Abstract
The P gene of measles virus (MV) encodes the P protein and three accessory proteins (C, V, and R). However, the role of these accessory proteins in the natural course of MV infection remains unclear. For this study, we generated a recombinant wild-type MV lacking the C protein, called wtMV(C−), by using a reverse genetics system (M. Takeda, K. Takeuchi, N. Miyajima, F. Kobune, Y. Ami, N. Nagata, Y. Suzaki, Y. Nagai, and M. Tashiro, J. Virol. 74:6643-6647). When 293 cells expressing the MV receptor SLAM (293/hSLAM) were infected with wtMV(C−) or parental wild-type MV (wtMV), the growth of wtMV(C−) was restricted, particularly during late stages. Enhanced green fluorescent protein-expressing wtMV(C−) consistently induced late-stage cell rounding and cell death in the presence of a fusion-inhibiting peptide, suggesting that the C protein can prevent cell death and is required for long-term MV infection. Neutralizing antibodies against alpha/beta interferon did not restore the growth restriction of wtMV(C−) in 293/hSLAM cells. When cynomolgus monkeys were infected with wtMV(C−) or wtMV, the number of MV-infected cells in the thymus was >1,000-fold smaller for wtMV(C−) than for wtMV. Immunohistochemical analyses showed strong expression of an MV antigen in the spleen, lymph nodes, tonsils, and larynx of a cynomolgus monkey infected with wtMV but dramatically reduced expression in the same tissues in a cynomolgus monkey infected with wtMV(C−). These data indicate that the MV C protein is necessary for efficient MV replication both in vitro and in cynomolgus monkeys.
Measles virus (MV) is a member of the genus Morbillivirus of the family Paramyxoviridae. The genome of MV contains six genes in the following order: 3′-leader-N-P-M-F-H-L-trailer-5′ (reviewed in reference 14). The P gene of MV encodes the P protein and three nonstructural proteins, namely, C, V, and R (27). The MV C protein is a small (186 amino acids), highly positively charged protein and is translated from the P mRNA in a different open reading frame from that for the P protein (4). Viruses in the genera Respirovirus, Henipavirus, and Morbillivirus and Tupaia paramyxovirus-like viruses express one or more C proteins, which are all relatively small basic proteins that are translated from the P mRNA in a different open frame from that for the P protein (for reviews, see references 25 and 31). The MV C protein is found both in the cytoplasm and in the nucleus (1, 4). With in vitro studies, the MV C protein has been shown to regulate viral RNA synthesis (43) and to impair the alpha/beta interferon (IFN-α/β) response (44, 55).
For investigations of the role of the MV C protein, an MV Edmonston (Ed) strain that lacks expression of the C protein (MV C-EdB) has been generated by reverse genetics (41). In in vitro studies, MV C-EdB replicates normally in Vero, CV-1, B95-8a, human neuroblastoma SK-N-MC, and primary mouse neuronal cells but not in human peripheral blood or HeLa cells (10, 40, 41, 44). In addition, MV C-EdB has exhibited restricted growth in human thymus tissue transplanted into SCID mice and reduced titers and mortality in CD46 transgenic mice (30, 40, 50). These results strongly suggest that the C protein is closely involved in virulence functions.
However, rodents are not natural hosts of MV, and infections of transplanted human tissues are of limited use as models of human infection. Moreover, the Ed strain was isolated several decades ago, has been passaged in nonlymphoid cell lines, and has gained the ability to use both CD46 (6, 33) and SLAM (9, 19, 49) as receptors. More importantly, this passaged virus is no longer pathogenic in monkey models (2, 8, 51, 54). This raises questions about the extent to which the Ed strain retains the original nature of MV. In contrast, MV strains that were isolated and passaged in B95a cells use SLAM as their sole receptor (9, 19, 36, 49) and retain their original pathogenicities (21, 22). In order to study the function of the C protein in the context of the natural course of MV pathogenesis, we generated an MV strain that lacks expression of the C protein, based on the highly pathogenic IC-B strain isolated from B95a cells (46), and designated this strain wtMV(C−).
For the present study, we examined the growth of wtMV(C−) and parental wild-type MV (wtMV) in vitro and in cynomolgus monkeys. The present results indicate that the MV C protein is necessary for the efficient growth of wild-type MV both in vitro and in macaques.
MATERIALS AND METHODS
Viruses and cells.
wtMV, corresponding to the IC-B strain of MV (21), was recovered from the plasmid p(+)MV323 encoding the antigenomic IC-B sequence; this strain was previously designated IC323 (46). B95a cells, which are an adherent marmoset B-cell line (21), were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal calf serum (FCS). Vero (African green monkey kidney) cells expressing human SLAM (Vero/hSLAM cells) (36) were grown in DMEM supplemented with 10% FCS and 400 μg of G418/ml for stable expression of the transfected hSLAM gene product. HepG2 cells (a human hepatoma cell line) were grown in DMEM supplemented with 10% FCS.
Plasmid construction and recovery of infectious virus from cDNA.
Point mutations were introduced by using the mutagenic primer 5′-CCATGTGAAAAACGGACTAGAATGC-3′ (the introduced nucleotide changes are underlined), and the mutated fragment was introduced into the Bpu1102I (antigenome position 127; numbering according to reference 48) and Bpu1102I (antigenome position 3146) window of p(+)MV323 after nucleotide sequencing. The resulting plasmid was designated p(+)MV(C−). The plasmid p(+)MV323-EGFP, which carries the full-genome cDNA of the IC-B strain and the enhanced green fluorescent protein (EGFP) gene, was described previously (16). To introduce the EGFP gene into p(+)MV(C−), we replaced the fragment of p(+)MV323-EGFP from Bpu1102I to Bpu1102I with the corresponding cDNA fragment of p(+)MV(C−). The resulting plasmid was designated p(+)MV(C−)-EGFP. The infectious recombinant MV strains wtMV(C−) and wtMV(C−)-EGFP were recovered from plasmids p(+)MV(C−) and p(+)MV(C−)-EGFP, respectively, as reported previously (42, 46).
Establishment of stable transformants.
The establishment of 293 cells stably expressing hSLAM was performed as previously described (47). Briefly, 293 cells in a 9-cm-diameter dish were transfected with 10 μg of the pCA-SLAM (36) and pKS336 plasmids by use of a mammalian transfection kit (Stratagene, La Jolla, Calif.). Two days later, the medium was replaced with DMEM supplemented with 10% FCS and 2 μg/ml of blasticidin-S (Funakoshi, Tokyo, Japan). Several colonies were selected and tested for susceptibility to wild-type MV.
Western blotting.
Cells were lysed in a lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.6% NP-40, 4 mM phenylmethylsulfonyl fluoride) and were disrupted by sonication for 1 min. After centrifugation, the lysates were electrophoresed in sodium dodecyl sulfate-polyacrylamide gels. The proteins in the gel were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). The C and P proteins were detected with antisera specific for the C protein (47) or MV strain Toyoshima (11) and with biotinylated anti-rabbit immunoglobulin (Amersham, Piscataway, N.J.) and streptavidin-alkaline phosphatase (Amersham).
Virus growth and syncytium formation in cell culture.
Monolayer cultures of B95a and 293/hSLAM cells in a 24-well plate were infected with recovered viruses at a multiplicity of infection (MOI) of 0.01 50% tissue culture infective doses (TCID50)/cell. At various times, the cells were scraped, and harvested cells in medium were subjected to three cycles of freezing and thawing. The infectivity titer was assessed as the TCID50 for B95a cells.
Addition of anti-interferon antibodies.
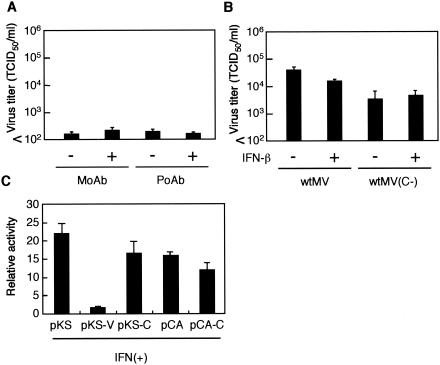
293/hSLAM cells in a 24-well plate were infected with wtMV(C−) at an MOI of 0.01. After incubation at 37°C for 1 h, the cells were washed three times and incubated with complete medium containing either a mixture of 5 μl (12.25 neutralizing units [NU]) of a mouse monoclonal antibody against human IFN-α (MBL, Nagoya, Japan) and 5 μl (15 NU) of a mouse monoclonal antibody against human IFN-β (Yamasa, Tokyo, Japan) or a mixture of 10 μl (800 NU) of rabbit polyclonal antisera against human IFN-α (National Institute of Infectious Diseases, Japan) and 10 μl (1,600 NU) of rabbit polyclonal antisera against human IFN-β (Toray, Tokyo, Japan). The cells were harvested at 3 days postinfection (dpi), and virus growth was assessed as the TCID50 for B95a cells.
Reporter assay.
An IFN-stimulated response element reporter assay was performed as previously described (47), with a minor modification. Briefly, HepG2 cells were transfected with a pKS336 or pCAGGS vector (34) encoding the C or V protein of the IC-B strain or with an empty vector, together with the pISRE-TA-Luc plasmid (Clontech, Palo Alto, Calif.) and the pSEAP-Control plasmid (Clontech), using standard calcium phosphate procedures. At 20 to 24 h posttransfection, the cells were incubated with or without 1,000 IU/ml of IFN-β for 6 h. A portion of cell lysates was assayed for luciferase activity by use of a luciferase assay system (Promega, Madison, Wis.) and a MiniLumat LB9506 luminometer (Berthold, Pforzheim, Germany). To monitor the transfection efficiency, we assayed a portion of each cell supernatant for secreted alkaline phosphatase (SEAP), using a SEAP assay kit (Toyobo, Osaka, Japan).
Infection of cynomolgus monkeys with recombinant MVs.
Cynomolgus monkeys were inoculated intranasally in groups of three animals each with 105 TCID50 of either wtMV or wtMV(C−). Peripheral blood mononuclear cells (PBMCs) were isolated by using Percoll gradients (Amersham). MV-infected cells among PBMCs and in tissues were counted as previously reported (46). All animal experiments were performed in compliance with institutional guidelines.
Immunohistochemistry.
Immunohistochemical analysis was performed as previously described (32). Briefly, tissues were excised, fixed in 10% formalin in phosphate buffer, embedded in paraffin, and cut into sections. The paraffin sections were deparaffinized in xylene, rehydrated in ethanol, treated with a 0.25% trypsin solution containing 0.5% CaCl2 for 30 min, and incubated in 1% hydrogen peroxide in methanol to block endogenous peroxidase activity. The sections were then incubated with normal goat serum for 5 min, followed by incubation with a rabbit serum against the MV N protein at 4°C overnight (N. Nagata, unpublished data). The sections were next incubated with biotin-conjugated anti-rabbit immunoglobulin G, followed by the application of streptavidin-peroxidase. The peroxidase reaction was developed in diaminobenzidine with hydrogen peroxide. Nuclei were counterstained with hematoxylin.
RESULTS
Recovery of recombinant virus lacking expression of the C protein.
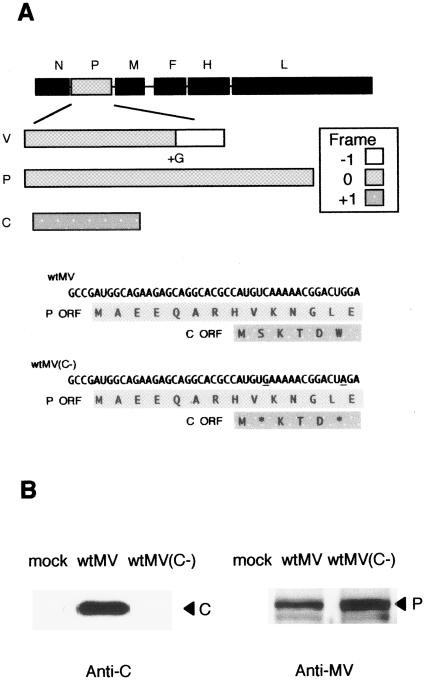
To knock out the expression of the C protein without changing the amino acid sequences of the P and V proteins, we introduced two stop codons into the open reading frame encoding the C protein. The first point mutation (C to G at position 1833) converted a UCA codon into a UGA stop codon, and the second point mutation (G to A at position 1845) converted a UGG codon into a UAG stop codon (Fig. 1A). To confirm that the introduced mutations silenced the expression of the C protein, we performed a Western blot analysis using total protein lysates from 293/hSLAM cells infected with wtMV(C−) or parental wtMV and detecting proteins with a rabbit antiserum specific for the C or P protein. The C protein was detected in wtMV-infected cells but not in wtMV(C−)-infected cells (Fig. 1B). In contrast, the P protein was detected in wtMV- and wtMV(C−)-infected cells (Fig. 1B), indicating that the mutations introduced into wtMV(C−) had no significant effect on P protein synthesis.
FIG. 1.
Construction and generation of recombinant wtMV lacking expression of the C protein. (A) Schematic diagram of genomic organization of wtMV showing the P cistron encoding the P, C, and V proteins and the mutations in the C open reading frame. Altered nucleotides are underlined. The expression of the C protein was eliminated by placing two stop codons, UGA [p(+)322 position 1832] and UAG [p(+)MV322 position 1844], downstream in the C open reading frame. (B) Western blot analysis using rabbit antisera against the MV C protein and MV virions. Lysates from cells infected with wtMV or wtMV(C−) or from mock-infected cells were probed with antisera against the MV C protein and the MV Toyoshima strain.
Growth of wtMV and wtMV(C−) in tissue culture cells.
In B95a cells, there was no significant difference in replication kinetics between the wtMV and wtMV(C−) viruses (Fig. 2A). In 293/SLAM cells, wtMV replicated efficiently for up to 3 days postinfection (dpi) and then gradually declined, whereas wtMV(C−) replicated for up to 2 dpi and then exhibited severely restricted growth (Fig. 2B). After 3 dpi, virtually no virus was detected in wtMV(C−)-infected 293/hSLAM cells (Fig. 2B).
FIG. 2.
Replication kinetics of wtMV and wtMV(C−). B95a cells (A) and 293/hSLAM cells (B) were infected with wtMV (circles) or wtMV(C−) (triangles) at an MOI of 0.01 TCID50/cell. Cells and media were harvested at 1, 2, 3, and 4 dpi, and infectivity titers were assessed as TCID50 using B95a cells.
Cytopathic effect in 293/hSLAM cells.
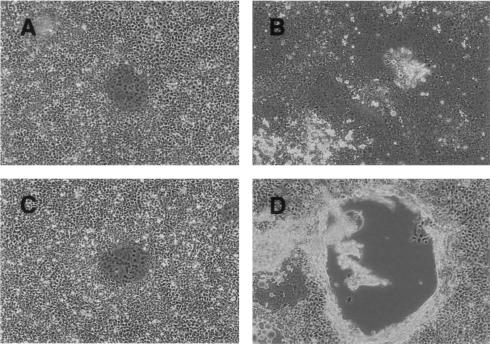
During the present experiments, we noticed a difference in cytopathic effects between wtMV(C−) and parental wtMV. When 293/hSLAM cells were infected with wtMV, syncytia developed at 1 dpi (Fig. 3A), and the syncytia had expanded to cover almost the entire dish at 3 dpi (Fig. 3B). When 293/hSLAM cells were infected with wtMV(C−), syncytia developed at 1 dpi (Fig. 3C), but the syncytia did not subsequently expand and had detached from the dishes due to shrinkage at 3 dpi (Fig. 3D).
FIG. 3.
Cytopathic effects of wtMV and wtMV(C−) in 293/hSLAM cells. 293/hSLAM cells were infected with wtMV (A and B) or wtMV(C−) (C and D), and cells were photographed under a microscope at 1 dpi (A and C) and 3 dpi (B and D).
Cell rounding and cell death induced by wtMV(C−).
To investigate the mechanism underlying the growth defect of wtMV(C−), we generated an EGFP-expressing recombinant virus, wtMV(C−)-EGFP, from the plasmid p(+)MV323-EGFP (16). When we measured the entry level of EGFP-expressing MV in the presence of a fusion-inhibiting peptide (FIP) by a previously presented method (16), we found that the shapes of infected cells differed between the two viruses. 293/hSLAM cells infected with wtMV-EGFP in the presence of the FIP were flat at 3 dpi (Fig. 4A). In contrast, 293/hSLAM cells infected with wtMV(C−)-EGFP in the presence of the FIP were spherical at 3 dpi (Fig. 4C). At 7 dpi, 293/hSLAM cells infected with wtMV-EGFP were still alive (Fig. 4B), whereas almost all cells infected with wtMV(C−)-EGFP had disappeared (Fig. 4D). This suggests that the MV C protein is necessary to prevent the early death of infected cells. To quantify early cell death, we counted EGFP-expressing cells under a fluorescence microscope at 3 dpi and 7 dpi. The percentage of wtMV(C−)-EGFP-infected cells was reduced to 12%, while that of wtMV-EGFP-infected cells increased to 216%, indicating the occurrence of cell division of wtMV-EGFP-infected cells.
FIG. 4.
Cell rounding and cell death observed in 293/hSLAM cells infected with wtMV(C−)-EGFP. 293/hSLAM cells were infected with wtMV-EGFP (A and B) or wtMV(C−)-EGFP (C and D) and incubated in the presence of the FIP. The expression of EGFP was monitored by using a fluorescence microscope at 3 dpi (A and C) and 7 dpi (B and D).
Examination of the effect of IFN-α/β.
Because it has been reported that the MV C protein inhibits the IFN-α/β response (44), we first examined whether IFN-α/β was responsible for the restricted growth of wtMV(C−) in 293/hSLAM cells. For this purpose, the growth of wtMV(C−) in 293/hSLAM cells was examined based on the presence of antibodies against IFN-α/β. However, neither mouse monoclonal antibodies against IFN-α/β nor antisera against IFN-α/β restored the growth of wtMV(C−) (Fig. 5A). Next, we examined the growth of wtMV and wtMV(C−) in the presence of IFN-β. For this purpose, 293/hSLAM cells were infected with wtMV or wtMV(C−) and incubated in the presence or absence of IFN-β for 2 days. However, exogenously added IFN-β did not inhibit the growth of wtMV or wtMV(C−) (Fig. 5B). These results indicate that IFN-α/β is not responsible for the growth restriction of wtMV(C−) in 293/hSLAM cells, and they are consistent with our previous observation that the MV C protein did not block IFN-α/β signaling in a cell line constitutively expressing the MV C protein (47). Since Shaffer et al. (44) reported that the C protein blocked IFN-α/β signal transduction in a transient expression system with MV C expression plasmids, we reexamined our previous data (47). We used HepG2 cells in those experiments, because HepG2 cells produced the most consistent results among the several cell lines tested. When HepG2 cells were transfected with the IFN-α/β-responsive plasmid pISRE-TA-luc and the empty plasmid pKS336 and were then incubated with IFN-β, the relative luciferase activity increased 20-fold compared to that of unstimulated cells (Fig. 5C). Under those conditions, cotransfection of a pKS336 plasmid expressing the MV V protein almost completely blocked IFN-α/β signaling (37, 47), while a pKS336 plasmid expressing the MV C protein did not strongly block IFN-α/β signaling, as reported previously (46). When the MV C protein was expressed by a different expression vector (pCAGGS), a similar result was obtained (Fig. 5C). We obtained almost the same results using 293T cells (data not shown). These data indicate that the MV C protein of the wild-type IC-B strain does not function as an IFN antagonist.
FIG. 5.
Effect of IFN on wtMV(C−) replication in 293/hSLAM cells and effect of the MV C protein on IFN-responsive reporter gene assay. (A) 293/hSLAM cells were infected with wtMV(C−) and incubated in the presence of a mixture of monoclonal antibodies against IFN-α and IFN-β (ΜοΑb) or a mixture of polyclonal antisera against IFN-α and IFN-β (PoAb). The cells were harvested at 3 dpi, and virus titers were assessed as TCID50 for B95a cells. Black bars represent the average titers obtained from triplicate samples. Standard deviations are also indicated. (B) 293/hSLAM cells in a 24-well plate were infected with wtMV or wtMV(C−) at an MOI of 0.01. After incubation at 37°C for 1 h, the cells were washed three times and incubated with complete medium containing 1,000 IU/ml of IFN-β. The cells were harvested at 2 dpi, and virus growth was assessed as the TCID50 for B95a cells. Black bars represent the average titers obtained from triplicate samples. Standard deviations are also indicated. (C) HepG2 cells were transfected with a plasmid containing an IFN-responsive luciferase reporter gene, the control pSEAP plasmid, and the pKS336 plasmid (pKS), the pKS336 plasmid expressing the MV V (pKS-V) or C (pKS-C) protein, the pCAGGS plasmid (pCA), or the pCAGGS plasmid expressing the MV C protein (pCA-C). After transfection, the cells were stimulated with 1,000 IU/ml of IFN-β or were left unstimulated. Relative expression levels were normalized to the SEAP activity and expressed as changes in activation. Black bars represent the average titers obtained from triplicate samples. Standard deviations are also indicated.
Experimental infection of cynomolgus monkeys with wtMV and wtMV(C−).
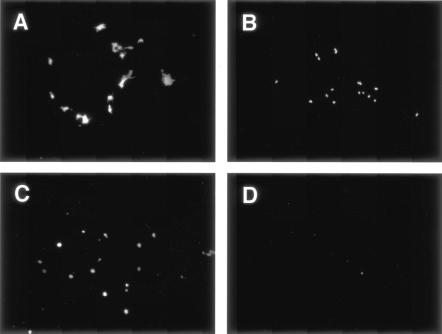
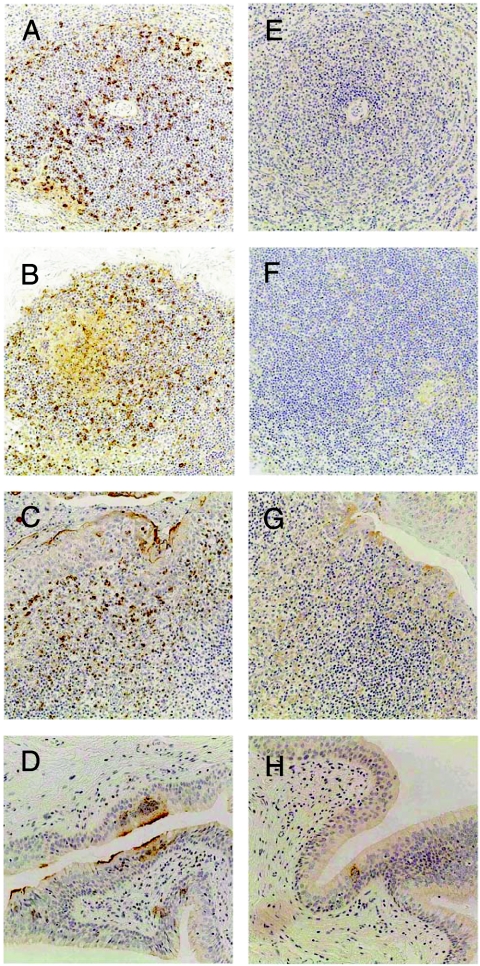
Because wtMV(C−) exhibited a specific growth defect in vitro, we examined its replication and pathogenesis in vivo. For this purpose, groups of three 3-year-old cynomolgus monkeys were inoculated intranasally with 105 TCID50 of wtMV or wtMV(C−), and the number of MV-infected cells per 105 PBMCs was counted at 3, 7, and 11 dpi, as reported previously (46). In addition, one animal from each group was autopsied at 3, 7, and 11 dpi, and the number of MV-infected cells per 105 tissue cells was counted. Koplik spots developed at 7 to 9 dpi in monkeys infected with wtMV but not in those infected with wtMV(C−). Lymphopenia and maculopapular rashes (46) were not observed at this time, even in wtMV-infected monkeys. The number of MV-infected cells was below the detectable level for all monkeys at 3 dpi (Table 1). At 7 dpi, up to 1,024 MV-infected cells/105 cells were detected in PBMCs or tissues from wtMV-infected monkeys. In contrast, ≤128 MV-infected cells/105 cells were detected in PBMCs or tissues from wtMV(C−)-infected monkeys. At 11 dpi, up to 16,384 MV-infected cells/105 cells were detected in PBMCs or tissues from wtMV-infected monkeys, whereas ≤64 MV-infected cells/105 cells were detected in PBMCs or tissues from wtMV(C−)-infected monkeys. Next, we used immunohistochemistry to examine the distribution of MV antigens in tissues from monkeys autopsied at 11 dpi. Large amounts of MV antigen were detected in the spleen, lymph nodes, tonsils, and larynx of a monkey infected with wtMV (Fig. 6), which is similar to the findings for human measles cases (56). In contrast, only a small amount of MV antigen was detected in tissues from a monkey infected with wtMV(C−) (Fig. 6), indicating that the replication of wtMV(C−) was strongly attenuated.
TABLE 1.
Distribution of infected cells in monkey tissues after inoculation of wtMV and wtMV(C−) viruses
| Days after inoculation | Virus | Monkey no. | No. of MV-infected cells/105 cells
|
||||
|---|---|---|---|---|---|---|---|
| PBMC | Cervical lymph node | Mesentric lymph node | Spleen | Thymus | |||
| 3 | wtMV | 4426 | <1 | <1 | <1 | <1 | <1 |
| wtMV | 4427 | <1 | |||||
| wtMV | 4430 | <1 | |||||
| wtMV(C−) | 4423 | <1 | <1 | <1 | <1 | NAa | |
| wtMV(C−) | 4424 | <1 | |||||
| wtMV(C−) | 4425 | <1 | |||||
| 7 | wtMV | 4427 | 16 | 1,024 | 1,024 | 362 | <1 |
| wtMV | 4430 | 362 | |||||
| wtMV(C−) | 4424 | <1 | 128 | <1 | <1 | <1 | |
| wtMV(C−) | 4425 | 64 | |||||
| 11 | wtMV | 4430 | 362 | 5,793 | 2,896 | 2,896 | 16,384 |
| wtMV(C−) | 4425 | 8 | 64 | 8 | 45 | 8 | |
NA, not applicable.
FIG. 6.
Spread of MV antigen in different tissues from infected cynomolgus monkeys. Sections were obtained from cynomolgus monkeys 11 days after wtMV (A to D) or wtMV(C−) (E to H) inoculation. The MV antigen (light brown) in spleens (A and E), lymph nodes (B and F), tonsils (C and G), and larynxes (D and H) was detected with a rabbit antiserum against the MV N protein. Nuclei were counterstained with hematoxylin.
DISCUSSION
Previous studies indicated that paramyxovirus C proteins are multifunctional proteins. Sendai virus (SeV) C proteins inhibit both viral transcription and replication (5, 18), inhibit the IFN-α/β response (12, 13), and are required for virus assembly and budding (15, 45). The human paramyxovirus type 3 (HPIV3) C protein inhibits viral transcription (29). The Nipah virus C protein has IFN-antagonist activity (39). It has been reported that the MV C protein regulates viral RNA synthesis (43) and inhibits the IFN-α/β response (44).
Previous studies have shown that the MV C-EdB strain has a growth defect in human peripheral blood cells and HeLa cells (10, 44) but not in Vero, CV-1, B95-8a, human neuroblastoma SK-N-MC, or primary mouse neuronal cells expressing CD46 (40, 41, 44), indicating that the growth of MV C-EdB is cell type specific. This is consistent with the present finding that wtMV(C−) grows normally in B95a cells but not in 293/hSLAM cells (Fig. 2 and 3). A cell-specific factor appears to regulate the growth of MV and appears to be more strictly necessary for the replication of MV lacking expression of the C protein. In this respect, it is interesting that a cellular 58-kDa protein has been shown to bind to the MV C protein (28). The identification of the 58-kDa protein may help to elucidate the function of the MV C protein.
Several reports have indicated that mutant paramyxoviruses, such as SeV lacking expression of the C protein or containing a mutation in the C protein, simian virus 5 lacking the SH gene or containing mutations in the P/V gene, and Newcastle disease virus lacking the V protein, induce apoptosis (17, 20, 23, 26, 38, 52, 53). These results suggest that the replication of paramyxoviruses induces apoptosis and that paramyxoviruses have evolved to encode a specific protein(s) that blocks apoptosis. It would be interesting to know whether the cell death induced by wtMV(C−) (Fig. 4) is apoptotic cell death. Experiments are under way to elucidate this point.
Although the C protein of the Ed strain has been shown to inhibit IFN-α/β signaling (44), we did not clearly detect an inhibition of IFN-α/β signaling by the C protein of the wild-type IC-B strain (Fig. 5C). In addition, we did not detect a strong inhibition of IFN-α/β signaling by the C protein of the Ed-tag strain under our experimental conditions. Shaffer et al. (44) used Vero cells, 100 U of recombinant IFN-β, pISRE and pGAS reporter plasmids made by R. E. Randall, and a C protein expression plasmid synthesized based on the Ed wild-type strain. On the other hand, we used HepG2 and 293T cells, 1,000 IU/ml of IFN-β, the pISRE-TA-Luc reporter plasmid (Clontech), and C protein expression plasmids based on the IC-B wild-type strain. At present, we cannot provide any reasonable explanations for the discrepancies between their results and our results. The possibility cannot be ruled out that these differences in materials might have affected the results. Recently, Ohno et al. (35) detected weak IFN-antagonist activities from the C proteins of MV strains. This is consistent with our present results.
Although a previous study indicated that MV EdB and MV C-EdB have similar viral spreads and viral loads in the central nervous systems of transgenic mice (40), the present results indicate a large difference between wtMV and wtMV(C−) in the spread of MV antigens in the spleens, lymph nodes, tonsils, and larynxes of cynomolgus monkeys. Large amounts of MV antigens were detected in tissues infected with wtMV but not in tissues infected with wtMV(C−) (Fig. 6). The finding that infectious wtMV viruses were isolated from PBMCs, lymph nodes, spleens, and thymuses of cynomolgus monkeys but that virus recovery was severely restricted in tissues from monkeys infected with wtMV(C−) is consistent with this (Table 1). It is interesting that >10% of the cells in the thymus were infected in a monkey infected with wtMV (Table 1). A massive infection of lymphocytes and the subsequent loss of infected cells may be one of the causes of the immune suppression that has been reported for human measles.
Several recombinant paramyxoviruses lacking the C protein have been generated by reverse genetics and analyzed in vitro and in vivo. A SeV virus lacking all four C-derived proteins replicated extremely inefficiently in vitro and in an attenuated manner in mice (24). HPIV3 lacking the C protein exhibits attenuated replication in LLC-MK2 cells and in rodents and primates (7). A rinderpest virus lacking the C protein showed specific growth defects in primary bovine skin fibroblasts (3). These findings are in good agreement with the present results and support the hypothesis that the C proteins are categorically nonessential gene products that greatly contribute to the in vitro replication capacity and in vivo multiplication and pathogenesis (31).
Acknowledgments
We thank Y. Yanagi for providing Vero/hSLAM cells and the p(+)MV323-EGFP plasmid, M. A. Billeter for providing 293-3-46 cells and the pEMC-La plasmid, and M. Kohase for providing rabbit antisera against human IFN-α and human IFN-β. We thank Y. Nagai for providing critical comments about the manuscript. We also thank A. Kato, A. Masumi, S. Saito, M. Hishiyama, and M. Tashiro for their helpful comments.
This work was supported in part by a grant-in-aid from the Ministry of Health, Labor, and Welfare and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Alkhatib, G., B. Massie, and D. J. Briedis. 1988. Expression of bicistronic measles virus P/C mRNA by using hybrid adenoviruses: levels of C protein synthesized in vivo are unaffected by the presence or absence of upstream P initiator codon. J. Virol. 62:4059-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180:950-958. [DOI] [PubMed] [Google Scholar]
- 3.Baron, M., and T. Barrett. 2000. Rinderpest viruses lacking the C and V proteins show specific defects in growth and transcription of viral RNAs. J. Virol. 74:2603-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53:908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran, J., J. B. Marq, and D. Kolakofsky. 1992. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology 189:647-656. [DOI] [PubMed] [Google Scholar]
- 6.Dörig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 7.Durbin, A. P., J. M. McAuliffe, P. L. Collins, and B. R. Murphy. 1999. Mutations in the C, D, and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology 261:319-330. [DOI] [PubMed] [Google Scholar]
- 8.Enders, J. F., S. L. Katz, and M. V. Milovanovic. 1960. Studies of an attenuated measles virus vaccine. I. Development and preparation of the vaccine: technics for assay of effects of vaccination. N. Engl. J. Med. 263:153-159. [DOI] [PubMed] [Google Scholar]
- 9.Erlenhoefer, C., W. J. Wurzer, S. Löffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escoffier, C., S. Manié, S. Vincent, C. P. Muller, M. A. Billeter, and D. Gerlier. 1999. Nonstructural C protein is required for efficient measles virus replication in human peripheral blood cells. J. Virol. 73:1695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda, A., M. Hishiyama, Y. Umino, and A. Sugiura. 1987. Immunocytochemical focus assay for potency determination of measles-mumps-rubella trivalent vaccine. J. Virol. Methods 15:279-284. [DOI] [PubMed] [Google Scholar]
- 12.Garcin, D., P. Laorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 73:6559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh, B., K. Takeuchi, T. Komatsu, J. Yokoo, Y. Kimura, A. Kurotani, A. Kato, and Y. Nagai. 1999. Knockout of the Sendai virus C gene eliminates the viral ability to prevent the interferon-α/β-mediated responses. FEBS Lett. 459:205-210. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. A. Martin, R. A. Lamb, B. Roizman, and S. E. Straus (ed.), Virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 15.Hasan, M. K., A. Kato, M. Muranaka, R. Yamaguchi, Y. Sakai, I. Hatano, M. Tashiro, and Y. Nagai. 2000. Versatility of accessory C proteins of Sendai virus: contribution to virus assembly as an additional role. J. Virol. 74:5619-5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto, K., N. Ono, H. Tatsuo, H. Minagawa, M. Takeda, K. Takeuchi, and Y. Yanagi. 2002. SLAM(CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 76:6743-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, B., G. Y. Lyn, J. E. Durbin, R. K. Durbin, and R. A. Lamb. 2001. The SH integral membrane protein of paramyxovirus simian virus 5 is required to block apoptosis in MDBK cells. J. Virol. 75:4068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horikami, S. M., R. E. Hector, S. Smallwood, and S. A. Moyer. 1997. The Sendai virus C protein binds the L polymerase protein to inhibit viral RNA synthesis. Virology 235:261-270. [DOI] [PubMed] [Google Scholar]
- 19.Hsu, E. C., C. Iorio, F. Sarangi, A. A. Khine, and C. D. Richardson. 2001. CDw150(SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 279:9-21. [DOI] [PubMed] [Google Scholar]
- 20.Itoh, M., H. Hotta, and M. Homma. 1998. Increased induction of apoptosis by a Sendai virus mutant is associated with attenuation of mouse pathogenicity. J. Virol. 72:2927-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobune, F., H. Takahashi, K. Terao, T. Ohkawa, Y. Ami, Y. Suzaki, N. Nagata, H. Sakata, K. Yamanouchi, and C. Kai. 1996. Nonhuman primate models of measles. Lab. Anim. Sci. 46:315-320. [PubMed] [Google Scholar]
- 23.Koyama, A. H., H. Irie, A. Kato, Y. Nagai, and A. Adachi. 2003. Virus multiplication and induction of apoptosis by Sendai virus: role of the C proteins. Microbes Infect. 5:373-378. [DOI] [PubMed] [Google Scholar]
- 24.Kurotani, A., K. Kiyotani, A. Kato, T. Shioda, Y. Sakai, K. Mizumoto, T. Yoshida, and Y. Nagai. 1998. Sendai virus C proteins are categorically nonessential gene products but silencing their expression severely impairs viral replication and pathogenesis. Genes Cells 3:111-124. [DOI] [PubMed] [Google Scholar]
- 25.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication, p. 1305-1340. In D. M. Knipe, P. M. Howley, D. E. Griffin, M. A. Martin, R. A. Lamb, B. Roizman, and S. E. Straus (ed.), Virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 26.Lin, Y., A. C. Bright, T. A. Rothermel, and B. He. 2003. Induction of apoptosis by paramyxovirus simian virus 5 lacking a small hydrophobic gene. J. Virol. 77:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liston, P., and D. J. Briedis. 1995. Ribosomal frameshifting during translation of measles virus P protein mRNA is capable of directing the synthesis of a unique protein. J. Virol. 69:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liston, P., C. DiFlumeri, and D. J. Briedis. 1995. Protein interactions entered into by the measles virus P, V, and C proteins. Virus Res. 38:241-259. [DOI] [PubMed] [Google Scholar]
- 29.Malur, A. G., M. A. Hoffman, and A. K. Banerjee. 2004. The human parainfluenza virus type 3 (HPIV 3) C protein inhibits viral transcription. Virus Res. 99:199-204. [DOI] [PubMed] [Google Scholar]
- 30.Mrkic, B., B. Odermatt, M. A. Klein, M. A. Billeter, J. Pavlovic, and R. Cattaneo. 2000. Lymphatic dissemination and comparative pathology of recombinant measles virus in genetically modified mice. J. Virol. 74:1364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai, Y., and A. Kato. 2004. Accessory genes of the Paramyxoviridae, a large family of nonsegmented negative strand RNA viruses, as a focus of active investigation by reverse genetics. Curr. Top. Microbiol. Immunol. 283:198-248. [DOI] [PubMed] [Google Scholar]
- 32.Nagata, N., T. Iwaswaki, Y. Ami, Y. Sato, I. Hatano, A. Harashima, Y. Suzaki, T. Yoshii, T. Hashikawa, T. Sata, Y. Horiuchi, S. Koike, T. Kurata, and A. Nomoto. 2004. A poliomyelitis model through mucosal infection in transgenic mice bearing human poliovirus receptor, TgPVR21. Virology 321:87-100. [DOI] [PubMed] [Google Scholar]
- 33.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 35.Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 85:2991-2999. [DOI] [PubMed] [Google Scholar]
- 36.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palosaari, H., J.-P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77:7635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, M.-S., A. García-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, M.-S., M. L. Shaw, J. Muñoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. García-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. A. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 267:80-89. [DOI] [PubMed] [Google Scholar]
- 41.Radecke, F., and M. A. Billeter. 1996. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology 217:418-421. [DOI] [PubMed] [Google Scholar]
- 42.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dötsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reutter, G. L., C. Coutese-Grogan, J. Wilson, and S. A. Moyer. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285:100-109. [DOI] [PubMed] [Google Scholar]
- 44.Shaffer, J. A., W. J. Bellini, and P. A. Rota. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315:389-397. [DOI] [PubMed] [Google Scholar]
- 45.Sugahara, F., T. Uchiyama, H. Watanabe, Y. Shimazu, M. Kuwayama, Y. Fujii, K. Kiyotani, A. Adachi, N. Kohno, T. Yoshida, and T. Sakaguchi. 2004. Paramyxovirus Sendai virus-like particle formation by expression of multiple viral proteins and acceleration of its release by C protein. Virology 325:1-10. [DOI] [PubMed] [Google Scholar]
- 46.Takeda, M., K. Takeuchi, N. Miyajima, F. Kobune, Y. Ami, N. Nagata, Y. Suzaki, Y. Nagai, and M. Tashiro. 2000. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 74:6643-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi, K., S. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon (IFN)-α/β but not IFN-γ signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545:177-182. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi, K., N. Miyajima, F. Kobune, and M. Tashiro. 2000. Comparative nucleotide sequence analyses of the entire genomes of B95a cell-isolated and Vero cell-isolated measles viruses from the same patient. Virus Genes 20:253-257. [DOI] [PubMed] [Google Scholar]
- 49.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 50.Valsamakis, A., H. Schneider, P. G. Auwaerter, H. Kaneshima, M. A. Billeter, and D. E. Griffin. 1998. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 72:7754-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Binnendijk, R. S., R. W. J. van der Heijden, G. van Amerongen, F. G. C. M. UytdeHaag, and A. D. M. E. Osterhaus. 1994. Viral replication and development of specific immunity in macaques after infection with different measles virus strains. J. Infect. Dis. 170:443-448. [DOI] [PubMed] [Google Scholar]
- 52.Wansley, E. K., J. M. Grayson, and G. D. Parks. 2003. Apoptosis induction and interferon signaling but not IFN-β promoter induction by an SV5 P/V mutant are rescued by coinfection with wild-type SV5. Virology 316:41-54. [DOI] [PubMed] [Google Scholar]
- 53.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitution in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 76:10109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamanouchi, K., Y. Egashira, N. Uchida, H. Komada, F. Kobune, M. Hayami, A. Fukuda, and A. Shishido. 1970. Giant cell formation in lymphoid tissues of monkeys inoculated with various strains of measles virus. Jpn. J. Med. Sci. Biol. 23:131-145. [DOI] [PubMed] [Google Scholar]
- 55.Yokota, S., H. Saito, T. Kubota, N. Yokosawa, K. Amano, and N. Fujii. 2003. Measles virus suppresses interferon-α signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-α receptor complex. Virology 306:135-146. [DOI] [PubMed] [Google Scholar]
- 56.Zaki, S. R., and W. J. Bellini. 1997. Measles, p. 233-244. In D. H. Connor, F. W. Chandler, D. A. Schwartz, H. J. Manz, E. E. Lack, J. K. Baird, and J. P. Utz (ed.), Pathology of infectious diseases. Appleton & Lange, Stamford, Conn.