Abstract
Background/Objectives: S-adenosylmethionine (SAMe) is a natural compound used to improve mood-related symptoms. Our aim was to determine the efficacy, safety, and optimal dose of SAMe in Central Nervous System (CNS) signs (e.g., mood, behavior). Methods: We conducted a PRISMA-based systematic review by searching PubMed, CINAHL, and Web of Science using MeSH search terms. Articles were independently reviewed by two researchers (with a third resolving conflicts) during title/abstract screening and full-text review. Data were extracted in the same approach, with a quality assessment of included articles. Results: Out of 1881 non-duplicated studies, 36 were included in the review focusing on CNS signs (mood, behavior, sleep). Most studies (n = 32) achieved a 4 or 5 out of 5 points, indicating high study quality. Overall, SAMe was effective in 24 of 36 studies, with adverse events mostly consisting of mild, transient gastrointestinal disturbances. Conclusions: Many patients in these studies did experience improvements in CNS signs from using SAMe alone or in combination with existing therapy. However, future studies are needed to further understand the long-term effects of SAMe in the CNS.
Keywords: S-adenosylmethionine, central nervous system
1. Introduction
Mental health conditions are common, with a global burden of one in eight people affected according to the World Health Organization (WHO) [1,2]. Since the COVID-19 pandemic, the prevalence of depression and anxiety have risen approximately 28% and 26%, respectively [3]. Furthermore, the pandemic disproportionally affected females and younger individuals related to these central nervous system (CNS) conditions [3], yet the efficacy of standard treatment is suboptimal. The Mental Health America (MHA) 2024 report found that only 36% of youth with at least one major depressive episode said treatment helped “a lot” while 65% said it helped “some” [4]. Additionally, many agents used to treat CNS conditions may cause several adverse effects, such as weight gain, nausea/vomiting, and sexual dysfunction. Therefore, there is a need for better treatment options that are both safe and effective in improving patient outcomes.
S-adenosylmethionine (SAMe) is a nutraceutical marketed for its potential beneficial effects in several areas of the body, including the CNS. SAMe is produced in the liver from L-methionine and adenosine triphosphate (ATP) and is known for its role as a methyl donor in a variety of biological processes [5]. Some of these include DNA and RNA gene expression and neurotransmitter secretion, including dopamine, norepinephrine, and serotonin, which help elevate mood and support cognitive processes [5,6]. The replenishment of depleted neurotransmitters in CNS signs, like major depressive disorder, is important; however, the beneficial effects of SAMe may also be due to its anti-inflammatory properties. This may be explained, in part, by the ability of SAMe to synthesize glutathione, which aids in cellular detoxification through removal of free radicals [6].
The beneficial effects of SAMe on depression and other mood disorders are not fully established. However, low levels of SAMe have been reported in patients with major depressive disorder (MDD), while higher levels may lead to symptom improvement. Furthermore, as mentioned above, its role in the regulation of glutathione, polyamines, and monoaminergic neurotransmitters are just a few of the ways it may affect important processes in the brain [7]. Nevertheless, reports of SAMe use in CNS signs have varying results [8,9,10]. An updated review of the literature is needed to understand how SAMe affects specific conditions in the CNS and how these may inform dosing. Therefore, we conducted a systematic review to evaluate the safety, efficacy, and optimal dose of SAMe in CNS signs (e.g., mood, behavior, depression, anxiety, etc.).
2. Methods
PRISMA methodology was used for this systematic review, which the researchers followed in full compliance (see PRISMA Checklist in the Supplementary Materials for full details) [11]. In collaboration with a research librarian, an initial search strategy was identified to address the research questions using the following MeSH search terms: “S-Adenosylmethionine” AND “Central Nervous System” OR “Behavioral Symptoms” OR “Anxiety” OR “Mood Disorders” OR “Sleep”. Based on these search terms, a research librarian refined the search in different search engines to maximize the accuracy and yield of the search in three databases: PubMed, CINAHL, and Web of Science. After confirmation from the research team, the research librarian finalized the search and ran the search with the dates of: 1 January 2004 to 17 April 2024. The search results were cleaned in Zotero (removing two retracted articles) and uploaded into Covidence (Melbourne, Australia). Covidence removed any duplicates identified.
Then, the research team began the review process. Three student research assistants and one faculty member were trained on the study protocol and Covidence system by the senior investigator (AC). The senior investigator also served as the project coordinator, checked for consistencies at each step, and resolved all disagreements and conflicts at each stage. All three phases were conducted in Covidence.
In the first phase, titles and abstracts were screened according to the (1) inclusion and exclusion criteria and (2) content. Inclusion criteria were as follows: research studies (randomized controlled trials, clinical trials, case studies or reports, cohort studies, prospective studies), English-language, and human studies. Exclusion criteria were as follows: review articles, guidelines, and expert opinion. Systematic reviews or meta-analyses also were excluded but were reviewed for any relevant articles. Secondly, articles were reviewed for content: all articles must include SAMe and a relevant CNS condition (mood, behavior, or sleep). Two research team members independently screened all titles and abstracts, categorizing them as “Yes”—meeting inclusion criteria, “No”—not meeting inclusion criteria, or “Maybe”—may meet inclusion criteria but needs further review. Any studies that were selected yes and/or maybe by two team members moved to full-text review. Any studies that were selected as no by two team members were excluded. Conflicts were resolved by a third researcher (AC).
In the second phase, full texts of all articles were pulled, read, and examined based on (1) criteria and (2) content. The same process with two independent reviewers and a third reviewer managing conflicts were followed. In this phase, the reason for exclusion was documented using a pre-specified list associated with the (1) criteria and (2) content. Any studies that were selected as “no” by two team members were excluded. All articles with “yes” were moved to the data-extraction phase.
In the third phase, a data-extraction template was built and utilized in Covidence to collect data to address the research questions. Reviewers also examined the quality of the study using the Mixed Methods Appraisal Tool (MMAT) (version 2018) [12], which allows for many different study designs to be evaluated for quality. Two research team members independently extracted the data and performed the quality appraisal, with the senior investigator reviewing all articles, resolving conflicts, and finalizing the data extraction into study tables. In addition, any missing data were noted in these tables. All research team members agreed on the final tables.
3. Results
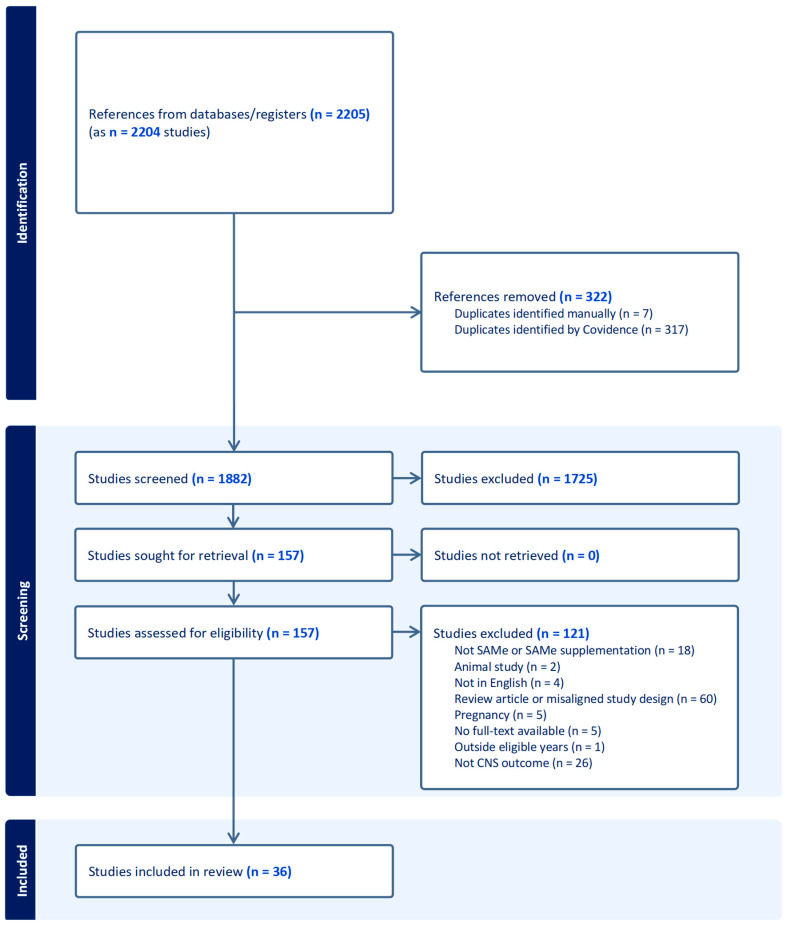
A total of 2207 articles were pulled from the search (PubMed = 521, CINAHL = 173, Web of Science = 1513). After clean-up and removal of duplicates, 1882 articles underwent the review process. The PRISMA (Figure 1) overviews the study process, resulting in 36 articles that underwent data extraction.
Figure 1.
PRISMA [11] diagram overviewing study inclusion and exclusion process.
Study Characteristics
The total number of participants across the 36 articles [5,7,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] was 1799 (not including any systematic reviews). In the studies that specified gender, 60.1% of participants were female. The median study length was 8 weeks.
CNS-related signs included depression (general, mild, moderate, resistant, bipolar, non-remittent, subthreshold), anxiety, mood, suicide attempt, ADHD, cognitive deficits, 22q11.2 deletion syndrome depressive disorder, creatine transporter deficiency, and schizophrenia. Assessments used to determine efficacy included various mood-related tests, EEG mapping and psychometry, MRI, and plasma SAMe levels.
Table 1 provides a summary of the outcomes. When used in CNS-related conditions, SAMe dosing ranged from 200 to 3200 mg, with the most common dose being 800 mg. In many studies, SAMe was titrated up to the target dose and/or divided into two to four doses daily.
Table 1.
Overview of study findings related to safety and efficacy.
| Condition | Efficacy Summary | Safety Summary | Dosing Ranges |
|---|---|---|---|
| CNS-Related Signs |
|
|
|
1 The case reports did not use a Naranjo scale or other validated assessments to examine the likelihood of the cause and effect. 2 This indicates the variability of assessing change in depressive symptoms.
Table 2 provides a full description of remaining study characteristics, including the specific CNS sign studied, intervention(s), and measurements used. For the intervention, comparators were only included if applicable. These comparators included placebo, a different SAMe dose, an antidepressant with or without a placebo, or a different nutraceutical product (e.g., UP165).
Table 2.
Characteristics of included studies examining the role of SAMe in CNS Signs.
| First Author (Year) Study Design|Location N of Patients|Study Length |
Intervention (with Dose) and Comparator | Disease (Sign) | Measurement of Mood/Depression |
|---|---|---|---|
| Abeysundera (2018) [13] Case report|Australia n = 1|2 weeks prior to incident |
No dose given | Depression | Differential diagnosis and lab levels |
| Alpert (2004) [14] Open trial|United States n = 30|6 weeks |
SSRI/Venlafaxine + SAMe: Initial: 400 mg twice daily At 2 weeks: 800 mg twice daily Comparator: None |
Resistant Major Depressive Disorder |
HAM-D–17, MADRS, CGI-I, CGI-S, SQ |
| Anderson (2016) [15] Case report|Canada n = 1|~1 month |
SAMe: 400 mg twice daily Comparator: None |
Anxiety (and hypothyroidism) | Not discussed |
| Arnold (2005) [16] Pharmacodynamic|Europe n = 12|15 days each medication + washout periods |
SAMe: 1600 mg/day SAMe: 400 mg/day Comparator: placebo |
Mood | EEG mapping and psychometry |
| Bambling (2015) [17] RCT|Australia n = 36|15 weeks |
SAMe: 1600 mg/day SAMe: 800 mg/day |
Major Depressive Disorder | BDI, ICD-DSM MINI, DASS, SCID, OQ45, WBS, QOLS |
| Carpenter (2011) [18] SR/MA|United States n = 14 studies on SAMe|N/A |
SAMe: 500–1050 mg/day | Major Depressive Disorder | Varied |
| Chitiva (2012) [19] Case report|United States n = 1|4 days prior to event |
No dose stated | Depression/suicide attempt | Not applicable |
| Cuomo (2020) [7] SR/MA|N/A n = 8 articles (1011 patients)|N/A |
SAMe: 200–3200 mg/day | Major Depressive Disorder | Varied |
| De Berardis (2013) [20] Non-randomized experimental|Europe n = 25|8 weeks |
Existing medication + SAMe: 800 mg/day | Major Depressive Disorder | HAM-D, CGI-I, SHAPS, SDS |
| Di Pierro (2015) [21] Open-label, randomized, observational|Europe n = 64 (60 completed)|12 months |
Betaine 250 mg/day + SAMe: 500 mg/day Comparator: Amitriptyline 75 mg/day | Mild Depression | Zung Self-Rating Depression Scale |
| Djokic (2017) [22] RCT|Europe n = 60|3 months |
Vit B complex + SAMe: 200 mg/day Comparator: placebo |
Depression (mild to moderate) | HAM-D, CGI-S, CGI-I |
| Dolcetta (2013) [23] Non-randomized experimental|Europe n = 14|12 months |
SAMe: 400–1600 mg/day; up to 80 mg/kg, depending on body weight and renal function | Mood Lesch–Nyhan Disease |
N/A |
| Galizia (2016) [24] SR/MA|UK n = 8 studies (934 patients)|N/A |
SAMe: 200–3200 mg/day | Depression | Varies |
| Green (2012) [25] RCT|Israel n = 12|6 weeks |
SAMe: 400 mg/day titrated up to 1600 mg/day (800 mg twice daily) Comparator: placebo |
22q11.2 deletion syndrome: depressive disorder, ADHD, cognitive deficits | Wechsler test: IQ, PANSS, YMRS, CGI-I, CDRS-R, ADHD-RS |
| Jaggumantri (2015) [26] Case report|Canada n = 2|Not described fully |
SAMe: 50 mg/kg, with a safe and tolerable dose identified as 17 mg/kg/day | Creatine transporter (SLC6A8) deficiency | MRI Various assessments and questionnaires |
| Kalman (2015) [27] RCT|United States n = 34 in efficacy analysis (out of 42 enrolled)|8 weeks |
SAMe: 400 mg/day Comparator: UP165 250 mg/day |
Mild depression or anxiety | BDI-II, BAI, SOS-10 |
| Levkovitz (2012) [28] RCT Re-Analysis|United States n = 55|6 weeks |
SAMe Weeks 1–2: 800 mg/day Weeks 3–6: 1600 mg/day |
Major Depressive Disorder | CPFQ |
| Limveeraprajak (2024) [5] SR/MA|N/A 23 trials (n = 2183)|N/A |
SAMe: 200–1600 mg/day | Depressive symptoms | Varied |
| Mischoulon (2012) [29] RCT|United States n = 35|6 weeks |
SAMe: 800–1600 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D Plasma SAMe levels |
| Mischoulon (2014) [30] RCT|United States n = 189|12 weeks |
SAMe: 1600–3200 mg/day Escitalopram: 10–20 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D |
| Murphy (2014) [31] RCT|United States n = 20 (17 completed)|6 weeks |
SAMe: Week 1: 800 mg/day Week 2: 400 mg/day Week 3: 800 mg/day Week 4: 1600 mg (only 3/7 days of week) | Persistent Treatment-Refractory Bipolar Depression | HAM-D, MADRS, YMRS |
| Olsufka (2017) [32] Case Report|United States n = 1|~1 week |
SAMe: 400 mg/day for 3 days then increased to 800 mg/day (up to day 10) | Depression | Not applicable |
| Papakostas (2010) [33] RCT|United States n = 73 (55 completed)|6 weeks |
Antidepressant + SAMe: 800 mg/day (up to 1600 mg/day) Comparator: antidepressant + placebo |
Major Depressive Disorder | HAM-D, CGI-S |
| Peng (2024) [34] SR/MA|Taiwan n = 14 studies (1522 patients)|N/A |
SAMe: 200–3200 mg/day | Depression | Varies |
| Saccarello (2020) [35] RCT|Europe n = 89|6 weeks |
Lactobacillus plantarum + SAMe: 200 mg/day Comparator: placebo |
Mild-to-moderate depression | Zung Self-Rating Depression Scale |
| Sakurai (2020) [36] RCT|United States n = 189|6 weeks |
SAMe: 1600 mg/day for 6 weeks (non-responders: 3200 mg/day for 6 weeks) Escitalopram: 10 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D, IDS-SR, CGI-S, CGI-I |
| Sarris (2014) [40] RCT|Australia n = 144|12 weeks |
SAMe: 1600–3200 mg/day Escitalopram: 10 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D |
| Sarris (2015) [41] RCT Re-Analysis|United States n = 189|12 weeks |
SAMe: 1600–3200 mg/day Escitalopram: 10–20 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D |
| Sarris (2018) [37] RCT|Australia n = 107 (77 completed)|8 weeks |
SAMe: 800 mg/day Comparator: placebo |
Non-remittent Major Depressive Disorder | MADRS |
| Sarris (2019) [38] RCT|Australia n = 158 | 8 weeks |
SAMe 800 mg + folinic acid + Omega-3 fatty acids + 5-HTP + Zinc picolinate + relevant co-factors/day Comparator: placebo |
Major Depressive Disorder | MADRS |
| Sarris (2020) [39] RCT|Australia n = 49 (41 completed)|8 weeks |
SAMe: 800 mg/day Comparator: placebo |
Major Depressive Disorder with mild-to-moderate symptoms | MADRS |
| Shippy (2004) [42] Non-randomized experimental study|United States n = 20 (15 completed)|8 weeks |
1000 μg Vit B12 + 800 mg Folic Acid + SAMe: 400 mg/day (200 mg bid) increased to 1600 mg/day (800 mg bid) Comparator: None |
Major Depressive Disorder | HAM-D (Response: ≥50% reduction in scores; Remission: HAM-D ≤ 7) |
| Strous (2009) [43] RCT|Israel n = 18 (15 completed)|8 weeks |
SAMe: Week 1: 400 mg/day Weeks 2–8: 800 mg/day Comparator: placebo |
Schizophrenia | PANSS, SANS, CGI, OAS, LHA, QLS |
| Targum (2018) [44] RCT|United States n = 234|8 weeks |
SAMe:800 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D, MADRS, IDS-SR |
| Targum (2020) [45] RCT (re-analysis)|United States n = 336|8 weeks |
SAMe:800 mg/day Comparator: placebo |
Major Depressive Disorder | HAM-D, MADRS, IDS-SR, CGI-S |
| Ullah (2022) [46] RCT (Crossover)|Europe n = 80 (65 completed)|3 months each |
Crossover between: 200 mg/day SAMe + lactobacillus and placebo |
Subthreshold depression Mild-to-moderate depression |
HAM-D, PHQ-9 |
Scales: Hamilton Rating Scale for Depression (HAM-D), Beck Depression Inventory (BDI) and BDI Version II (BDI-II), Mini International Neuropsychiatric Interview (ICD-DSM MINI), Depression Anxiety Stress Scale (DASS), Structured Clinical Interview for DSM (SCID), Outcome Questionnaire 45 (OQ45), Warwick–Edinburgh Mental Well-being Scale (WBS), Quality of Life Scale (QOLS), Clinical Global Impression of Improvement (CGI-I), Clinical Global Impression–Severity scale (CGI-S), Kellner Symptom Questionnaire (SQ), Snaith–Hamilton Pleasure Scale (SHAPS), Sheehan Disability Scale (SDS), Positive and Negative Syndrome Scale (PANSS), Young Mania Rating Scale (YMRS), Children’s Depression Rating Scale–Revised (CDRS-R); ADHD Rating Scale IV (ADHD-RS), Beck Anxiety Inventory (BAI), Schwartz Outcome Scale (SOS-10), cognitive and physical symptoms questionnaire (CPFQ), Montgomery–Asberg Depression Rating Scale (MADRS), Inventory of Depressive Symptomatology–Self Rated (IDS-SR), Calgary Scale for Depression in Schizophrenia (SANS), Overt Aggression Scale (OAS), Life History of Aggression Scale (LHA), Quality of Life Scale (QLS), Patient Health Questionnaire-9 (PHQ-9) Other Acronyms: Systematic Review (SR), Meta-Analysis (MA), Randomized Controlled Trial (RCT), Attention Deficit/Hyperactivity Disorder (ADHD).
Of the studies included, 33 assessed mood and depression (1 bipolar depression), 2 assessed anxiety, 1 assessed ADHD and cognitive deficits, and 1 assessed schizophrenia. Dosing strategies differed between CNS signs. More specifically, doses used for anxiety were 400 mg once or twice daily, 400 mg daily titrated to 800 mg twice daily for ADHD, and 400 mg daily titrated to 800 mg daily for schizophrenia. For depression, SAMe was dosed between 200 and 3200 mg daily, with studies titrating from lower to higher doses over a period of 3 days, 1 week, and 2 weeks.
The most common measurements of mood were versions of HAM-D (n = 14 studies), versions of CGI (n = 8 studies), and MADRS (n = 7 studies).
The compiled study outcomes for efficacy can be seen in Table 3 and safety in Table 4. If assessed by the study, all efficacy and safety data related to SAMe use were included. A total of 33 studies examined efficacy outcomes, while 29 studies included safety outcomes.
Table 3.
Study efficacy outcomes for studies examining the use of SAMe in CNS signs.
| First Author (Year) | Efficacy |
|---|---|
| Alpert (2004) [14] | SAMe: Intent-to-treat analyses based on the HAM-D
|
| Anderson (2016) [15] |
|
| Arnold (2005) [16] |
|
| Bambling (2015) [17] | 1600 mg and 800 mg SAMe were effective:
OQ45 change: significant reduction in functional distress scores [df = 18; p < 0.001] QOL change: significant increase in scores [df = 18; p < 0.001] |
| Carpenter (2011) [18] | Positive Results in Mild-to-Moderate (n = 9 studies):
|
| Cuomo 2020 [7] |
|
| De Berardis (2013) [20] |
|
| Di Pierro (2015) [21] | No improvement for either group at 3 months Effectiveness demonstrated at 6 and 12 months for both groups SAMe vs. amitriptyline:
|
| Djokic (2017) [22] | Significant differences between SAMe and placebo in HAM-D and CGI-S scores at 3 months (p < 0.001) In SAMe:
|
| Dolcetta (2013) [23] | 4 patients tolerated the full dose and demonstrated efficacy:
|
| Galizia (2016) [24] |
|
| Green (2012) [25] |
|
| Jaggumantri (2015) [26] | Patient 1:
|
| Kalman (2015) [27] | SAMe significantly:
|
| Levkovitz (2012) [28] | SAMe:
|
| Limveeraprajak (2024) [5] | SAMe superior to placebo (SMD = −0.58, 95% CI = −0.93 to −0.23, I2 = 68%), even when two trials with a high risk of bias were excluded (SMD = −0.61, 91% CI = −1.05 to −0.17, I2 = 74%)
|
| Mischoulon (2012) [29] | SAMe:
|
| Mischoulon (2014) [30] | SAMe vs. escitalopram vs. placebo:
|
| Murphy (2014) [31] | SAMe vs. placebo:
|
| Papakostas (2010) [33] | SAMe + antidepressant vs. placebo + antidepressant:
|
| Peng (2024) [34] | SAMe vs. placebo:
|
| Saccarello (2020) [35] | SAMe + Lactobacillus plantarum vs. placebo:
|
| Sakurai (2020) [36] | SAMe vs. escitalopram vs. placebo:
|
| Sarris (2014) [40] | SAMe vs. escitalopram vs. placebo:
Remission rates (HAM-D < 7): Significantly different between groups (χ22,102 = 8.57; p = 0.014)
|
| Sarris (2015) [41] | SAMe vs. escitalopram vs. placebo in HAM-D:
|
| Sarris (2018) [37] | SAMe + antidepressant vs. placebo + antidepressant:
|
| Sarris (2019) [38] | Nutraceutical product vs. placebo:
|
| Sarris (2020) [39] | SAMe:
|
| Shippy (2004) [42] |
|
| Strous (2009) [43] | SAMe vs. placebo: Significant improvements in SAMe patients only:
|
| Targum (2018) [44] | SAMe + antidepressant or placebo + antidepressant: No statistically significant treatment differences Note: study did not achieve primary endpoint due to subject selection differences First half of the study participants: favored SAMe
|
| Targum (2020) [45] | MADRS and HAM-D: SAMe was significantly better than placebo (F = 6.39; df = 1; p = 0.012), effect size = 0.404 |
| Ullah (2022) [46] |
|
Acronyms: Standardized Mean Difference (SMD).
Table 4.
Study safety outcomes for studies examining the use of SAMe in CNS signs.
| First Author (Year) | Safety |
|---|---|
| Abeysundera (2018) [13] | Conclusion was that the patient experienced substance-/medication-induced mood disorder (when adding SAMe to the SSRI) |
| Alpert (2004) [14] | GI and headache side effects were most common No significant changes in weight, folate, B12, or homocysteine levels |
| Arnold (2005) [16] | Good tolerability |
| Bambling (2015) [17] | 10 subjects dropped out |
| Carpenter (2011) [18] |
|
| Chitiva (2012) [19] | Patient attempted suicide after taking SAMe for 4 days |
| Cuomo 2020 [7] | Mild, transient, non-relevant side effects |
| De Berardis (2013) [20] | SAMe was well tolerated Most common adverse events:
|
| Di Pierro (2015) [21] | SAMe group had fewer side effects |
| Dolcetta (2013) [23] | Excess of excitement experienced at lower dosage, which led to discontinuations Increase in anxiety (n = 7) |
| Green (2012) [25] | No manic or psychotic symptoms No significant differences in side effects between groups Most common side effects were GI symptoms |
| Jaggumantri (2015) [26] | Patient 1:
|
| Kalman (2015) [27] | No significant adverse events |
| Limveeraprajak (2024) [5] | Generally well-tolerated |
| Mischoulon (2014) [30] | No significant differences in side effects between groups (p > 0.05) SAMe: GI, stomach discomfort, diarrhea |
| Murphy (2014) [31] | Discontinued after the 800 mg/day SAMe dosage due to brief episode of auditory hallucinations (n = 1) No other issues, including mania |
| Olsufka (2017) [32] | Treatment-emergent hypomania due to use of SAMe
|
| Papakostas (2010) [33] | No serious adverse events |
| Peng (2024) [34] | No significant difference between dropouts due to adverse effects (RR: 0.92, 95% CI: 0.49 to 1.73) |
| Saccarello (2020) [35] | Limited adverse events, which researchers believed were not related to products |
| Sakurai (2020) [36] | 3200 mg/day SAMe:
|
| Sarris (2014) [40] | Well-tolerated No significant adverse events |
| Sarris (2018) [37] | 5 SAMe group withdrawals possibly related to treatment: nausea, heightened anxiety, sleep issues |
| Sarris (2019) [38] |
|
| Sarris (2020) [39] | No significant differences in adverse events between groups (p = 0.53) |
| Shippy (2004) [42] | No dropouts due to side effects |
| Strous (2009) [43] | 3 patients were discontinued from the study due to potential adverse effects of the study medication No significant differences between SAMe and placebo for all adverse events (all p > 0.05) |
| Targum (2018) [44] | High completion rate with 113 SAMe-assigned subjects (95.8%) Predominant adverse events were mild and primarily related to GI tract (<2% of patients) |
| Targum (2020) [45] | SAMe well-tolerated Predominant adverse events were mild and primarily related to GI tract |
Improvements in scales or measurements of mood/CNS signs were seen in 24 studies. For studies that utilized HAM-D to assess SAMe, response rates were seen in up to 74% of participants and remission rates were seen in up to 93%. Six studies using the CGI tool (https://ia800200.us.archive.org/19/items/ecdeuassessmentm1933guyw/ecdeuassessmentm1933guyw_bw.pdf, accessed on 22 August 2024) found significant improvement with SAMe on these scores, while two studies showed no significant difference. For the MADRS tool, five studies reported that SAMe had no significant improvement, while the other two studies showed that SAMe had significant improvement.
Studies evaluating safety reported minimal adverse events, with gastrointestinal (GI) complaints identified as the most common (n = 10). In general, GI disturbances were reported as mild to moderate and transient. No studies reported severe GI side effects. Other adverse events reported were excitation, increased anxiety, mania/hypomania, sleep disruption, behavioral changes, headache, and fluid retention. One case report described an instance of medication-induced mood disorder in a patient taking an SSRI and SAMe combination. Another case report documented an attempted suicide. Nine studies reported no significant differences in adverse effects between SAMe and comparator, and one study reported fewer adverse effects in the SAMe group.
Study quality assessments can be found in Table 5. Most studies (n = 32) achieved 4 or 5 out of 5 points, indicating high study quality. A few studies (n = 4) had lower quality due to incomplete information as case reports, but they did not score any lower than 3 out of 5 points.
Table 5.
Quality assessment of CNS studies (n = 36 studies).
| First Author Year | Clear Research Questions | Data Address Question | Total MMAT Score (out of 5) |
|---|---|---|---|
| Abeysundera 2018 [13] | Yes | Can’t Tell a | 4 |
| Alpert 2004 [14] | Yes | Yes | 3 |
| Anderson 2016 [15] | No | No | 3 |
| Arnold 2005 [16] | Yes | Yes | 5 |
| Bambling 2015 [17] | Yes | Yes | 4 |
| Carpenter 2011 [18] | Yes | Yes | 4 |
| Chitiva 2012 [19] | Yes | Can’t Tell a | 4 |
| Cuomo 2020 [7] | Yes | Yes | 5 |
| De Berardis 2013 [20] | Yes | Yes | 5 |
| Di Pierro 2015 [21] | Yes | Yes | 4 |
| Djokic 2017 [22] | Yes | Yes | 5 |
| Dolcetta 2013 [23] | Yes | Yes | 5 |
| Galizia 2016 [24] | Yes | Yes | 5 |
| Green 2012 [25] | Yes | Yes | 4 |
| Jaggumantri 2015 [26] | Yes | Can’t Tell a | 4 |
| Kalman 2015 [27] | Yes | Yes | 5 |
| Levkovitz 2012 [28] | Yes | Yes | 5 |
| Limveeraprajak 2024 [5] | Yes | Yes | 5 |
| Mischoulon 2012 [29] | Yes | Yes | 3 |
| Mischoulon 2014 [30] | Yes | Yes | 5 |
| Murphy 2014 [31] | Yes | Yes | 5 |
| Olsufka 2017 [32] | Yes | Can’t Tell a | 4 |
| Papakostas 2010 [33] | Yes | Yes | 5 |
| Peng 2024 [34] | Yes | Yes | 5 |
| Saccarello 2020 [35] | Yes | Yes | 5 |
| Sakurai 2020 [36] | Yes | Yes | 5 |
| Sarris 2014 [40] | Yes | Yes | 5 |
| Sarris 2015 [41] | Yes | Yes | 5 |
| Sarris 2018 [37] | Yes | Yes | 5 |
| Sarris 2019 [38] | Yes | Yes | 5 |
| Sarris 2020 [36] | Yes | Yes | 5 |
| Shippy 2004 [42] | Yes | Yes | 5 |
| Strous 2009 [43] | Yes | Yes | 4 |
| Targum 2018 [44] | Yes | No b | 3 |
| Targum 2020 [45] | Yes | Yes | 5 |
| Ullah 2022 [46] | Yes | Yes | 5 |
a Case report, so there is limited evidence whether the data address the question. Further, the authors did not use a Naranjo scale to evaluate the likelihood that the medication caused the event, limiting the ability to evaluate this. However, the authors did use the literature and the case progression to substantiate the potential event. b Inclusion criteria resulted in differences between groups, limiting the validity of the results.
4. Discussion
Our findings suggest that SAMe shows potential therapeutic benefit in CNS-related signs, with major depressive disorder (MDD) being the most commonly studied among these. Improvements in mood were seen in a variety of validated scales, including HAM-D, MADRS, and CGI. Moreover, SAMe showed efficacy both as a monotherapy and as an adjunct to conventional antidepressants [7,24].
Nevertheless, results varied for SAMe use in the CNS. One study showed that it had no benefit on attention deficit/hyperactivity disorder (ADHD) symptoms [25]. Moreover, while some studies showed no significant difference between SAMe and a conventional antidepressant [5,7,24,34,36,41], others found it had no significant benefit compared to placebo [24,29,30,31,34,36,38,39]. As a comparison, a systematic review and network meta-analysis by Kishi et al. found that the efficacy of specific antidepressants (bupropion, escitalopram, vilazodone, etc.) was not better than placebo in preventing relapse at 6 months for adults with MDD [47]. Furthermore, while sertraline, vortioxetine, and desvenlafaxine had better efficacy, they were significantly more poorly tolerated compared to placebo. In children and adolescents, a meta-analysis by Rao et al. again noted that specific antidepressants (duloxetine, fluoxetine, venlafaxine, and escitalopram) were significantly more effective in treating depression compared to placebo [48]. However, of those agents, intolerability was also significantly higher in duloxetine and venlafaxine as well as several other antidepressants. Overall, choosing pharmacologic treatment for MDD is a delicate balance of weighing efficacy and safety. Because, in many cases, SAMe has demonstrated equivalence to antidepressants in terms of efficacy, it would be a valuable option for patients who wish to reduce or avoid adverse effects.
When considering SAMe as a treatment option, there are several factors to consider in an attempt to understand some of the varying results seen in the studies. Some of this variability may be explained by the differing dosing strategies, SAMe formulations, patient populations, and disease states. Furthermore, SAMe was studied in various combinations, such as with antidepressants (e.g., escitalopram) [14,20,33,37,44], Lactobacillus species [35,46], or other nutraceutical products [21,22,38,42]. Another factor to take into consideration is that SAMe may need time to reach its full effect for mood, similar to traditional antidepressants [21]. Finally, it is important to recognize that studies on MDD and other mood disorders have shown discrepancies in objective versus subjective assessments of mood and cognition [49,50]. Yet, many trial methodologies do not use both types of measurement in their design, making it difficult to make a comprehensive assessment.
To understand the most favorable dosage, it is important to note that SAMe is considered a hormetic nutrient because it demonstrates a biphasic dose response [51]. Hormesis is a biological phenomenon where exposure to low doses of a stressor induces adaptive, protective responses that enhance resilience against more severe stressors [52]. At low to moderate doses, SAMe exhibits potent antioxidant and anti-inflammatory properties because of its role in maintaining cellular redox homeostasis and modulating stress-response genes such as HO-1 [53]. The ability of SAMe to upregulate the Nrf2 pathway further highlights its crucial role in counteracting oxidative stress and neuroinflammation [54,55]. However, like other hormetic compounds, high doses of SAMe can be detrimental, leading to inhibition of antioxidant defenses and neurotoxicity. Thus, the therapeutic efficacy of SAMe hinges on careful dose regulation and balance.
While the optimal dose to optimize efficacy and minimize adverse effects of SAMe is not entirely known, benefits for mood and depressive symptoms were seen ranging from 200 mg to 3200 mg. Many studies started at lower doses and titrated to the target dose after a few days to 2 weeks or if they did not respond to the lower dose. Based on the available efficacy and safety from the included studies, we recommend considering doses starting at 200 mg to 800 mg for mild–moderate depression and 800 mg daily in one to two divided doses for resistant or non-remittent depression. Lower starting doses are warranted in patients with concomitant medications that may increase serotonin levels. Generally, we do not recommend a dose of 3200 mg due to higher rates of adverse effects. However, titrating to a target dose of 1600 mg per day is reasonable based on patient response. Finally, weight-based dosing with doses between 17 mg and 80 mg per kilogram per day may also be appropriate [23,26].
In addition to depression, SAMe showed benefit in other CNS conditions despite the limited amount of studies. When considering dosing for patients with anxiety, improvements in anxiety symptoms are seen with doses between 400 mg and 800 mg daily in one or two divided doses. Similarly, for patients with schizophrenia, improvements in aggression, depressive symptoms, and quality of life were seen at a dose of 400 mg daily titrated to 800 mg daily after 1 week. Interestingly, in the study by Green et al., SAMe improved depressive symptoms, although it did not improve ADHD symptoms [25]. This highlights that SAMe may have more consistent and substantial benefits when used in depression. In addition, the potential side effect of excitation caused by SAMe may limit its use in ADHD.
Furthermore, the oral or IM routes may be preferred over IV administration [5]. While the IV route has shown to be significantly worse in CNS diseases and should preferentially be avoided, it is still important to note that bioavailability may be highly variable between oral formulations [5].
Among the studies included, SAMe had a reasonable safety profile. Most common side effects of SAMe included mild to moderate gastrointestinal disturbances (constipation, abdominal discomfort, nausea) and headache. One study even reported fewer adverse events in the SAMe group [21]. Other adverse effects reported included increased anxiety [18,23,37], excitation [23], and fluid retention [36]. Rare adverse effects included anxiety [18,23,37], mania/hypomania [18,32], and psychomotor excitation [18]. Suicide attempt after SAMe use was documented with a single case report [19].
This systematic review has considerable strengths. First, PRISMA methodology was used, providing robustness to the study design as a systematic review. It also covers several different CNS-related signs, providing a more comprehensive and current assessment of the therapeutic potential of SAMe in these contexts. Furthermore, most studies (32 of 36) achieved a high study quality rating, allowing for more optimal evaluation of the evidence. Limitations of our review included the heterogeneity of study designs, populations, dosages, and outcomes measured. Because of this, it is difficult to generalize findings. In addition, our review process is not free from potential human error, as inconsistencies could be present in the application of inclusion/exclusion criteria. Finally, participant bias may be present, especially in subjective assessments of mood. However, many studies included an active or placebo comparator group to minimize this.
One of the current challenges of SAMe use in CNS health includes its lack of long-term efficacy and safety data past a 12-month period. Moreover, most evidence has been collected during 6 to 8 weeks, limiting our understanding of its chronic use. Another challenge is understanding the conditions for which it is most effective. While there is some evidence for its use in schizophrenia and bipolar disorder, in general, benefit was found mostly on depressive symptoms in these conditions. Thus, clinicians should be cautious in using SAMe for CNS signs outside of depression and anxiety.
Future studies are needed to assess long-term data of SAMe use on CNS health with and without concurrent antidepressant or nutraceutical therapy. In addition, issues in bioavailability between oral formulations must be addressed in order to provide accurate dosing recommendations and ensure optimal treatment. Thus, quality control in the manufacturing and production processes is necessary for safe and effective use of all supplements and nutraceuticals, including SAMe.
5. Conclusions
Current evidence suggests that use of SAMe improves depressive symptoms both as a monotherapy and with concurrent antidepressant or nutraceutical therapy. Clinicians should consider individual patient factors (e.g., specific disease treated, SAMe formulation and dose, patient comorbidities, medication history, goals of therapy, etc.) that could support the use of SAMe. While other therapies are currently used in first line management of CNS disease areas, SAMe may provide a useful option if they fail, are not appropriate, or if the patient prefers a nutraceutical approach. Future studies are needed to assess SAMe’s long-term efficacy and safety in depression, to further understand its effects alongside other therapies, and to address inconsistencies in oral formulations to ensure optimal dosing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16183148/s1, PRISMA Checklist.
Author Contributions
Conceptualization, A.M.H.C.; methodology, A.M.H.C. and J.A.D.; software, A.M.H.C. and J.A.D.; formal analysis, A.M.H.C. and K.E.R.B.; investigation, H.M., E.C., N.G., J.A.D. and A.M.H.C.; data curation, A.M.H.C.; writing—original draft preparation, K.E.R.B. and A.M.H.C.; writing—review and editing, K.E.R.B., H.M., E.C., N.G., J.A.D. and A.M.H.C.; supervision, A.M.H.C. and J.A.D.; project administration, A.M.H.C. and J.A.D. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created outside of what is published in this article. The study protocol, data collection forms, data extracted from included studies, and all other materials used in this review can be made available upon request. No amendments were made to the study protocol.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Mental Disorders. [(accessed on 22 August 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders.
- 2.Institute for Health Metrics and Evaluation 2021 Global Burden of Disease Study Results. [(accessed on 22 August 2024)]. Available online: https://vizhub.healthdata.org/gbd-results/
- 3.World Health Organization Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2 March 2022. [(accessed on 22 August 2024)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
- 4.Reinert M., Fritze D., Nguyen T. The State of Mental Health in America 2024. Mental Health America; Alexandria, VA, USA: 2024. [Google Scholar]
- 5.Limveeraprajak N., Nakhawatchana S., Visukamol A., Siripakkaphant C., Suttajit S., Srisurapanont M. Efficacy and acceptability of S-adenosyl-L-methionine (SAMe) for depressed patients: A systematic review and meta- analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2024;132:110985. doi: 10.1016/j.pnpbp.2024.110985. [DOI] [PubMed] [Google Scholar]
- 6.Sachinvala N.D., Teramoto N., Stergiou A. Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D(3) in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression. Brain Sci. 2020;10:600. doi: 10.3390/brainsci10090600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuomo A., Beccarini Crescenzi B., Bolognesi S., Goracci A., Koukouna D., Rossi R., Fagiolini A. S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): A clinician-oriented systematic review. Ann. Gen. Psychiatry. 2020;19:50. doi: 10.1186/s12991-020-00298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A., Gerbarg P., Bottiglieri T., Massoumi L., Carpenter L.L., Lavretsky H., Muskin P.R., Brown R.P., Mischoulon D. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry. 2017;78:e656–e667. doi: 10.4088/JCP.16r11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rambaldi A., Gluud C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst. Rev. 2006;1:CD002235. doi: 10.1002/14651858.CD002235.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Noureddin M., Sander-Struckmeier S., Mato J.M. Early treatment efficacy of S-adenosylmethionine in patients with intrahepatic cholestasis: A systematic review. World J. Hepatol. 2020;12:46–63. doi: 10.4254/wjh.v12.i2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong Q.N., Pluye P., Fàbregues S., Bartlett G., Boardman F., Cargo M., Dagenais P., Gagnon M.P., Griffiths F., Nicolau B., et al. Mixed Methods Appraisal Tool (MMAT), Version 2018. Canadian Intellectual Property Office, Industry Canada; Gatineau, QC, Canada: 2018. Registration of Copyright (#1148552) [Google Scholar]
- 13.Abeysundera H., Gill R. Possible SAMe-induced mania. BMJ Case Rep. 2018;2018:bcr2018224338-bcr. doi: 10.1136/bcr-2018-224338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpert J.E., Papakostas G., Mischoulon D., Worthington J.J., Petersen T., Mahal Y., Burns A., Bottiglieri T., Nierenberg A.A., Fava M. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: An open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J. Clin. Psychopharmacol. 2004;24:661–664. doi: 10.1097/01.jcp.0000145339.45794.cd. [DOI] [PubMed] [Google Scholar]
- 15.Anderson S., Panka J., Rakobitsch R., Tyre K., Pulliam K. Anxiety and Methylenetetrahydrofolate Reductase Mutation Treated With S-Adenosyl Methionine and Methylated B Vitamins. Integr. Med. Clin. J. 2016;15:48–52. [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold O., Saletu B., Anderer P., Assandri A., di Padova C., Corrado M., Saletu-Zyhlarz G.M. Double-blind, placebo-controlled pharmacodynamic studies with a nutraceutical and a pharmaceutical dose of ademetionine (SAMe) in elderly subjects, utilizing EEG mapping and psychometry. Eur. Neuropsychopharmacol. 2005;15:533–543. doi: 10.1016/j.euroneuro.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Bambling M., Parham S.C., Coulson S., Vitetta L. S-adenosylmethionine (SAMe) and Magnesium Orotate as adjunctives to SSRIs in sub-optimal treatment response of depression in adults: A pilot study. Adv. Integr. Med. 2015;2:56–62. doi: 10.1016/j.aimed.2015.04.003. [DOI] [Google Scholar]
- 18.Carpenter D.J. St. John’s wort and S-adenosyl methionine as “natural” alternatives to conventional antidepressants in the era of the suicidality boxed warning: What is the evidence for clinically relevant benefit? Altern. Med. Rev. 2011;16:17–39. [PubMed] [Google Scholar]
- 19.Chitiva H., Audivert F., Alvarez C. Suicide attempt by self-burning associated with ingestion of S-adenosylmethionine: A review of the literature and case report. J. Nerv. Ment. Dis. 2012;200:99–101. doi: 10.1097/NMD.0b013e31823fafdf. [DOI] [PubMed] [Google Scholar]
- 20.De Berardis D., Marini S., Serroni N., Rapini G., Iasevoli F., Valchera A., Signorelli M., Aguglia E., Perna G., Salone A., et al. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: An open label, fixed dose, single-blind study. Sci. World J. 2013;2013:204649. doi: 10.1155/2013/204649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Pierro F., Settembre R. Preliminary results of a randomized controlled trial carried out with a fixed combination of S-adenosyl-L-methionine and betaine versus amitriptyline in patients with mild depression. Int. J. Gen. Med. 2015;8:73–78. doi: 10.2147/IJGM.S79518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djokic G., Korcok D., Djordjevic V., Agic A., Rankovic A., Djukic D. The effects of S-adenosyl-L-methionine-vitamin B complex on mild and moderate depressive symptoms. Hippokratia. 2017;21:140–143. [PMC free article] [PubMed] [Google Scholar]
- 23.Dolcetta D., Parmigiani P., Salmaso L., Bernardelle R., Cesari U., Andrighetto G., Baschirotto G., Nyhan W.L., Hladnik U. Quantitative evaluation of the clinical effects of S-adenosylmethionine on mood and behavior in Lesch-Nyhan patients. Nucleosides Nucleotides Nucleic Acids. 2013;32:174–188. doi: 10.1080/15257770.2013.774012. [DOI] [PubMed] [Google Scholar]
- 24.Galizia I., Oldani L., Macritchie K., Amari E., Dougall D., Jones T.N., Lam R.W., Massei G.J., Yatham L.N., Young A.H. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst. Rev. 2016;10:CD011286. doi: 10.1002/14651858.CD011286.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green T., Steingart L., Frisch A., Zarchi O., Weizman A., Gothelf D. The feasibility and safety of S-adenosyl-L-methionine (SAMe) for the treatment of neuropsychiatric symptoms in 22q11.2 deletion syndrome: A double-blind placebo-controlled trial. J. Neural Transm. 2012;119:1417–1423. doi: 10.1007/s00702-012-0831-x. [DOI] [PubMed] [Google Scholar]
- 26.Jaggumantri S., Dunbar M., Edgar V., Mignone C., Newlove T., Elango R., Collet J.P., Sargent M., Stockler-Ipsiroglu S., van Karnebeek C.D.M. Treatment of Creatine Transporter (SLC6A8) Deficiency with Oral S-Adenosyl Methionine as Adjunct to L-arginine, Glycine, and Creatine Supplements. Pediatr. Neurol. 2015;53:360–363.e362. doi: 10.1016/j.pediatrneurol.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Kalman D.S., Feldman S., Vazquez R.R., Krieger D.R. A Prospective Randomized Double-Blind Study Evaluating UP165 and S-Adenosyl-l-Methionine on Depression, Anxiety and Psychological Well-Being. Foods. 2015;4:130–139. doi: 10.3390/foods4020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levkovitz Y., Alpert J.E., Brintz C.E., Mischoulon D., Papakostas G.I. Effects of S-adenosylmethionine augmentation of serotonin-reuptake inhibitor antidepressants on cognitive symptoms of major depressive disorder. Eur. Psychiatry. 2012;27:518–521. doi: 10.1016/j.eurpsy.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Mischoulon D., Alpert J.E., Arning E., Bottiglieri T., Fava M., Papakostas G.I. Bioavailability of S-adenosyl methionine and impact on response in a randomized, double-blind, placebo-controlled trial in major depressive disorder. J. Clin. Psychiatry. 2012;73:843–848. doi: 10.4088/JCP.11m07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mischoulon D., Price L.H., Carpenter L.L., Tyrka A.R., Papakostas G.I., Baer L., Dording C.M., Clain A.J., Durham K., Walker R., et al. A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J. Clin. Psychiatry. 2014;75:370–376. doi: 10.4088/JCP.13m08591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy B.L., Babb S.M., Ravichandran C., Cohen B.M. Oral SAMe in persistent treatment-refractory bipolar depression: A double-blind, randomized clinical trial. J. Clin. Psychopharmacol. 2014;34:413–416. doi: 10.1097/JCP.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 32.Olsufka W., Abraham M.-A. Treatment-emergent hypomania possibly associated with over-the-counter supplements. Ment. Health Clin. 2017;7:160–163. doi: 10.9740/mhc.2017.07.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papakostas G.I., Mischoulon D., Shyu I., Alpert J.E., Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am. J. Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 34.Peng T.-R., Cheng H.-Y., Wu T.-W. S-Adenosylmethionine (SAMe) as an adjuvant therapy for patients with depression: An updated systematic review and meta-analysis. Gen. Hosp. Psychiatry. 2024;86:118–126. doi: 10.1016/j.genhosppsych.2024.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Saccarello A., Montarsolo P., Massardo I., Picciotto R., Pedemonte A., Castagnaro R., Brasesco P.C., Guida V., Picco P., Fioravanti P., et al. Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Prim. Care Companion CNS Disord. 2020;22:23164. doi: 10.4088/PCC.19m02578. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai H., Carpenter L.L., Tyrka A.R., Price L.H., Papakostas G.I., Dording C.M., Yeung A.S., Cusin C., Ludington E., Bernard-Negron R., et al. Dose increase of S-Adenosyl-Methionine and escitalopram in a randomized clinical trial for major depressive disorder. J. Affect. Disord. 2020;262:118–125. doi: 10.1016/j.jad.2019.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarris J., Byrne G.J., Bousman C., Stough C., Murphy J., MacDonald P., Adams L., Nazareth S., Oliver G., Cribb L., et al. Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: An 8-week double-blind, randomized, controlled trial. Eur. Neuropsychopharmacol. 2018;28:1126–1136. doi: 10.1016/j.euroneuro.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 38.Sarris J., Byrne G.J., Stough C., Bousman C., Mischoulon D., Murphy J., Macdonald P., Adams L., Nazareth S., Oliver G., et al. Nutraceuticals for major depressive disorder- more is not merrier: An 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 2019;245:1007–1015. doi: 10.1016/j.jad.2018.11.092. [DOI] [PubMed] [Google Scholar]
- 39.Sarris J., Murphy J., Stough C., Mischoulon D., Bousman C., MacDonald P., Adams L., Nazareth S., Oliver G., Cribb L., et al. S-Adenosylmethionine (SAMe) monotherapy for depression: An 8-week double-blind, randomised, controlled trial. Psychopharmacology. 2020;237:209–218. doi: 10.1007/s00213-019-05358-1. [DOI] [PubMed] [Google Scholar]
- 40.Sarris J., Papakostas G.I., Vitolo O., Fava M., Mischoulon D. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: Efficacy and effects of histamine and carnitine as moderators of response. J. Affect. Disord. 2014;164:76–81. doi: 10.1016/j.jad.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 41.Sarris J., Price L.H., Carpenter L.L., Tyrka A.R., Ng C.H., Papakostas G.I., Jaeger A., Fava M., Mischoulon D. Is S-Adenosyl Methionine (SAMe) for Depression Only Effective in Males? A Re-Analysis of Data from a Randomized Clinical Trial. Pharmacopsychiatry. 2015;48:141–144. doi: 10.1055/s-0035-1549928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shippy R.A., Mendez D., Jones K., Cergnul I., Karpiak S.E. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38. doi: 10.1186/1471-244X-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strous R.D., Ritsner M.S., Adler S., Ratner Y., Maayan R., Kotler M., Lachman H., Weizman A. Improvement of aggressive behavior and quality of life impairment following S-adenosyl-methionine (SAM-e) augmentation in schizophrenia. Eur. Neuropsychopharmacol. 2009;19:14–22. doi: 10.1016/j.euroneuro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Targum S.D., Cameron B.R., Ferreira L., MacDonald I.D. An augmentation study of MSI-195 (S-adenosylmethionine) in Major Depressive Disorder. J. Psychiatr. Res. 2018;107:86–96. doi: 10.1016/j.jpsychires.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Targum S.D., Cameron B.R., Ferreira L., MacDonald I.D. Early score fluctuation and placebo response in a study of major depressive disorder. J. Psychiatr. Res. 2020;121:118–125. doi: 10.1016/j.jpsychires.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Ullah H., Di Minno A., Esposito C., El-Seedi H.R., Khalifa S.A.M., Baldi A., Greco A., Santonastaso S., Cioffi V., Sperandeo R., et al. Efficacy of a food supplement based on S-adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild-to-moderate depression: A monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2022;156:113930. doi: 10.1016/j.biopha.2022.113930. [DOI] [PubMed] [Google Scholar]
- 47.Kishi T., Ikuta T., Sakuma K., Okuya M., Hatano M., Matsuda Y., Iwata N. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: A systematic review and network meta-analysis. Mol. Psychiatry. 2023;28:402–409. doi: 10.1038/s41380-022-01824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao Y., Yang R., Zhao J., Cao Q. Efficacy and tolerability of antidepressant drugs in treatment of depression in children and adolescents: A network meta-analysis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022;51:480–490. doi: 10.3724/zdxbyxb-2022-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalak J., Niemeyer H., Tschacher W., Baumann N., Chi Zhang X., Adolph D. Subjective and Objective Measures of Activity in Depressed and Non-depressed Individuals in Everyday Life. J. Exp. Psychopathol. 2022;13:20438087221092582. doi: 10.1177/20438087221092582. [DOI] [Google Scholar]
- 50.Serra-Blasco M., Torres I.J., Vicent-Gil M., Goldberg X., Navarra-Ventura G., Aguilar E., Via E., Portella M.J., Figuereo I., Palao D., et al. Discrepancy between objective and subjective cognition in major depressive disorder. Eur. Neuropsychopharmacol. 2019;29:46–56. doi: 10.1016/j.euroneuro.2018.11.1104. [DOI] [PubMed] [Google Scholar]
- 51.Módis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., Hellmich M.R., Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scuto M., Rampulla F., Reali G.M., Spanò S.M., Trovato Salinaro A., Calabrese V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants. 2024;13:484. doi: 10.3390/antiox13040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S.Y., Hong S.W., Kim M.O., Kim H.S., Jang J.E., Leem J., Park I.S., Lee K.U., Koh E.H. S-adenosyl methionine prevents endothelial dysfunction by inducing heme oxygenase-1 in vascular endothelial cells. Mol. Cells. 2013;36:376–384. doi: 10.1007/s10059-013-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y., Ma R., Deng Q., Wang W., Cao C., Yu C., Li S., Shi L., Tian J. S-adenosylmethionine improves cognitive impairment in D-galactose-induced brain aging by inhibiting oxidative stress and neuroinflammation. J. Chem. Neuroanat. 2023;128:102232. doi: 10.1016/j.jchemneu.2023.102232. [DOI] [PubMed] [Google Scholar]
- 55.Cordaro M., Trovato Salinaro A., Siracusa R., D’Amico R., Impellizzeri D., Scuto M., Ontario M.L., Crea R., Cuzzocrea S., Di Paola R., et al. Hidrox(®) Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants. 2021;10:818. doi: 10.3390/antiox10050818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created outside of what is published in this article. The study protocol, data collection forms, data extracted from included studies, and all other materials used in this review can be made available upon request. No amendments were made to the study protocol.