Abstract
The human papillomavirus (HPV) E7 protein promotes S-phase reentry in a fraction of postmitotic, differentiated keratinocytes. Here we report that these cells contain an inherent mechanism that opposes E7-induced DNA replication. In organotypic raft cultures of primary human keratinocytes, neither cyclin E nor p21cip1 is detectable in situ. However, E7-transduced differentiated cells not in S phase accumulate abundant cyclin E and p21cip1. We show that normally p21cip1 protein is rapidly degraded by proteasomes. In the presence of E7 or E6/E7, p21cip1, cyclin E, and cyclin E2 proteins were all up-regulated. The accumulation of p21cip1 protein is a posttranscriptional event, and ectopic cyclin E expression was sufficient to trigger it. In constract, cdk2 and p27kip1 were abundant in normal differentiated cells and were not significantly affected by E7. Cyclin E, cdk2, and p21cip1 or p27kip1 formed complexes, and relatively little kinase activity was found associated with cyclin E or cdk2. In patient papillomas and E7 raft cultures, all p27kip1-positive cells were negative for bromodeoxyuridine (BrdU) incorporation, but only some also contained cyclin E and p21cip1. In contrast, all cyclin E-positive cells also contained p27kip1. When the expression of p21cip1 was reduced by rottlerin, a PKC δ inhibitor, p27kip1- and BrdU-positive cells remained unchanged. These observations show that high levels of endogenous p27kip1 can prevent E7-induced S-phase reentry. This inhibition then leads to the stabilization of cyclin E and p21cip1. Since efficient initiation of viral DNA replication requires cyclin E and cdk2, its inhibition accounts for heterogeneous viral activities in productively infected lesions.
Human papillomaviruses (HPVs) comprise a large family of ubiquitous human pathogens that infect cutaneous and mucosal squamous epithelial cells and are etiologically linked to carcinomas of the anogenital tract. However, the most common lesions associated with these viruses are benign and self-limiting warts, condylomata, and papillomas. Productive infection depends on squamous differentiation. As viral DNA replication depends heavily on the cellular DNA replication machinery, HPVs must reactivate the corresponding host genes (reviewed in reference 9). Indeed, unscheduled cellular DNA synthesis occurs in a subset of differentiated cells in papillomas caused by the nononcogenic HPVs, typically HPV type 6 (HPV-6) and HPV-11. We have also shown that the HPV-18 E7 gene alone, under the control of the differentiation-dependent native promoter, is necessary and sufficient to induce S-phase reentry by differentiated primary human keratinocytes (PHKs) in an organotypic model system (abbreviated hereafter as epithelial raft cultures) (7). Thus, a major natural function of E7 is to reestablish an S-phase milieu to allow HPV replication in postmitotic, differentiated keratinocytes.
Cell cycle progression is normally regulated by cyclins, cyclin-dependent kinases (cdks), and cdk inhibitors (reviewed in reference 64). Underphosphorylated retinoblastoma susceptibility protein (pRb) recruits histone deacetylases and binds to the family of transcription factors E2F/DP on the promoter of a number of genes involved in DNA replication and cell cycle progression, leading to their transcriptional repression (reviewed in reference 15; 4, 39, 43). Ordinarily, D-type G1 cyclins are induced after mitogen stimulation to activate cdk4 or cdk6. The cyclin D-cdk complexes phosphorylate and inactivate pRb, activating E2F-responsive gene. One of the genes controlled by E2F is cyclin E (21, 53), which binds to and activates cdk2. Cyclin E-cdk2 is present maximally in late G1 and early S and is essential for S-phase entry (54). It phosphorylates pRb further, shoring up the G1-to-S-phase transition (25). This kinase also functions downstream of pRb (27, 35, 38). A few cellular proteins and papillomaviral E1 and E2 proteins have been identified as targets of this kinase, and each is involved in some aspect of DNA replication (10, 40, 41, 44, 71, 81).
The activities of cyclin-cdk complexes are themselves regulated by inhibitors (CKIs). For example, p21cip1 and the related p27kip1 are potent inhibitors of cyclin E- and cyclin A-dependent kinases (reviewed in reference 64). p21cip1 mediates the p53-dependent G1 checkpoint upon DNA damage. In addition, p21cip1 mRNA is up-regulated in postmitotic cells in many different tissue types in vivo and in vitro through p53-independent pathways (11, 24, 42, 77). Of particular relevance is our observation that p21cip1 mRNA is expressed at high levels in differentiated keratinocytes in normal human skin, raft cultures, or biopsies of benign papillomas, but the p21cip1 protein cannot be detected by in situ techniques unless HPV or, more specifically, HPV E7 is also present (28, 61, 68). Similarly, p21cip1 mRNA is also highly expressed in mouse epithelium (57). Under submerged culture conditions that induce squamous differentiation, mouse keratinocytes express high levels of p21cip1 mRNA but low levels of p21cip1 protein which, however, can be increased when the proteasome pathway is blocked (14). p27kip1 controls G0/G1-to-S transition in normal cycling cells (64). In addition, p27kip1 is also induced when normal or E7-transduced keratinocytes differentiate upon growth as suspension culture in methylcellulose-containing medium (59) and has been detected in HPV-associated patient lesions (76). But the significance of its presence regarding HPV pathobiology has not been investigated.
The E7 protein of oncogenic HPVs interacts with a large group of cellular targets (reviewed in reference 83). The best-characterized target is the pRb-E2F pathway. The E7 protein inactivates and promotes the degradation of the underphosphorylated form of pRb (3, 31), bypassing the need for cyclin D-cdk4 or -6. Consequently, E7 induces cyclin E and cyclin A in growth-arrested NIH 3T3 cells (79). In addition, several reports indicate that E7 can also associate with p21cip1 and p27kip proteins in vitro, abrogating their inhibitory activities (20, 30, 78). Similarly, the E6 protein of oncogenic HPVs also interacts with a number of cellular proteins. In particular, it functions as a cofactor of E6-AP to ubiquitinate p53, resulting in its rapid degradation (reviewed in reference 69). These properties of the viral oncoproteins have provided mechanistic interpretations for HPV-associated oncogenesis in patients when the viral oncogenes are inappropriately expressed in normally quiescent epithelial stem cells (82).
We have reported novel virus-host interactions in epithelial raft cultures that simulate benign lesions (28, 29, 61). Upon expression of HPV-18 E7 from its differentiation-dependent native viral enhancer and promoter (or upstream regulatory region [URR]) (56), at least two populations of PCNA-positive, postmitotic, differentiated keratinocytes are observed. In one, high levels of cyclin E and p21cip1 proteins simultaneously accumulate, but there is no active DNA replication. In the other, p21cip1 and cyclin E proteins are below detection, but active DNA synthesis takes place. These observations have been confirmed in papillomatous clinical specimens. In addition, there is an inverse relationship between p21cip1 or cyclin E induction and high levels of HPV DNA or RNA in these lesions regardless of the associated HPV types. We concluded that the induction of p21cip1 protein inhibits cyclin E-cdk2, prevents both viral and host DNA synthesis, and accounts, at least in part, for the heterogeneous nature of viral activities in the spinous cells of benign HPV lesions. However, the cause for leading to these distinct cell populations was not understood. We also proposed that the coinduction of these two normally short-lived cell cycle regulatory proteins was attributed to protein stabilization in an inactive cyclin E-cdk2-p21cip1 complex, but this hypothesis has not been tested. In the present study, we present data supporting this interpretation as well as evidence implicating p27kip1 as a possible trigger for initiating cyclin E-p21cip1 costabilization. Fortuitously, the differentiation-dependent expression of the endogenous p21cip1 and p27kip1 proteins in effect constitutes an inherent mechanism by which host cells suppress viral replication.
MATERIALS AND METHODS
Tissue specimens.
Neonatal foreskins were collected from Cooper Green Hospital (Birmingham, Ala.). Juvenile laryngeal papillomas were collected from The Children's Hospital of Alabama (Birmingham, Ala.) as part of routine therapies. The latter tissues were incubated with 50 μg of bromodeoxyuridine (BrdU) per ml in Dulbecco's modified Eagle's medium with 10% fetal bovine serum for 6 h before being fixed in 10% buffered formalin and embedded in paraffin.
Retroviruses, cells, and organotypic raft cultures.
PHKs recovered from neonatal foreskins were grown in serum-free medium (Life Technologies, Inc., Rockville, Md.), infected with recombinant Moloney murine leukemia retroviruses, and used to seed the epithelial raft cultures as previously described (7, 56). Recombinant retroviruses containing HPV-18 URR-E7 or HPV-18 URR-E6/E7 have been described elsewhere (7). These vectors express the bacterial neomycin resistance gene from the SV40 promoter, whereas the HPV gene or genes are under the control of the native HPV enhancer and promoter (URR). To express cyclin E under the control of URR, the retrovirus pLNSX (45) was modified. In this vector, the neomycin resistance gene is expressed from the retroviral promoter in the long terminal repeat. The SV40 promoter in the vector was substituted by HPV-18 URR using the BamHI and HindIII restriction sites. Then, human cyclin E cDNA from pCS2+-cyclin E (kindly provided by James M. Roberts) was excised using HindIII and cloned in the sense or antisense orientation downstream of the HPV-18 URR.
Epithelial raft cultures were harvested after 9 or more days at the medium-air interface by fixation in 10% buffered formalin, embedded in paraffin, and then cut into 4-μm sections. Treatment with inhibitors is described below with each experiment. To mark newly synthesized cellular DNA, the cultures were incubated in raft culture medium containing 50 μg of BrdU/ml for 12 or 24 h before harvest, as described in the figure legend for each experiment.
Antibodies and chemicals.
Rabbit polyclonal antibodies used for immunoprecipitation were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.) and were anti-p21cip1 (sc-397), anti-p27kip1 (sc-528), and anti-cdk2 (sc-163). The primary monoclonal antibodies used for immunoblotting (1:1,000 dilution in all cases) and in situ immunodetections (with dilutions used indicated) were cyclin E (catalog no. 14591C; PharMingen, San Diego, Calif.; 1:25), p21cip1 (catalog no. OP64; Oncogene Research, Cambridge, Mass.; 1:25), p27kip1 (catalog no. NCL-p27; Novocastra, Newcastle upon Tyne, United Kingdom; 1:20), p53 (catalog no. NCL-p53-DO1; Novocastra; 1:20), and BrdU (catalog no. 18-0103; Zymed Laboratories, San Francisco, Calif.; 1:50). Lactacystin, PD150606, and rottlerin were purchased from Calbiochem (San Diego, Calif.). Each was dissolved in dimethyl sulfoxide as a concentrated stock solution and was added to the raft culture medium for the duration and at the final concentrations indicated for each experiment.
Protein extraction and immunoprecipitation.
To prepare protein extracts from raft cultures, the rafts were frozen in liquid nitrogen after 9 to 11 days of culturing and detached from the metal grids with a scalpel. Two to five rafts were minced in a mortar containing liquid nitrogen and collected in 100 to 500 μl of cold radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 10 mM phosphate buffer [pH 7.3], 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 2 mM EDTA). After 5 min on ice, the tubes were vortexed briefly and centrifuged for 5 min at 10,000 × g to separate the insoluble material. Protein concentrations were measured by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) using known concentrations of bovine serum albumin (Sigma-Aldrich, St. Louis, Mo.) as a standard. For immunoprecipitation, 400 μg of total raft extracts was first preincubated with approximately 3 mg of protein A-Sepharose CL-4B (Amersham Pharmacia Biotech AB, Uppsala, Sweden) in a rocking shaker for 1 h at 4°C. Following a brief, low-speed centrifugation, the supernatant was transferred to a new tube containing fresh protein A-Sepharose CL-4B beads and 3 μg of the appropriate polyclonal antibody. The mix was further incubated for 2 h at 4°C. The Sepharose beads were collected after a 1-min low-speed centrifugation and washed three times with RIPA buffer. The beads were finally resuspended in Laemmli sample buffer containing β-mercaptoethanol.
Histone H1 kinase assays.
Raft cultures were homogenized using a glass tissue grinder in lysis buffer consisting of 250 mM NaCl, 50 mM HEPES (pH 7.0), 5 mM EDTA, 1 mM dithiothreitol, 10 μM ZnCl2, 0.5% Nonidet P-40, and one tablet of protease inhibitors cocktail (Complete Mini; Roche Biochemicals, Indianapolis, Ind.) per 10 ml. Insoluble material was removed and protein concentrations were measured as described above. Next, 400 μg of extracts was diluted to a volume of 0.4 ml with lysis buffer and precleared with 3 mg of protein A-Sepharose CL-4B beads as described above. One milligram of fresh protein A-Sepharose CL-4B and 4 μg of either cyclin E polyclonal antibody (sc-248; Santa Cruz Biotechnology) or cdk2 polyclonal antibody (sc-163; Santa Cruz Biotechnology) were added to the precleared extracts and were incubated with rocking at 4°C for 2 h. The beads were collected by brief centrifugation and were washed three times with lysis buffer and twice with 50 mM Tris (pH 7.0), 10 mM MgCl2. In vitro kinase assays were performed using [γ-32P]ATP and H1 histone as substrates as described before (58). The incorporated radioactivity was quantitated using a Storm phosphorimager (Molecular Dynamics, Sunnyvale, Calif.).
Western blotting.
Proteins were resolved by electrophoresis through sodium dodecyl sulfate–12% polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech) using semi-dry blotting techniques. The membranes were blocked for 1 h in 5% nonfat milk, probed for 1 to 2 h with the primary antibodies and, after extensive washing, reprobed with horseradish peroxidase (HRP)-conjugated secondary antibodies. The bands were revealed using enhanced chemiluminescence procedures according to the manufacturer's recommendations (Amersham Pharmacia Biotech).
Immunohistochemical and immunofluorescence detection.
Deparaffinized sections were incubated in 10 mM citrate buffer (pH 6) at 95°C for 10 min for antigen retrieval. Immunohistochemical detections of BrdU were performed using the primary antibody in conjunction with the Histostain-SP broad-spectrum kit following the manufacturer's instructions (Zymed Laboratories Inc.). The sections were then counterstained lightly with hematoxylin. Images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, Mich.) attached to an Olympus BH-2 microscope.
For double immunofluorescence involving BrdU, the sections were treated for antigen retrieval, blocked, and then probed overnight with the monoclonal antibody to the protein antigen of interest. The sections were then washed with phosphate-buffered saline and reprobed with biotinylated anti-mouse immunoglobulin G (IgG). After washing, the sections were reacted with streptavidin-Texas Red (Vector Laboratories, Burlingame, Calif.), blocked again, and probed with fluorescein-conjugated anti-BrdU antibody (Roche Biochemicals).
For double immunofluorescence of cyclin E and p27kip1, the sections were first probed overnight with anti-p27kip1 IgG2a (NCL-p27; Novocastra) and then with anti-mouse IgG2a-HRP conjugate (Roche Biochemicals). The p27kip1 signals were enhanced with fluorescein conjugated with tyramide following the manufacturer's instructions (NEN Life Science Products, Boston, Mass.). The HRP was inactivated by treatment with 3% H2O2, and the sections were reblocked and incubated overnight with anti-cyclin E IgG1 monoclonal antibody (14591C; Pharmingen). Next, they were probed with anti-mouse IgG1-HRP conjugate (Roche Biochemicals), and the signal was amplified by the deposition of Cy3-tyramide conjugates (NEN Life Science Products). For double immunofluorescence of cyclin E and p21cip1, the sections were first probed overnight with the cyclin E antibody mentioned above and developed with Texas Red as described above. Then, they were reprobed with anti-p21cip1 conjugated with fluorescein (OP64F; Oncogene) and then with anti-fluorescein conjugated with HRP (1426346; Roche Molecular Biochemicals). Finally, the signal was amplified by the deposition of fluorescein-tyramide conjugates as described above. The images were captured with an Olympix 2000 digital camera (Life Sciences Resources, Cambridge, United Kingdom) attached to an Olympus Provis AX70 microscope. All the images were digitally processed using Photoshop 5.0 (Adobe Systems, San Jose, Calif.).
RNA extraction and Northern blot analysis.
Total RNA from raft cultures was extracted using Trizol reagent (Life Technologies) following the manufacturer's instructions. About 100 μg of total RNA was obtained from two raft cultures pooled together. Twenty micrograms of total RNA was used for Northern blot analysis as described before (2). p21cip1-specific probes were synthesized by the random primer method using [α-32P]dCTP and the RTS Radprime DNA labeling system (Life Technologies) using a 2.1-kb p21cip1 cDNA as template (a gift from Yue Xiong).
mRNA in situ hybridization.
Biotinylated riboprobes were prepared from a pGEM1 vector containing the p21cip1 cDNA (61) using the Renaissance RNA biotin labeling kit (NEN Life Science Products). Treatment and preparation of the tissue sections were performed as described before (61). Hybridization was performed overnight at 37°C in Hybrisol VII (Ventana Medical Systems), and washings were performed at 37°C in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 50% formamide, and 0.5% Triton X-100 for 30 min. After washing, the slides were blocked with 4× SSC and 1% casein and probed with streptavidin-HRP. Finally, the color was developed using a 3-amino-q-ethyl carbazole (AEC) kit (Zymed Laboratories Inc.), and the slides were lightly counterstained with hematoxylin.
RESULTS
p21cip1 protein is stabilized in normal, differentiated keratinocytes by the proteasome inhibitor lactacystin.
To test our hypothesis that the p21cip1 protein is normally short-lived in differentiated keratinocytes in squamous epithelium, we treated normal raft cultures or cultures transduced with HPV-18 URR-E7 or HPV-18 URR-E6/E7 with 10 μM lactacystin or 50 μM PD150606 for 12 h immediately prior to harvest on day 9. Lactacystin is a specific inhibitor of the 26S subunit of the proteasome (16), while PD150606 targets the calcium binding site of calpain, a calcium-dependent protease (73). The cultures were preexposed to BrdU for 12 h prior to treatment with lactacystin to mark cells in S phase prior to the addition of the drug.
Typical results of double immunofluorescence detection of p21cip1 protein and BrdU are shown in Fig. 1A. In untreated raft cultures of untransduced PHKs, p21cip1 protein was not detectable in any strata (Fig. 1A, top row, left panel). However, after treatment with lactacystin, high levels of p21cip1 protein were detected in nearly all the keratinocytes, including basal BrdU-positive cells (top row, middle panel). In untreated cultures expressing HPV-18 E7 or HPV-18 E6/E7 from the HPV-18 URR, a significant subset of postmitotic cells was strongly positive for p21cip1 protein while another subset was positive for BrdU (middle and bottom rows, left panels), as reported previously (28). As with the control raft cultures, upon treatment with lactacystin, nearly all the cells were positive for p21cip1 protein, including BrdU-positive cells in all cell strata (middle and bottom rows, middle panels). In contrast, the calpain inhibitor PD150606 had no effect on p21cip1 accumulation in the control or in E7-expressing raft cultures (Fig. 1A, right panels). We also added BrdU during the last 12 h of a 24- or 36-h lactacystin treatment. The sections were then probed by immunohistochemistry. Bright-field micrographs revealed that DNA replication was abrogated in all the cells while the histology of the tissues remained unaffected (Fig. 1B). These results are consistent with an interpretation that p21cip1 protein stabilization effectively inhibits both the initiation and elongation of cellular DNA synthesis. However, additional deleterious effects of lactacystin cannot be excluded.
FIG. 1.
Effect of lactacystin or PD150606 on p21cip1 protein accumulation and DNA synthesis in raft cultures. (A) On day 9, triplicate sets of control (PHK) or E7- or E6/E7-expressing raft cultures were incubated with BrdU for 12 h. Then, one set was left untreated while the remaining sets were treated either with 10 μM lactacystin, an inhibitor of proteasomes, or with 50 μM PD150606, an inhibitor of calpain. The incubation proceeded for another 12 h. Four-micrometer sections of the formalin-fixed, paraffin-embedded cultures were subjected to double immunofluorescence assays. p21cip1 protein was visualized using Texas Red, and BrdU was visualized with fluorescein (green). Arrows point to the basal stratum. (B) On day 9, one set of raft cultures was left untreated, while two additional sets were treated with 10 μM lactacystin. After 12 or 24 h, BrdU was added to the culture medium without removing the lactacystin, and the cultures were incubated for another 12 h. Four-micrometer sections of formalin-fixed, paraffin-embedded raft cultures were subjected to immunohistochemical probing to detect BrdU incorporation and then counterstained lightly with hematoxylin to reveal tissue histology.
We next performed Western blotting on crude protein extracts of raft cultures to provide a more quantitative comparison than in situ assays. The results were in agreement (Fig. 2A). p21cip1 was very low in control cultures, whereas p21cip1 protein was elevated in both URR-E7- and URR-E6/E7-transduced raft cultures relative to control cultures. Upon lactacystin treatment, the amounts of p21cip1 increased but were similar in all four cultures. Thus the increase was largest for the control cultures and least for the E6/E7 cultures.
FIG. 2.
Cellular proteins, mRNA, and cellular DNA replication in raft cultures in the presence or in the absence of lactacystin. (A) Western blot of control raft cultures (PHK, vector), E7-, or E6/E7-containing raft cultures that were either left untreated or treated with 10 μM lactacystin for the last 12 h prior to harvest on day 9. Fifty micrograms of each of these extracts was Western blotted for immunodetection of p21cip1, p27kip1, p53, and cyclin E. (B) Northern blot for p21cip1 RNA from untreated raft cultures. Twenty micrograms of total RNA from raft cultures was analyzed by using a probe specific for p21cip1. 18S rRNA was visualized by ethidium bromide staining and was included as a control of loading and RNA integrity. (C) Western blots of total raft extracts. Fifty micrograms of total protein extracts from indicated raft cultures was Western blotted and probed with the antibodies to cyclin E, cyclin E2, and cdk2 as indicated. (D) Lack of effects by lactacystin on the accumulation of cyclin E in raft cultures. Thin sections from the same raft cultures as those in Fig. 1A were probed for cyclin E (Texas Red) and BrdU (fluorescein, green) by double immunofluorescence. Sporadic, nonspecific trapping of the cyclin E antibody was observed in the granular layer and in the acellular squamous layer. Arrows point at the basal stratum.
p21cip1 protein accumulation induced by E7 is a posttranscriptional event.
The above observations and our previous in situ data indicated that the p21cip1 protein accumulation induced by E7 is attributable to posttranscriptional stabilization. To substantiate this conclusion, we performed two additional experiments. First, we measured the level of p53 protein by Western blotting, as p53 protein is up-regulated in our HPV-18 URR-E7-transduced raft cultures (28). Second, we performed Northern blotting to measure the relative p21cip1 mRNA abundance.
Western blots showed that the elevated levels of p21cip1 observed in E7 or E6/E7 raft cultures did not correlate with the amounts of p53 protein in the tissues (Fig. 2A). p53 was detected in all cultures except those containing HPV-18 URR-E6/E7, in keeping with the ability of E6 to promote p53 degradation. The sporadic weak induction of p53 in differentiated keratinocytes in E7-transduced raft cultures (28) was not discernable by Western blot. Interestingly, p53 was not significantly affected by lactacystin, suggesting that it can be degraded by other mechanisms as well. These data clearly ruled out p53 being a major contributor in transcriptional induction of p21cip1 in differentiated cells. Northern blotting revealed that the levels of p21cip1 mRNA did not change significantly in the presence of E7 or E6/E7 when compared with the control rafts (Fig. 2B). Collectively, the data support the conclusion that the increase in the steady-state levels of p21cip1 protein in the presence of E7 is a posttranscriptional event.
As described earlier (7, 28, 29) and throughout the present study, the E6/E7-transduced cultures elicited the same cellular responses as the E7-transduced cultures, except that they were more effective. This quantitative difference could be due to either higher titers of the E6/E7 virus or more efficient E7 translation from E6/E7 mRNAs relative to the construct containing E7 alone (67). It is also possible that the E6 and truncated E6* proteins enhance E7 functions. Although these alternatives were not pursued in the present work, the data from the E6/E7 cultures are included for completeness.
Level of cyclin E is not altered by lactacystin treatment.
We also probed the same raft cultures, with or without lactacystin, used in p21cip1 protein studies for cyclin E and BrdU by double immunofluorescence. In the control cultures, cyclin E was below the detection threshold of indirect immunofluorescence or immunohistochemistry even though some of the proliferating basal and parabasal cells may have been in G1/S phase boundary, when cyclin E is normally present at the highest level. This may be construed to be a consequence of failing to capture the brief period during which cyclin E is abundant. Cyclin E is degraded by proteasomes (74). However, after treatment with lactacystin, cyclin E remained below detection in the control cultures and in BrdU-positive cells of E7 cultures (Fig. 2D). Concordant with in situ observations, Western blotting showed that the levels of cyclin E in control or E7-containing raft culture extracts were not discernibly affected by lactacystin (Fig. 2A). Thus, most of the cyclin E present in the E7-expressing raft cultures is already stable and inhibiting protein degradation cannot significantly increase it.
In the Western blotting, several closely spaced cyclin E antibody-reactive bands were detected (Fig. 2A and C). The faster migrating bands, possibly hypophosphorylated forms, are those most prominently up-regulated by E7 or E6/E7. A band of molecular mass significantly smaller than cyclin E was also detected (data not shown). By using a polyclonal antibody specific for cyclin E2, which shares significant homology with cyclin E (23, 75), we concluded that the fast-migrating band is likely a cyclin E degradation product. However, we did detect a marked induction of cyclin E2 in E7-containing cultures (Fig. 2C), as also reported in human fibroblasts (75). Similar to cyclin E, the putative unphosphorylated cyclin E2 is also the most prominently up-regulated. The cyclin E2 antibody, however, did not react with its antigen in tissue sections and it was not further investigated. In the same blot, the amounts of cdk2 were equally abundant in all cultures (Fig. 2C). Thus, cdk2 is constitutively expressed in raft cultures and is not a limiting factor for the G1/S transition. This result also indicates that cdk2 alone cannot stabilize p21cip1 protein in the absence of cyclin E despite its ability to interact with p21cip1 in vitro (6).
Ectopic expression of cyclin E induces low level of p21cip1 protein accumulation.
To demonstrate that cyclin E induced by E7 is sufficient to increase p21cip1 protein, cyclin E cDNA was cloned downstream of differentiation-dependent HPV-18 URR (56). Using a recombinant retrovirus, this construct was transduced into the raft cultures. Cyclin E expression was detected in a subset of differentiated cells (Fig. 3A). The observation that not all the differentiated cells had detectable cyclin E might be at least in part due to the varied URR activities from individual proviruses integrated at different chromosomal locations. This varied but nonetheless differentiation-dependent URR activity has previously been observed with the bacterial β-galactosidase as a reporter (56). As predicted, p21cip1 protein was also induced in a subset of differentiated cells. Double immunofluorescence showed that p21cip1 was only found in those cells that also contained cyclin E (Fig. 3C), but some cyclin E-positive cells were negative for p21cip1. This is probably because the levels of both cyclin E and p21cip1 proteins were low and were below the threshold of p21cip1 detection in some of the cells. Western blotting of crude protein extracts from these raft cultures indeed showed a significant and concordant increase in both cyclin E and p21cip1 proteins (Fig. 3B). Interestingly, unlike reports using growth-arrested cell lines (1, 35, 36, 38, 60), the expression of cyclin E by itself in differentiated keratinocytes was insufficient to induce S-phase entry, since BrdU-positive cells were only found in the basal and parabasal layer (Fig. 3A). This is further indication that the suprabasal cells in our normal raft cultures are postmitotic.
FIG. 3.
Effect of ectopic cyclin E expression on p21cip1 protein accumulation and DNA replication. (A) Double immunofluorescence to detect cyclin E (Texas Red) and BrdU (fluorescein, green) in raft culture sections. Cyclin E was ectopically expressed in the differentiated keratinocytes using the retrovirus pLN-URR-cyclin E. In the control, the cyclin E cDNA was cloned in the opposite direction from the URR promoter. The transduced raft cultures were exposed to BrdU for 12 h prior to fixation. (B) Western blot of total extracts from raft cultures containing pLN-URR-cyclin E or the antisense control. Forty micrograms of total protein was loaded per lane and probed with cyclin E and p21cip1 antibodies. (C) Double immunofluorescence assay to detect cyclin E (Texas Red) and p21cip1 (fluorescein, green). The left and right panels reveal p21cip1 and cyclin E, respectively, while the middle panel shows a merged image. Arrows point at the basal stratum.
A significant fraction of the cyclin E in E7-expressing epithelial raft cultures is found in a complex with cdk2 and p21cip1.
The data presented so far suggest that cyclin E induction is tightly linked to p21cip1 accumulation, consistent with the formation of a complex. To confirm this interpretation and to quantify the amount of the complex in different cultures, a polyclonal antibody against the carboxyl-terminal region of p21cip1 was used to perform immunoprecipitation studies. The presence of cyclin E and p21cip1 in the precipitates was confirmed by Western blotting (Fig. 4A). A significant fraction of cyclin E present in the E7-expressing raft extracts was also detected in the supernatants, whereas little or no p21cip1 protein remained in the supernatant. The reason for this difference in the partition of these two proteins will be addressed later. To prove that cdk2 is also part of the cyclin E-p21cip1 complex, we repeated the immunoprecipitation experiment by using an antibody against the carboxyl terminus of cdk2. As shown in Fig. 4B, both p21cip1 and cyclin E coimmunoprecipitated with cdk2.
FIG. 4.

Coimmunoprecipitation of cyclin E, p21, and cdk2 from raft culture extracts. (A) Coimmunoprecipitation with a polyclonal antibody directed against the carboxyl terminus of p21cip1. Three hundred micrograms of total extracts from various raft cultures was put through one round of immunoprecipitation. The precipitates and the supernatants were Western blotted and probed with the indicated antibodies. (B) Coimmunoprecipitation with a polyclonal antibody directed against the carboxyl terminus of cdk2. Four hundred micrograms of total extracts from raft cultures was immunoprecipitated, Western blotted, and probed with the indicated antibodies. Forty micrograms (10% of input) of the total extracts was analyzed in parallel.
The majority of cyclin E or cdk2 immunoprecipitate in E7-expressing raft cultures does not have an associated kinase activity.
p21cip1 strongly inhibits cdk2 and PCNA (18, 64, 72), but this inhibition can be overcome by E7 in cycling cells and in a cell-free system (20, 30). However, the BrdU-incorporation results suggest that the p21cip1 inhibition is not abolished in E7-expressing raft cultures except in a small fraction of differentiated keratinocytes. To quantify the cdk2 kinase activity present in the rafts, we performed in vitro kinase assays with immune precipitates obtained with cdk2 or cyclin E antibody using histone H1 as a substrate. Additional aliquots were used to measure the relative levels of cyclin E in the same extracts by Western blotting. The results were quantified using a PhosphorImager (Fig. 5A). The kinase activities among the control and E7-expressing raft cultures were within twofold of one another. However, cyclin E was five- to sevenfold as abundant in E7-containing rafts as that in normal rafts (Fig. 5B). Taken together, these data demonstrate that the majority of the cyclin E-cdk2 complex is inactive in the presence of E7 or E6/E7 due to the association with p21cip1 or p27kip1 (see below).
FIG. 5.

H1 histone kinase activities associated with cdk2 or with cyclin E. (A) Four hundred micrograms of total raft culture extracts was immunoprecipitated with the appropriate antibodies. After extensive washing, the H1 histone kinase activity present in the immunopellets was determined by using an in vitro kinase assay followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and phosphorimaging. The intensity of each band was quantified and is shown as a percentage of the strongest band (100%). (B) The relative level of cyclin E on each extract was determined by Western blotting and quantified by densitometry. The intensity of each band is expressed as a percentage of the strongest band (100%). Actin was included as a control of loading.
p27kip1 protein is normally present in most differentiated keratinocytes.
Although p21cip1 is constitutively transcribed in the differentiated keratinocytes (28, 61), only a subset of the differentiated cells in E7-expressing raft cultures accumulated p21cip1 protein. Moreover, the proportion of cyclin E in the supernatant after precipitation with a p21cip1 antibody (Fig. 4A) would have predicted a substantial increase in cdk2 activity in E7-expressing cultures relative to that in control cultures. However, this was clearly not the case (Fig. 5). We hypothesized that another cdk inhibitor might be heterogeneously distributed among the differentiated cells and it might be responsible for initiating the accumulation of cyclin E and p21cip1 proteins. Our candidate was p27kip1, which normally controls G0/G1-to-S phase transition in cycling cells (64). Double immunofluorescence of normal raft cultures indicated that p27kip1 was present in the majority of the differentiated cells but not in the basal or parabasal cells, regardless of whether they were actively incorporating BrdU (Fig. 6A, left panel). The signals were stronger in the upper strata than in the lower strata. Western blotting showed that p27kip1 was present in all cultures (Fig. 2A). Its level appeared to be slightly higher in the presence of E7 or E6/E7. However, it did not appreciably increase upon lactacystin treatment (Fig. 2A). Thus, the p27kip1 protein is stable in normal, differentiated cells, unlike p21cip1, which is unstable.
FIG. 6.
Distribution of p27kip1 protein in raft cultures and in a laryngeal papilloma. (A) Double immunofluorescence assay to reveal the distributions of cells positive for p27kip1 (Texas Red) or BrdU (fluorescein, green) in sections from vector-only, E7-, and E6/E7-expressing raft cultures that were exposed to BrdU for 12 h prior to fixation in formalin. (B) Double immunofluorescence assay for p27kip1 and BrdU on a laryngeal papilloma caused by HPV-6 which was exposed to BrdU for 6 h prior to fixation in formalin. Two areas are shown. (C) Coimmunoprecipitation of cyclin E and p27kip1. Three hundred micrograms of total extracts from controls (PHK, vector-only), E7-, and E6/E7-expressing raft cultures was immunoprecipitated with a polyclonal antibody against the carboxyl terminus of p27kip1. The immunoprecipitates and the supernatants were Western blotted and probed with the indicated antibodies. (D) Colocalization of p27kip1 (green) and cyclin E (red) by double immunofluorescence in normal (PHK) or E6/E7-containing raft cultures. Both p27kip1 and cyclin E signals were enhanced using tyramide deposition, as described in Materials and Methods. The signals from cyclin E (red) or p27kip1 (green) are shown individually in the left and right panels. The merged images are shown on the center panels. Arrows point at the basal stratum.
p27kip1 protein is present in cells not in S phase, regardless of the level of cyclin E.
To examine the functional significance of p27kip in E7-expressing raft cultures, we used double immunofluorescence to probe URR-E7 and URR-E6/E7 raft cultures exposed to BrdU. The pattern of p27kip1 distribution was similar to that in the control cultures without E7. As demanded by our hypothesis, p27kip1-positive cells did not contain BrdU, and vice versa (Fig. 6A). We also examined HPV-6-induced laryngeal papillomas and observed an identical pattern of distribution (Fig. 6B). We were not able to confirm these findings with benign lesions associated with oncogenic HPV-16 or HVP-18, since it is not a recommended practice for physicians to remove low-grade genital lesions, due to high spontaneous regression rates. These observations suggest that p27kip1 may play an important role in determining the fate of E7-expressing differentiated keratinocytes.
Immunoprecipitation of the raft culture extracts with a polyclonal antibody against the carboxyl terminus of p27kip demonstrated that a fraction of the cyclin E was found in a complex with p27 (Fig. 6C). This observation explains why a significant amount of cyclin E remained in the supernatant after immunoprecipitation with an antibody to p21cip1 (Fig. 4A). To determine whether p27kip1 plays a role in initiating the accumulation of cyclin E and p21cip1 protein in E7-expressing cultures, we examined the relative distribution of p27kip1 and cyclin E (Fig. 6D). Our hypothesis would predict that, among the p27kip1-positive population, some would be positive for cyclin E while others are yet to accumulate cyclin E to detectable levels. Indeed, in E7- or E6/E7-transduced raft cultures, cyclin E antibody reactivity coincided with that of p27kip1, but only a fraction of the p27kip1-positive cells contained cyclin E. In untransduced raft cultures, only p27kip1 was detected in the differentiated strata (Fig. 6D and data not shown). Since cyclin E and p21cip1 always colocalize in E7-expressing raft cultures (29), these results infer that some of the p27kip1-positive differentiated keratinocytes also accumulated detectable p21cip1 protein.
Reduction of p21cip1 levels in differentiated keratinocytes by rottlerin does not increase BrdU-positive cells.
The above observation strongly suggests that p21cip1 protein accumulation by E7-induced cyclin E may be a consequence of S-phase arrest caused initially by abundant p27kip1 protein that is naturally present in the differentiated cells. If this is true, then one should be able to reduce p21cip1 protein accumulation without significantly increasing the fractions of differentiated cells in S phase in the E7-expressing raft cultures. To test this hypothesis, we sought to affect the endogenous p21cip1 mRNA level. It has been reported that the expression of p21cip1 gene is up-regulated by pathways requiring protein kinase C (PKC) in several different cell lines (19, 77, 80), but keratinocytes have not been examined. Since PKC is activated upon squamous differentiation (13, 52), we reasoned that the constitutive transcription of the p21cip1 gene in the differentiated keratinocytes might also be linked to PKC activation.
We treated E6/E7-transduced raft cultures with rottlerin, a naturally occurring specific inhibitor of the novel PKC δ isotype (22). p21cip1 transcription was indeed markedly reduced relative to levels in untreated cultures, as assessed by in situ hybridization with biotinylated p21cip1 antisense-strand riboprobes. A sense-strand probe did not elicit any signals in either culture (Fig. 7A). As an additional proof for the effect of rottlerin on p21cip1 expression, we treated PHK raft cultures with rottlerin for 24 h and then with 10 μM lactacystin during the last 12 h to reveal the amount of p21cip1 protein that can be stabilized. Figure 7B (bottom panel) shows that p21cip1 protein, in the culture treated with both inhibitors, was significantly reduced. In contrast, the culture not treated with either inhibitor had no detectable p21cip1 protein (data not shown, but see Fig. 1A), whereas that treated with lactacystin alone was positive for p21cip1 in virtually all the cells, as demonstrated previously (Fig. 1A). These data establish that the constitutive expression of the p21cip1 gene in differentiated keratinocytes is, at least in part, linked to the activity of PKC δ.
FIG. 7.
Effects of PKC inhibition on the expression of p21cip1 and p27kip1 and on unscheduled DNA synthesis in E6/E7 raft cultures. (A) In situ hybridization of p21cip1 mRNA in E6/E7 raft cultures treated with 20 μM rottlerin or mock treated with solvent. p21cip1 sense- and antisense-strand biotinylated riboprobes were used to probe the sections. The positive signal appears as a reddish cytoplasmic deposition. (B) Day 9 untransduced PHK rafts were treated with lactacystin alone, as described for Fig. 1A, or with rottlerin and lactacystin as follows. The raft culture was incubated in the presence of 20 μM rottlerin for 12 h. Then, 10 μM lactacystin was added to the culture medium for another 12 h without removing the rottlerin. The sections were probed for p21cip1 (green). Arrows point at the basal stratum. (C) E6/E7 raft cultures were treated on day 9 with 10 or 20 μM rottlerin for 12 h. BrdU was then added to the medium and the incubation was continued for another 12 h without removing rottlerin. Immunofluorescence assays to detect p21cip1 (red) and BrdU (green) are shown in the top panels, while p27kip1 (green) is shown in the bottom panels.
To examine the effect of p21cip1 mRNA reduction on unscheduled DNA synthesis in E6/E7-transduced cultures, we examined the patterns of BrdU incorporation and those of p21cip1 and p27kip1 accumulation. Cultures were either mock treated or treated with 10 or 20 μM rottlerin for 24 h. BrdU was added to the cultures for the last 12 h immediately before harvest. Immunofluorescence showed that p21cip1 protein was reduced in the E6/E7-transduced raft cultures after rottlerin treatment (Fig. 7C, top row). However, despite the significant reduction of p21cip1 protein, there was little or no change in the fraction of BrdU-positive cells (Fig. 7C, top row). Moreover, neither the fractions of p27kip1-positive cells nor the p27kip1 signal strength was discernibly affected by rottlerin treatment (Fig. 7C, bottom row). These results support the notion that p27kip1 causes the accumulation of p21cip1 and plays the primary role in preventing S-phase reentry by inhibiting cyclin E-cdk2.
DISCUSSION
We have previously shown that HPV infection can induce a subset of postmitotic, differentiated keratinocytes to reenter into S phase, whereas a separate population accumulates exceptionally high levels of cyclin E and p21cip1 proteins, and that the viral gene responsible is E7 (28, 29, 61). We suggested that cyclin E and p21cip1 protein accumulation is due to costabilization into a kinase-inactive, replication-incompetent complex. In this report, we examined the regulation of p21cip1 expression and presented multiple lines of evidence confirming our interpretation.
Through the use of the proteasome inhibitor lactacystin, we have established that p21cip1 protein is indeed present but is turned over very rapidly in all strata of normal raft cultures (Fig. 1A). Sheaff et al. have also shown that free p21cip1 protein is very unstable and does not require ubiquitination in order to be degraded by the proteasomes (63). Our data show that E7 is able to indirectly overcome this rapid degradation in many of the cells through the induction of cyclin E that sequesters p21cip1 in an inactive complex that also includes cdk2. First, the presence of E7 does not affect the level of p21cip1 mRNA found in the raft cultures, but it increases the levels of p21cip1 protein (Fig. 1A and 2A and B; see also reference 28). Second, ectopic expression of cyclin E alone is sufficient to cause an induction of p21cip1, although it is less efficient than E7 (Fig. 3). This latter result is expected because ectopic cyclin E expression does not involve a cascade of signals, whereas induction by E7 does. For example, E7 also significantly induces cyclin E2 (Fig. 2C) which can also bind p21cip1 (23, 34, 75). Cyclin E2 was absent from this experiment. Third, we have shown that cyclin E, cdk2, and p21cip1 form a complex and the amount of this complex is higher in E7-expressing cultures than in control cultures (Fig. 4). Fourth, an in vitro kinase assay demonstrated that the cdk2 or cyclin E complex had low activities despite the large increase in cyclin E (Fig. 5). Furthermore, we have previously demonstrated a genetic link between cyclin E and p21cip1 induction in raft cultures. An E7 dDLLC mutation, which lacks the consensus pRb binding motif LXCXE and is unable to bind to pRb or p107 in vitro, was also unable to induce either cyclin E or p21cip1 in the differentiated keratinocytes (8). Conversely, several DNA replication-incompetent E7 mutants that bind pRb and induce cyclin E also accumulate p21cip1 protein (8).
We should mention that the coregulation of cyclin E with CKI in CKI-arrested cells is not unique to our raft cultures. In HeLa cells, a cervical carcinoma cell line expressing abundant HPV-18 E6 and E7 proteins, cyclin E increases while cdk2 kinase activity decreases when p21cip1 is conditionally induced or when p21cip1 or p27kip1 is transduced into the cells via an adenovirus (51). We have confirmed the coregulation of cyclin E with p21cip1 proteins in a fibrosarcoma cell line, p21-9, which is devoid of HPV oncogenes (data not shown). Upon induction of p21cip1 from an inducible promoter, the cell cycle was halted while cyclin E mRNA remained unchanged (5). Thus, in some aspects, these cell lines are analogous to our E7 raft cultures except that, in our system, the transcription of both p21cip1 and p27kip1 was induced by squamous differentiation rather than by ectopic overexpression. In the raft cultures, the sequestration of cyclin E into inactive CKI-containing complexes (Fig. 5) not only blocked the entry into the S phase, as seen in cycling cells (26), but it also prevented the subsequent transcriptional inactivation of the E2F pathway by cyclin A (33). The failure of this feedback control then led to the continuous synthesis of cyclin E with concomitant stabilization of p21cip1, accounting for the unusually high amounts of both proteins in a subset of the differentiated keratinocytes.
It is also interesting that lactacystin did not stabilize cyclin E to a detectable level in any of the raft cultures, in sharp contrast to the stabilization of p21cip1 protein by lactacystin in all cells (Figs. 1A and 2A and D). Both free cyclin E and the form phosphorylated by cdk2 are rapidly degraded, but the bound unphosphorylated form is stable (49, 66, 74). Our observations are consistent with the interpretation that, in both normal and E7 cultures, cells capable of entering S phase and cells already in S phase express low amounts of cyclin E and, for a short period of time such that the protein levels remain undetectable, even after stabilization with a proteasome inhibitor. This situation is in contrast to the E7-transduced raft cultures in which most of cyclin E is already in stable complexes with cdk2 and p21cip1 or p27kip1 in cells prevented from S-phase reentry (Fig. 4 and 6). Thus, very little is targeted for degradation, accounting for the lack of effects by the proteasome inhibitor. Moreover, these results also suggest that E7 does not continuously deregulate cyclin E expression once the cells enter S phase. Thus, the function of E7 is to promote S-phase entry. In support of this latter conclusion, cyclin A was only observed in BrdU-positive cells in the E7 raft cultures (data not shown).
The most intriguing finding of our study is that endogenous p27kip1, another potent cdk2 inhibitor, appears to play an important role in HPV biology. Unlike p21cip1, p27kip1 has to be phosphorylated by cdk2 in order to be ubiquitinated and turned over (46, 47, 50, 55, 62, 65, 70). Several observations strongly suggest the accumulation of cyclin E-p21cip1 protein to be secondary to S-phase arrest caused by high levels of the endogenous p27kip1 protein.
First, we showed that p27kip1 protein was not observed in basal or parabasal cells but was easily detected in most differentiated cells in the presence or in the absence of E7 and also in patient laryngeal papilloma specimens (Fig. 6). This high stability explains why the proteasome inhibitor did not increase p27kip1 to any appreciable extent (Fig. 2A). Second, all p27kip1-positive cells, regardless of whether they also accumulated detectable levels of cyclin E and hence p21cip1 proteins, were negative for BrdU incorporation, whereas all cyclin E-positive cells were also positive for p27kip1 (Fig. 6) and p21cip1 (29). Thus, there are three populations of differentiated cells in E7 cultures and in patient specimens: those in S phase without detectable p27kip1, p21cip1, or cyclin E; those not in S phase but with detectable p27kip1 protein; and those not in S phase but with detectable levels of p27kip1, p21cip1, and cyclin E proteins. These observations show that the presence of p27kip1 is incompatible with S-phase reentry by the differentiated keratinocytes. This interpretation is supported by the observation that rottlerin, which caused a reduction in the distribution and signal strength of p21cip1 but not p27kip1 protein, did not increase or decrease the BrdU-positive cells in the differentiated strata (Fig. 7). Third, immunoprecipitation assays revealed that, as did p21cip1, p27kip1 sequestered a fraction of the E7-induced cyclin E (Figs. 6C). Moreover, despite a large increase in cyclin E relative to control cultures, the cdk2 or cyclin E immune precipitates from E7-expressing cultures had a low kinase (Fig. 5), strongly suggesting that the cdk2 kinase was inhibited by the associated p27kip1 or p21cip1. The observation that p27kip1 in the E7-expressing cultures did not decrease but may have slightly increased relative to the control cultures (Fig. 2A and 6A) also agrees with the low cdk2 kinase activities of the cdk2 complexes. Had E7-induced cyclin E exceeded that of the preexisting p27kip1 to constitute an active cyclin E-cdk2 complex, the kinase would have then phosphorylated p27kip1, causing its degradation. This is clearly not the case.
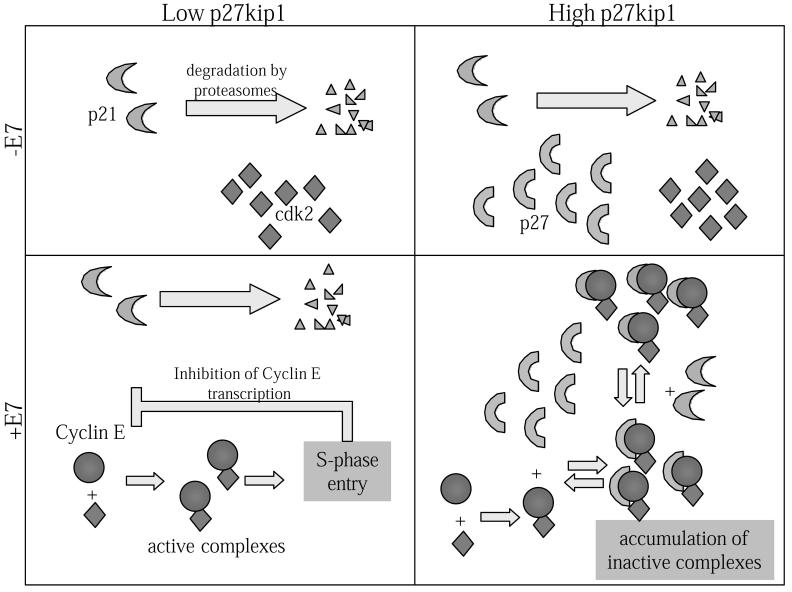
We conclude that, in differentiated keratinocytes in warty lesions and in raft cultures, the E7 protein cannot overcome the inhibitory effect of elevated p27kip1 or p21cip1 proteins, consistent with the report by Niculescu et al. on HeLa cells (51). This is probably because the amount of E7 is not sufficient to inactivate all the p27kip1 and p21cip1 through a stoichiometric mechanism. We propose a model to explain the three distinct cell populations in E7-expressing cells just described (Fig. 8). We surmise that during squamous differentiation, p27kip1 is stably synthesized in most of the postmitotic cells, regardless of the presence or absence of HPVs. p27kip1 then sequesters HPV E7-induced cyclin E in a stable and kinase-inactive complex with cdk2. At an early stage of arrest, the concentration of stabilized cyclin E is too low to be detected or to stabilize the p21cip1 protein, which is also low due to its instability in these cells. However, with time, as the amount of cyclin E increases, an equilibrium is established between p27kip and p21cip1 in complex with cyclin E/cdk2. Eventually, a significant fraction of cyclin E-cdk2 is in a complex with p21cip1, and both cyclin E and p21cip1 become detectable in situ. The sequestration of cyclin E-cdk2 into the kinase-inactive complexes containing either CKI then effectively inhibits or, at a minimum, significantly delays S-phase reentry by differentiated keratinocytes. However, in p27kip1-negative, postmitotic cells, E7-induced cyclin E is able to overcome the low steady-state level of p21cip1 protein to promote S-phase reentry. Since efficient initiation of HPV DNA replication also requires the activity of cyclin E-cdk2 (37, 41), the presence of elevated CKIs would then also inhibit viral DNA replication, accounting for the heterogeneous viral activities in benign patient lesions (9).
FIG. 8.
A model to account for the different populations of differentiated keratinocytes detected in the raft cultures containing HPV-18 URR-E7 and in patient papillomas. In differentiated keratinocytes, cdk2 is stably present. While p21cip1 protein is quickly degraded and below the threshold of in situ detection, the p27kip1 protein is detectable in most of the differentiated cells. In cells with undetectable levels of p27kip1 protein, E7-induced cyclin E easily overcomes the very low steady-state levels of p21cip1 protein and promotes S-phase reentry. Once in S phase, the transcription of cyclin E is turned off and there is no accumulation of either cyclin E or p21cip1 protein. In cells that contain high levels of p27kip1 protein, the E7-induced cyclin E is trapped in an inactive complex with cdk2 and p27kip1. When sufficient cyclin E accumulates, an equilibrium is established between cyclin E-cdk2-p27kip1 and cyclin E-cdk2-p21cip1 complexes. S-phase reentry is inhibited and the continuous expression of cyclin E eventually leads to the accumulation of cyclin E and p21cip1 proteins, in addition to p27kip1 in the same cell. This scenario accounts for the three populations observed in the differentiated strata of E7 raft cultures: those in S phase without detectable cyclin E or p21icp1; those not in S phase but with a high level of the p27kip1 protein; and those not in S phase and containing high levels of p27kip1, cyclin E, and p21cip1 proteins.
In summary, we have demonstrated that the differentiated keratinocytes contain an inherent mechanism that suppresses E7-induced DNA replication in postmitotic cells. p21cip1 or p27kip1 knockout mice indicate that neither of these CKIs is essential for achieving tissue differentiation (12, 17, 32, 48). Perhaps their purpose in differentiated cells is at least in part to prevent unscheduled S-phase reentry under unusual circumstances. In the case of HPV infections, viral propagation occurs in cells that have only low levels of the inhibitory CKIs. This attenuating process could work to the benefit of the long-term virus-host coexistence but can also be eventually exploited as a new alternative for treatment of papillomaviral diseases.
ACKNOWLEDGMENTS
This research was supported by U.S. PHS grant CA36200. The Digital Imaging Microscopy Facility was established with grant funds provided in large measure by the UAB Health Services Foundation and by grant DE/CA 11910.
We thank Ge Jin for tissue embedding and sectioning and Martha Hayes for performing mRNA in situ hybridization. We also thank Yue Xiong for providing p21cip1 cDNA and the antibody against cyclin E2, James Roberts for providing cyclin E cDNA, and Igor Roninson for the p21-9 cell line. We gratefully acknowledge the nurses in Cooper Green Hospital for collecting foreskins and Brian J. Wiatrak for collecting remnant laryngeal papillomas. Finally, we thank Wade Harper for critical discussion.
REFERENCES
- 1.Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997;16:5322–5333. doi: 10.1093/emboj/16.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1995. [Google Scholar]
- 3.Berezutskaya E, Yu B, Morozov A, Raychaudhuri P, Bagchi S. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 1997;8:1277–1286. [PubMed] [Google Scholar]
- 4.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 5.Chang B D, Watanabe K, Broude E V, Fang J, Poole J C, Kalinichenko T V, Roninson I B. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Jackson P K, Kirschner M W, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S, Schmidt-Grimminger D C, Murant T, Broker T R, Chow L T. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9:2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 8.Chien W M, Parker J N, Schmidt-Grimminger D C, Broker T R, Chow L T. Casein kinase II phosphorylation of the human papillomavirus-18 E7 protein is critical for promoting S-phase entry. Cell Growth Differ. 2000;11:425–435. [PubMed] [Google Scholar]
- 9.Chow L T, Broker T R. Small DNA tumor viruses. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 267–301. [Google Scholar]
- 10.Cueille N, Nougarede R, Mechali F, Philippe M, Bonne-Andrea C. Functional interaction between the bovine papillomavirus virus type 1 replicative helicase E1 and cyclin E-Cdk2. J Virol. 1998;72:7255–7262. doi: 10.1128/jvi.72.9.7255-7262.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datto M B, Li Y, Panus J F, Howe D J, Xiong Y, Wang X. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 13.Denning M F, Dlugosz A A, Williams E K, Szallasi Z, Blumberg P M, Yuspa S H. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6:149–157. [PubMed] [Google Scholar]
- 14.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 15.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 16.Fenteany G, Schreiber S L. Lactacystin, proteasome function, and cell fate. J Biol Chem. 1998;273:8545–8548. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]
- 17.Fero M L, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L H, Broudy V, Perlmutter R M, Kaushansky K, Roberts J M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 18.Flores-Rozas H, Kelman Z, Dean F B, Pan Z Q, Harper J W, Elledge S J, O'Donnell M, Hurwitz J. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc Natl Acad Sci USA. 1994;91:8655–8659. doi: 10.1073/pnas.91.18.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey M R, Saxon M L, Zhao X, Rollins A, Evans S S, Black J D. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21 (waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem. 1997;272:9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- 20.Funk J O, Waga S, Harry J B, Espling E, Stillman B, Galloway D A. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11:2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 22.Gschwendt M, Muller H J, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 23.Gudas J M, Payton M, Thukral S, Chen E, Bass M, Robinson M O, Coats S. Cyclin E2, a novel G1 cyclin that binds Cdk2 and is aberrantly expressed in human cancers. Mol Cell Biol. 1999;19:612–622. doi: 10.1128/mcb.19.1.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 25.Harbour J W, Luo R X, Santi A D, Postigo A A, Dean D C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 26.Hengst L, Gopfert U, Lashuel H A, Reed S I. Complete inhibition of Cdk/cyclin by one molecule of p21 (Cip1) Genes Dev. 1998;12:3882–3888. doi: 10.1101/gad.12.24.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jallepalli P V, Kelly T J. Cyclin-dependent kinase and initiation at eukaryotic origins: a replication switch? Curr Opin Cell Biol. 1997;9:358–363. doi: 10.1016/s0955-0674(97)80008-7. [DOI] [PubMed] [Google Scholar]
- 28.Jian Y, Schmidt-Grimminger D C, Chien W M, Wu X, Broker T R, Chow L T. Post-transcriptional induction of p21cip1 protein by human papillomavirus E7 inhibits unscheduled DNA synthesis reactivated in differentiated keratinocytes. Oncogene. 1998;17:2027–2038. doi: 10.1038/sj.onc.1202142. [DOI] [PubMed] [Google Scholar]
- 29.Jian Y, Van Tine B A, Chien W M, Shaw G M, Broker T R, Chow L T. Concordant induction of cyclin E and p21cip1 in differentiated keratinocytes by the human papillomavirus E7 protein inhibits cellular and viral DNA synthesis. Cell Growth Differ. 1999;10:101–111. [PubMed] [Google Scholar]
- 30.Jones D L, Alani R M, Münger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11:2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones D L, Thompson D A, Münger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 32.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 33.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G J, Livingston D M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 34.Lauper N, Beck A R, Cariou S, Richman L, Hofmann K, Reith W, Slingerland J M, Amati B. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637–2643. doi: 10.1038/sj.onc.1202477. [DOI] [PubMed] [Google Scholar]
- 35.Leng X, Connell-Crowley L, Goodrich D, Harper J W. S-phase entry upon ectopic expression of G1 cyclin-dependent kinases in the absence of retinoblastoma protein phosphorylation. Curr Biol. 1997;7:709–712. doi: 10.1016/s0960-9822(06)00301-0. [DOI] [PubMed] [Google Scholar]
- 36.Leone G, DeGregori J, Jakoi L, Cook J G, Nevins J R. Collaborative role of E2F transcriptional activity and G1 cyclin dependent kinase activity in the induction of S phase. Proc Natl Acad Sci USA. 1999;96:6626–6631. doi: 10.1073/pnas.96.12.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin B Y, Ma T, Liu J S, Kuo S R, Jin L, Broker T R, Harper J W, Chow L T. HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication. J Biol Chem. 2000;275:6167–6174. doi: 10.1074/jbc.275.9.6167. [DOI] [PubMed] [Google Scholar]
- 38.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 39.Luo R X, Postigo A A, Dean D C. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 40.Ma T, Van Tine B A, Yue W, Garrett M, Nelson D, Adams P D, Wang J, Chow L T, Harper J W. Cell cycle-regulated phosphorylation of p220NPAT by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 2000;14:2298–2313. doi: 10.1101/gad.829500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma T, Zou N, Lin B Y, Chow L T, Harper J W. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc Natl Acad Sci USA. 1999;96:382–387. doi: 10.1073/pnas.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 43.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 44.Masai H, Matsui E, You Z, Ishimi Y, Tamai K, Arai K. Human Cdc7-related kinase complex. In vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a critical threonine residue of Cdc7 by Cdks. J Biol Chem. 2000;275:29042–29052. doi: 10.1074/jbc.M002713200. [DOI] [PubMed] [Google Scholar]
- 45.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–982. [PMC free article] [PubMed] [Google Scholar]
- 46.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G F, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morisaki H, Fujimoto A, Ando A, Nagata Y, Ikeda K, Nakanishi M. Cell cycle-dependent phosphorylation of p27 cyclin-dependent kinase (Cdk) inhibitor by cyclin E/Cdk2. Biochem Biophys Res Commun. 1997;240:386–390. doi: 10.1006/bbrc.1997.7590. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama K, Nagahama H, Minamishima Y A, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Nakayama K, Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen H, Gitig D M, Koff A. Cell-free degradation of p27kip1, a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteasome. Mol Cell Biol. 1999;19:1190–1201. doi: 10.1128/mcb.19.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niculescu A B, Chen X, Smeets M, Hengst L, Prives C, Reed S I. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh N H, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtani K, DeGregori J, Nevins J R. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 56.Parker J N, Zhao W, Askins K J, Broker T R, Chow L T. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 1997;8:751–762. [PubMed] [Google Scholar]
- 57.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledge S J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 58.Ruesch M N, Laimins L A. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J Virol. 1997;71:5570–5578. doi: 10.1128/jvi.71.7.5570-5578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruesch M N, Laimins L A. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology. 1998;250:19–29. doi: 10.1006/viro.1998.9359. [DOI] [PubMed] [Google Scholar]
- 60.Santoni-Rugiu E, Falck J, Mailand N, Bartek J, Lukas J. Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol Cell Biol. 2000;20:3497–3509. doi: 10.1128/mcb.20.10.3497-3509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt-Grimminger D C, Wu X, Jian Y, Broker T R, Chow L T. Post-transcriptional induction of p21cip1 protein in condylomata and dysplasias is inversely related to human papillomavirus activities. Am J Pathol. 1998;152:1015–1024. [PMC free article] [PubMed] [Google Scholar]
- 62.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 63.Sheaff R J, Singer J D, Swanger J, Smitherman M, Clurman B E. Proteasomal turnover of p21(Cip1) does not require p21(Cip1) ubiquitination. Mol Cell. 2000;5:403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- 64.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 65.Shirane M, Harumiya Y, Ishida N, Hirai A, Miyamoto C, Hatakeyama S, Nakayama K, Kitagawa M. Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J Biol Chem. 1999;274:13886–13893. doi: 10.1074/jbc.274.20.13886. [DOI] [PubMed] [Google Scholar]
- 66.Singer J D, Gurian-West M, Clurman B, Roberts J M. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 1999;13:2375–2387. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stacey S N, Jordan D, Williamson A J K, Brown M, Coote J H, Arrand J R. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J Virol. 2000;74:7284–7297. doi: 10.1128/jvi.74.16.7284-7297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steenbergen R D, Parker J N, Isern S, Snijders P J, Walboomers J M, Meijer C J, Broker T R, Chow L T. Viral E6–E7 transcription in the basal layer of organotypic cultures without apparent p21cip1 protein precedes immortalization of human papillomavirus type 16- and 18-transfected human keratinocytes. J Virol. 1998;72:749–757. doi: 10.1128/jvi.72.1.749-757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas M, Pim D, Banks L. The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 70.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voitenleitner C, Rehfuess C, Hilmes M, O'Rear L, Liao P C, Gage D A, Ott R, Nasheuer H P, Fanning E. Cell cycle-dependent regulation of human DNA polymerase alpha-primase activity by phosphorylation. Mol Cell Biol. 1999;19:646–656. doi: 10.1128/mcb.19.1.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waga S, Hannon G J, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 73.Wang K K, Nath R, Posner A, Raser K J, Buroker-Kilgore M, Hajimohammadreza I, Probert A W J, Marcoux F W, Ye Q, Takano E, Hatanaka M, Maki M, Caner H, Collins J L, Fergus A, Lee K S, Lunney E A, Hays S J, Yuen P. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci USA. 1996;93:6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winston J T, Chu C, Harper J W. Culprits in the degradation of cyclin E apprehended. Genes Dev. 1999;13:2751–2757. doi: 10.1101/gad.13.21.2751. [DOI] [PubMed] [Google Scholar]
- 75.Zariwala M, Liu J, Xiong Y. Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene. 1998;17:2787–2798. doi: 10.1038/sj.onc.1202505. [DOI] [PubMed] [Google Scholar]
- 76.Zehbe I, Ratsch A, Alunni-Fabbroni M, Burzlaff A, Bakos E, Durst M, Wilander E, Tommasino M. Overriding of cyclin-dependent kinase inhibitors by high and low risk human papillomavirus types: evidence for an in vivo role in cervical lesions. Oncogene. 1999;18:2201–2211. doi: 10.1038/sj.onc.1202549. [DOI] [PubMed] [Google Scholar]
- 77.Zeng Y X, El-Deiry W S. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene. 1996;12:1557–1564. [PubMed] [Google Scholar]
- 78.Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz J W, Jansen-Dürr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13:2323–2330. [PubMed] [Google Scholar]
- 79.Zerfass K, Schulze A, Spitkovsky D, Friedman V, Henglein B, Jansen-Dürr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J Virol. 1995;69:6389–6399. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zezula J, Sexl V, Hutter C, Karel A, Schutz W, Freissmuth M. The cyclin-dependent kinase inhibitor p21cip1 mediates the growth inhibitory effect of phorbol esters in human venous endothelial cells. J Biol Chem. 1997;272:29967–29974. doi: 10.1074/jbc.272.47.29967. [DOI] [PubMed] [Google Scholar]
- 81.Zhao J, Kennedy B K, Lawrence B D, Barbie D A, Matera A G, Harlow E. NPAT links cyclin E-Cdk2 to the regulation of replication-dependent histone gene transcription. Genes Dev. 2000;14:2283–2297. [PMC free article] [PubMed] [Google Scholar]
- 82.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 83.Zwerschke W, Jansen-Dürr P. Cell transformation by the E7 oncoprotein of human papillomavirus type 16: interactions with nuclear and cytoplasmic target proteins. Adv Cancer Res. 2000;78:1–29. doi: 10.1016/s0065-230x(08)61022-2. [DOI] [PubMed] [Google Scholar]