Członkowski A., Członkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp. Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- 236.Machado A., Herrera A.J., Venero J.L., Santiago M., De Pablos R.M., Villarán R.F., Espinosa-Oliva A.M., Argüelles S., Sarmiento M., Delgado-Cortés M.J., et al. Peripheral inflammation increases the damage in animal models of nigrostriatal dopaminergic neurodegeneration: Possible implication in Parkinson’s disease incidence. Park. Dis. 2011;2011:393769. doi: 10.4061/2011/393769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fu Q., Song R., Yang Z., Shan Q., Chen W. 6-Hydroxydopamine induces brain vascular endothelial inflammation. IUBMB Life. 2017;69:887–895. doi: 10.1002/iub.1685. [DOI] [PubMed] [Google Scholar]
- 238.Walker D.G., Lue L.F., Tang T.M., Adler C.H., Caviness J.N., Sabbagh M.N., Serrano G.E., Sue L.I., Beach T.G. Changes in CD200 and intercellular adhesion molecule-1 (ICAM-1) levels in brains of Lewy body disorder cases are associated with amounts of Alzheimer’s pathology not α-synuclein pathology. Neurobiol. Aging. 2017;54:175–186. doi: 10.1016/j.neurobiolaging.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tan C., Liu X., Chen J. Microarray Analysis of the Molecular Mechanism Involved in Parkinson’s Disease. Park. Dis. 2018;2018:1590465. doi: 10.1155/2018/1590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Andican G., Konukoglu D., Bozluolcay M., Bayulkem K., Firtiina S., Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol. Belg. 2012;112:155–159. doi: 10.1007/s13760-012-0015-3. [DOI] [PubMed] [Google Scholar]
- 241.Lerche S., Zimmermann M., Wurster I., Roeben B., Fries F.L., Deuschle C., Waniek K., Lachmann I., Gasser T., Jakobi M., et al. CSF and Serum Levels of Inflammatory Markers in PD: Sparse Correlation, Sex Differences and Association With Neurodegenerative Biomarkers. Front. Neurol. 2022;13:834580. doi: 10.3389/fneur.2022.834580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lerche S., Zimmermann M., Roeben B., Wurster I., Fries F.L., Deuschle C., Waniek K., Lachmann I., Jakobi M., Joos T.O., et al. Inflammatory CSF profiles and longitudinal development of cognitive decline in sporadic and GBA-associated PD. NPJ Park. Dis. 2023;9:38. doi: 10.1038/s41531-023-00476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stockwell B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Berndt C., Alborzinia H., Amen V.S., Ayton S., Barayeu U., Bartelt A., Bayir H., Bebber C.M., Birsoy K., Bottcher J.P., et al. Ferroptosis in health and disease. Redox Biol. 2024;75:103211. doi: 10.1016/j.redox.2024.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen Z., Jiang J., Fu N., Chen L. Targetting ferroptosis for blood cell-related diseases. J. Drug Target. 2022;30:244–258. doi: 10.1080/1061186X.2021.1971237. [DOI] [PubMed] [Google Scholar]
- 248.Fortuna V., Lima J., Oliveira G.F., Oliveira Y.S., Getachew B., Nekhai S., Aschner M., Tizabi Y. Ferroptosis as an emerging target in sickle cell disease. Curr. Res. Toxicol. 2024;7:100181. doi: 10.1016/j.crtox.2024.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tang D., Kroemer G. Ferroptosis. Curr. Biol. 2020;30:R1292–R1297. doi: 10.1016/j.cub.2020.09.068. [DOI] [PubMed] [Google Scholar]
- 250.Fujii J., Homma T., Osaki T. Superoxide Radicals in the Execution of Cell Death. Antioxidants. 2022;11:501. doi: 10.3390/antiox11030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhao Y., Li Y., Zhang R., Wang F., Wang T., Jiao Y. The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy. OncoTargets Ther. 2020;13:5429–5441. doi: 10.2147/OTT.S254995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhou Y., Lin W., Rao T., Zheng J., Zhang T., Zhang M., Lin Z. Ferroptosis and Its Potential Role in the Nervous System Diseases. J. Inflamm. Res. 2022;15:1555–1574. doi: 10.2147/JIR.S351799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nishizawa H., Matsumoto M., Shindo T., Saigusa D., Kato H., Suzuki K., Sato M., Ishii Y., Shimokawa H., Igarashi K. Ferroptosis is controlled by the coordinated transcriptional regulation of glutathione and labile iron metabolism by the transcription factor BACH1. J. Biol. Chem. 2020;295:69–82. doi: 10.1074/jbc.RA119.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nishizawa H., Yamanaka M., Igarashi K. Ferroptosis: Regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 2023;290:1688–1704. doi: 10.1111/febs.16382. [DOI] [PubMed] [Google Scholar]
- 256.Xu Y., Huang X., Geng X., Wang F. Meta-analysis of iron metabolism markers levels of Parkinson’s disease patients determined by fluid and MRI measurements. J. Trace Elem. Med. Biol. 2023;78:127190. doi: 10.1016/j.jtemb.2023.127190. [DOI] [PubMed] [Google Scholar]
- 257.Sofic E., Lange K.W., Jellinger K., Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci. Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-B. [DOI] [PubMed] [Google Scholar]
- 258.Pearce R.K., Owen A., Daniel S., Jenner P., Marsden C.D. Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J. Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 259.Liu L., Cui Y., Chang Y.Z., Yu P. Ferroptosis-related factors in the substantia nigra are associated with Parkinson’s disease. Sci. Rep. 2023;13:15365. doi: 10.1038/s41598-023-42574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Angelova P.R., Choi M.L., Berezhnov A.V., Horrocks M.H., Hughes C.D., De S., Rodrigues M., Yapom R., Little D., Dolt K.S., et al. Alpha synuclein aggregation drives ferroptosis: An interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27:2781–2796. doi: 10.1038/s41418-020-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mahoney-Sanchez L., Bouchaoui H., Boussaad I., Jonneaux A., Timmerman K., Berdeaux O., Ayton S., Kruger R., Duce J.A., Devos D., et al. Alpha synuclein determines ferroptosis sensitivity in dopaminergic neurons via modulation of ether-phospholipid membrane composition. Cell Rep. 2022;40:111231. doi: 10.1016/j.celrep.2022.111231. [DOI] [PubMed] [Google Scholar]
- 262.Thapa K., Khan H., Kanojia N., Singh T.G., Kaur A., Kaur G. Therapeutic Insights on Ferroptosis in Parkinson’s disease. Eur. J. Pharmacol. 2022;930:175133. doi: 10.1016/j.ejphar.2022.175133. [DOI] [PubMed] [Google Scholar]
- 263.Hare D.J., Double K.L. Iron and dopamine: A toxic couple. Brain. 2016;139:1026–1035. doi: 10.1093/brain/aww022. [DOI] [PubMed] [Google Scholar]
- 264.Sun J., Lin X.M., Lu D.H., Wang M., Li K., Li S.R., Li Z.Q., Zhu C.J., Zhang Z.M., Yan C.Y., et al. Midbrain dopamine oxidation links ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons. J. Clin. Investig. 2023;133:e165228. doi: 10.1172/JCI165228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hauser D.N., Dukes A.A., Mortimer A.D., Hastings T.G. Dopamine quinone modifies and decreases the abundance of the mitochondrial selenoprotein glutathione peroxidase 4. Free Radic. Biol. Med. 2013;65:419–427. doi: 10.1016/j.freeradbiomed.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pezzella A., d’Ischia M., Napolitano A., Misuraca G., Prota G. Iron-mediated generation of the neurotoxin 6-hydroxydopamine quinone by reaction of fatty acid hydroperoxides with dopamine: A possible contributory mechanism for neuronal degeneration in Parkinson’s disease. J. Med. Chem. 1997;40:2211–2216. doi: 10.1021/jm970099t. [DOI] [PubMed] [Google Scholar]
- 267.Lin K.J., Chen S.D., Lin K.L., Liou C.W., Lan M.Y., Chuang Y.C., Wang P.W., Lee J.J., Wang F.S., Lin H.Y., et al. Iron Brain Menace: The Involvement of Ferroptosis in Parkinson Disease. Cells. 2022;11:3829. doi: 10.3390/cells11233829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kortekaas R., Leenders K.L., van Oostrom J.C., Vaalburg W., Bart J., Willemsen A.T., Hendrikse N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 269.Gray M.T., Woulfe J.M. Striatal blood-brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Al-Bachari S., Naish J.H., Parker G.J.M., Emsley H.C.A., Parkes L.M. Blood-Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020;11:593026. doi: 10.3389/fphys.2020.593026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lan G., Wang P., Chan R.B., Liu Z., Yu Z., Liu X., Yang Y., Zhang J. Astrocytic VEGFA: An essential mediator in blood-brain-barrier disruption in Parkinson’s disease. Glia. 2022;70:337–353. doi: 10.1002/glia.24109. [DOI] [PubMed] [Google Scholar]
- 272.McCarthy R.C., Kosman D.J. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS ONE. 2014;9:e89003. doi: 10.1371/journal.pone.0089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang Z.L., Yuan L., Li W., Li J.Y. Ferroptosis in Parkinson’s disease: Glia-neuron crosstalk. Trends Mol. Med. 2022;28:258–269. doi: 10.1016/j.molmed.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 274.Feng Z., Min L., Chen H., Deng W., Tan M., Liu H., Hou J. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 2021;43:101984. doi: 10.1016/j.redox.2021.101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liang P., Zhang X., Zhang Y., Wu Y., Song Y., Wang X., Chen T., Liu W., Peng B., Yin J., et al. Neurotoxic A1 astrocytes promote neuronal ferroptosis via CXCL10/CXCR3 axis in epilepsy. Free Radic. Biol. Med. 2023;195:329–342. doi: 10.1016/j.freeradbiomed.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 276.Ishii T., Warabi E., Mann G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic. Biol. Med. 2019;133:169–178. doi: 10.1016/j.freeradbiomed.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 277.Li Y., Xiao D., Wang X. The emerging roles of ferroptosis in cells of the central nervous system. Front. Neurosci. 2022;16:1032140. doi: 10.3389/fnins.2022.1032140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jacques M.T., Saso L., Farina M. LPS-Activated Microglial Cell-Derived Conditioned Medium Protects HT22 Neuronal Cells against Glutamate-Induced Ferroptosis. Int. J. Mol. Sci. 2023;24:2910. doi: 10.3390/ijms24032910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ryan S.K., Zelic M., Han Y., Teeple E., Chen L., Sadeghi M., Shankara S., Guo L., Li C., Pontarelli F., et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2023;26:12–26. doi: 10.1038/s41593-022-01221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Friedrich I., Reimann K., Jankuhn S., Kirilina E., Stieler J., Sonntag M., Meijer J., Weiskopf N., Reinert T., Arendt T., et al. Cell specific quantitative iron mapping on brain slices by immuno-microPIXE in healthy elderly and Parkinson’s disease. Acta Neuropathol. Commun. 2021;9:47. doi: 10.1186/s40478-021-01145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Park M.W., Cha H.W., Kim J., Kim J.H., Yang H., Yoon S., Boonpraman N., Yi S.S., Yoo I.D., Moon J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021;41:101947. doi: 10.1016/j.redox.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Demuynck R., Efimova I., Naessens F., Krysko D.V. Immunogenic ferroptosis and where to find it? J. Immunother. Cancer. 2021;9:e003430. doi: 10.1136/jitc-2021-003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chang N.P., DaPrano E.M., Lindman M., Estevez I., Chou T.W., Evans W.R., Nissenbaum M., McCourt M., Alzate D., Atkins C., et al. Neuronal DAMPs exacerbate neurodegeneration via astrocytic RIPK3 signaling. JCI Insight. 2024;9:e177002. doi: 10.1172/jci.insight.177002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kigerl K.A., de Rivero Vaccari J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang W., Green M., Choi J.E., Gijon M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Friedmann Angeli J.P., Xavier da Silva T.N., Schilling B. CD8(+) T cells PUF(A)ing the flames of cancer ferroptotic cell death. Cancer Cell. 2022;40:346–348. doi: 10.1016/j.ccell.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 287.Liao P., Wang W., Wang W., Kryczek I., Li X., Bian Y., Sell A., Wei S., Grove S., Johnson J.K., et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365–378. doi: 10.1016/j.ccell.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhou J., Pathak J.L., Cao T., Chen B., Wei W., Hu S., Mao T., Wu X., Watanabe N., Li X., et al. CD4 T cell-secreted IFN-gamma in Sjogren’s syndrome induces salivary gland epithelial cell ferroptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2024;1870:167121. doi: 10.1016/j.bbadis.2024.167121. [DOI] [PubMed] [Google Scholar]
- 289.Lin Z., Zou S., Wen K. The crosstalk of CD8+ T cells and ferroptosis in cancer. Front. Immunol. 2023;14:1255443. doi: 10.3389/fimmu.2023.1255443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Liang J., Shen Y., Wang Y., Huang Y., Wang J., Zhu Q., Tong G., Yu K., Cao W., Wang Q., et al. Ferroptosis participates in neuron damage in experimental cerebral malaria and is partially induced by activated CD8(+) T cells. Mol. Brain. 2022;15:57. doi: 10.1186/s13041-022-00942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Luoqian J., Yang W., Ding X., Tuo Q.Z., Xiang Z., Zheng Z., Guo Y.J., Li L., Guan P., Ayton S., et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell. Mol. Immunol. 2022;19:913–924. doi: 10.1038/s41423-022-00883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Xue Y., Lu F., Wang W. Ferroptotic cells augment T-cell activation and neuroinflammation. Ageing Neurodegener. Dis. 2022;2:15. doi: 10.20517/and.2022.17. [DOI] [Google Scholar]
- 293.Zhang Y., Sun C., Zhao C., Hao J., Zhang Y., Fan B., Li B., Duan H., Liu C., Kong X., et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res. 2019;1706:48–57. doi: 10.1016/j.brainres.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 294.Qiao Y., Sun C., Kan S., He L., Wang Y., Gao H., Zhang Y., Cheng Y., Wang S., Zhao L., et al. SRS 16-86 promotes diabetic nephropathy recovery by regulating ferroptosis. Exp. Physiol. 2024;109:1199–1210. doi: 10.1113/EP091520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bai T., Li M., Liu Y., Qiao Z., Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 296.Leng Y., Luo X., Yu J., Jia H., Yu B. Ferroptosis: A Potential Target in Cardiovascular Disease. Front. Cell Dev. Biol. 2021;9:813668. doi: 10.3389/fcell.2021.813668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yang Z., He Y., Wu D., Shi W., Liu P., Tan J., Wang R., Yu B. Antiferroptosis therapy alleviated the development of atherosclerosis. MedComm. 2024;5:e520. doi: 10.1002/mco2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lopes-Coelho F., Martins F., Hipolito A., Mendes C., Sequeira C.O., Pires R.F., Almeida A.M., Bonifacio V.D.B., Pereira S.A., Serpa J. The Activation of Endothelial Cells Relies on a Ferroptosis-Like Mechanism: Novel Perspectives in Management of Angiogenesis and Cancer Therapy. Front. Oncol. 2021;11:656229. doi: 10.3389/fonc.2021.656229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lakshminarayanan V., Beno D.W., Costa R.H., Roebuck K.A. Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 and tumor necrosis factor-alpha in endothelial and epithelial cells. J. Biol. Chem. 1997;272:32910–32918. doi: 10.1074/jbc.272.52.32910. [DOI] [PubMed] [Google Scholar]
- 300.Lo S.K., Janakidevi K., Lai L., Malik A.B. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am. J. Physiol. 1993;264:L406–L412. doi: 10.1152/ajplung.1993.264.4.L406. [DOI] [PubMed] [Google Scholar]
- 301.Bhunia A.K., Arai T., Bulkley G., Chatterjee S. Lactosylceramide mediates tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 (ICAM-1) expression and the adhesion of neutrophil in human umbilical vein endothelial cells. J. Biol. Chem. 1998;273:34349–34357. doi: 10.1074/jbc.273.51.34349. [DOI] [PubMed] [Google Scholar]
- 302.Takacs P., Kauma S.W., Sholley M.M., Walsh S.W., Dinsmoor M.J., Green K. Increased circulating lipid peroxides in severe preeclampsia activate NF-kappaB and upregulate ICAM-1 in vascular endothelial cells. FASEB J. 2001;15:279–281. doi: 10.1096/fj.00-0549fje. [DOI] [PubMed] [Google Scholar]
- 303.Zadeh M.S., Kolb J.P., Geromin D., D’Anna R., Boulmerka A., Marconi A., Dugas B., Marsac C., D’Alessio P. Regulation of ICAM-1/CD54 expression on human endothelial cells by hydrogen peroxide involves inducible NO synthase. J. Leukoc. Biol. 2000;67:327–334. doi: 10.1002/jlb.67.3.327. [DOI] [PubMed] [Google Scholar]
- 304.Regal-McDonald K., Xu B., Barnes J.W., Patel R.P. High-mannose intercellular adhesion molecule-1 enhances CD16(+) monocyte adhesion to the endothelium. Am. J. Physiol. Heart Circ. Physiol. 2019;317:H1028–H1038. doi: 10.1152/ajpheart.00306.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.McDonald K.R., Hernandez-Nichols A.L., Barnes J.W., Patel R.P. Hydrogen peroxide regulates endothelial surface N-glycoforms to control inflammatory monocyte rolling and adhesion. Redox Biol. 2020;34:101498. doi: 10.1016/j.redox.2020.101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Scott D.W., Dunn T.S., Ballestas M.E., Litovsky S.H., Patel R.P. Identification of a high-mannose ICAM-1 glycoform: Effects of ICAM-1 hypoglycosylation on monocyte adhesion and outside in signaling. Am. J. Physiol. Cell Physiol. 2013;305:C228–C237. doi: 10.1152/ajpcell.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lv X., Luo C., Wu J., Huang Y., Quan J., Gong Q., Tong Z. Integration of single-cell RNA sequencing of endothelial cells and proteomics to unravel the role of ICAM1-PTGS2 communication in apical periodontitis: A laboratory investigation. Int. Endod. J. 2024;57:1228–1246. doi: 10.1111/iej.14080. [DOI] [PubMed] [Google Scholar]
- 308.Berman S.B., Hastings T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: Implications for Parkinson’s disease. J. Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 309.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Xu Y., Li Y., Wang C., Han T., Liu H., Sun L., Hong J., Hashimoto M., Wei J. The reciprocal interactions between microglia and T cells in Parkinson’s disease: A double-edged sword. J. Neuroinflamm. 2023;20:33. doi: 10.1186/s12974-023-02723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ravenhill S.M., Evans A.H., Crewther S.G. Escalating Bi-Directional Feedback Loops between Proinflammatory Microglia and Mitochondria in Ageing and Post-Diagnosis of Parkinson’s Disease. Antioxidants. 2023;12:1117. doi: 10.3390/antiox12051117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Barcia C., Sanchez Bahillo A., Fernandez-Villalba E., Bautista V., Poza Y.P.M., Fernandez-Barreiro A., Hirsch E.C., Herrero M.T. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia. 2004;46:402–409. doi: 10.1002/glia.20015. [DOI] [PubMed] [Google Scholar]
- 313.Carson M.J., Sutcliffe J.G., Campbell I.L. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J. Neurosci. Res. 1999;55:127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 314.Kawanokuchi J., Shimizu K., Nitta A., Yamada K., Mizuno T., Takeuchi H., Suzumura A. Production and functions of IL-17 in microglia. J. Neuroimmunol. 2008;194:54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 315.Dohgu S., Takata F., Matsumoto J., Kimura I., Yamauchi A., Kataoka Y. Monomeric alpha-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc. Res. 2019;124:61–66. doi: 10.1016/j.mvr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 316.Chen Y., Fang Z.M., Yi X., Wei X., Jiang D.S. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023;14:205. doi: 10.1038/s41419-023-05716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Bloomer R.J., Schilling B.K., Karlage R.E., Ledoux M.S., Pfeiffer R.F., Callegari J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med. Sci. Sports Exerc. 2008;40:1385–1389. doi: 10.1249/MSS.0b013e31816f1550. [DOI] [PubMed] [Google Scholar]
- 318.Segura C., Eraso M., Bonilla J., Mendivil C.O., Santiago G., Useche N., Bernal-Pacheco O., Monsalve G., Sanchez L., Hernandez E., et al. Effect of a High-Intensity Tandem Bicycle Exercise Program on Clinical Severity, Functional Magnetic Resonance Imaging, and Plasma Biomarkers in Parkinson’s Disease. Front. Neurol. 2020;11:656. doi: 10.3389/fneur.2020.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Thirupathi A., Marqueze L.F., Outeiro T.F., Radak Z., Pinho R.A. Physical Exercise-Induced Activation of NRF2 and BDNF as a Promising Strategy for Ferroptosis Regulation in Parkinson’s Disease. Neurochem. Res. 2024;49:1643–1654. doi: 10.1007/s11064-024-04152-6. [DOI] [PubMed] [Google Scholar]
- 320.Ahern J., Boyle M.E., Thompson W.K., Fan C.C., Loughnan R. Dietary and Lifestyle Factors of Brain Iron Accumulation and Parkinson’s Disease Risk. medRxiv. 2024. preprint. [DOI]
- 321.Muro S., Gajewski C., Koval M., Muzykantov V.R. ICAM-1 recycling in endothelial cells: A novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105:650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 322.Zhang F., Pan L., Lian C., Xu Z., Chen H., Lai W., Liang X., Liu Q., Wu H., Wang Y., et al. ICAM-1 may promote the loss of dopaminergic neurons by regulating inflammation in MPTP-induced Parkinson’s disease mouse models. Brain Res. Bull. 2024;214:110989. doi: 10.1016/j.brainresbull.2024.110989. [DOI] [PubMed] [Google Scholar]
- 323.Huang Y., Liu Z., Cao B.B., Qiu Y.H., Peng Y.P. Treg Cells Attenuate Neuroinflammation and Protect Neurons in a Mouse Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2020;15:224–237. doi: 10.1007/s11481-019-09888-5. [DOI] [PubMed] [Google Scholar]
- 324.Muro S., Cui X., Gajewski C., Murciano J.C., Muzykantov V.R., Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am. J. Physiol. Cell Physiol. 2003;285:C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 325.Sari E., Tunc-Sarisozen Y., Mutlu H., Shahbazi R., Ucar G., Ulubayram K. ICAM-1 targeted catalase encapsulated PLGA-b-PEG nanoparticles against vascular oxidative stress. J. Microencapsul. 2015;32:687–698. doi: 10.3109/02652048.2015.1073384. [DOI] [PubMed] [Google Scholar]
- 326.Lutton E.M., Razmpour R., Andrews A.M., Cannella L.A., Son Y.J., Shuvaev V.V., Muzykantov V.R., Ramirez S.H. Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury. Sci. Rep. 2017;7:3846. doi: 10.1038/s41598-017-03309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Leonard B.M., Shuvaev V.V., Bullock T.A., Galpayage Dona K.N.U., Muzykantov V.R., Andrews A.M., Ramirez S.H. Engineered Dual Antioxidant Enzyme Complexes Targeting ICAM-1 on Brain Endothelium Reduce Brain Injury-Associated Neuroinflammation. Bioengineering. 2024;11:200. doi: 10.3390/bioengineering11030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Vuorte J., Lindsberg P.J., Kaste M., Meri S., Jansson S.E., Rothlein R., Repo H. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J. Immunol. 1999;162:2353–2357. doi: 10.4049/jimmunol.162.4.2353. [DOI] [PubMed] [Google Scholar]
- 329.Xie C., Alcaide P., Geisbrecht B.V., Schneider D., Herrmann M., Preissner K.T., Luscinskas F.W., Chavakis T. Suppression of experimental autoimmune encephalomyelitis by extracellular adherence protein of Staphylococcus aureus. J. Exp. Med. 2006;203:985–994. doi: 10.1084/jem.20051681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang P., Tao C., Shimura T., Huang A.C., Kong N., Dai Y., Yao S., Xi Y., Wang X., Fang J., et al. ICAM1 antibody drug conjugates exert potent antitumor activity in papillary and anaplastic thyroid carcinoma. iScience. 2023;26:107272. doi: 10.1016/j.isci.2023.107272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Guo P., Huang J., Zhu B., Huang A.C., Jiang L., Fang J., Moses M.A. A rationally designed ICAM1 antibody drug conjugate eradicates late-stage and refractory triple-negative breast tumors in vivo. Sci. Adv. 2023;9:eabq7866. doi: 10.1126/sciadv.abq7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Spencer J.P., Jenner A., Aruoma O.I., Evans P.J., Kaur H., Dexter D.T., Jenner P., Lees A.J., Marsden D.C., Halliwell B. Intense oxidative DNA damage promoted by L-dopa and its metabolites. Implications for neurodegenerative disease. FEBS Lett. 1994;353:246–250. doi: 10.1016/0014-5793(94)01056-0. [DOI] [PubMed] [Google Scholar]
- 333.Prigione A., Begni B., Galbussera A., Beretta S., Brighina L., Garofalo R., Andreoni S., Piolti R., Ferrarese C. Oxidative stress in peripheral blood mononuclear cells from patients with Parkinson’s disease: Negative correlation with levodopa dosage. Neurobiol. Dis. 2006;23:36–43. doi: 10.1016/j.nbd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 334.Stansley B.J., Yamamoto B.K. L-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology. 2013;67:243–251. doi: 10.1016/j.neuropharm.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hörmann P., Delcambre S., Hanke J., Geffers R., Leist M., Hiller K. Impairment of neuronal mitochondrial function by L-DOPA in the absence of oxygen-dependent auto-oxidation and oxidative cell damage. Cell Death Discov. 2021;7:151. doi: 10.1038/s41420-021-00547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang F., Liu M., Tuo J., Zhang L., Zhang J., Yu C., Xu Z. Levodopa-induced dyskinesia: Interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation. Front. Immunol. 2023;14:1253273. doi: 10.3389/fimmu.2023.1253273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sarkar S., Roy A., Choudhury S., Banerjee R., Dey S., Kumar H. Levodopa-induced Dyskinesia in Parkinson’s Disease: Plausible Inflammatory and Oxidative Stress Biomarkers. Can. J. Neurol. Sci. 2024;51:104–109. doi: 10.1017/cjn.2023.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data was created. All citations are availale online.