Abstract
The human cytomegalovirus (HCMV) TRS1 and IRS1 genes rescue replication of vaccinia virus (VV) that has a deletion of the double-stranded RNA binding protein gene E3L (VVΔE3L). Like E3L, these HCMV genes block the activation of key interferon-induced, double-stranded RNA (dsRNA)-activated antiviral pathways. We investigated the hypothesis that the products of these HCMV genes act by binding to dsRNA. pTRS1 expressed by cell-free translation or by infection of mammalian cells with HCMV or recombinant VV bound to dsRNA. Competition experiments revealed that pTRS1 preferentially bound to dsRNA compared to double-stranded DNA or single-stranded RNA. 5′- and 3′-end deletion analyses mapped the TRS1 dsRNA-binding domain to amino acids 74 through 248, a region of identity to pIRS1 that contains no homology to known dsRNA-binding proteins. Deletion of the majority of this region (Δ86-246) completely abrogated dsRNA binding. To determine the role of the dsRNA-binding domain in the rescue of VVΔE3L replication, wild-type or deletion mutants of TRS1 were transfected into HeLa cells, which were then infected with VVΔE3L. While full-length TRS1 rescued VVΔE3L replication, deletion mutants affecting a carboxy-terminal region of TRS1 that is not required for dsRNA binding failed to rescue VVΔE3L. Analyses of stable cell lines revealed that the carboxy-terminal domain is necessary to prevent the shutoff of protein synthesis and the phosphorylation of eIF2α after VVΔE3L infection. Thus, pTRS1 contains an unconventional dsRNA-binding domain at its amino terminus, but a second function involving the carboxy terminus is also required for countering host cell antiviral responses.
One early response to acute viral infections is the production of alpha/beta interferons by the host immune system. These interferons induce transcription of many genes, including protein kinase R (PKR) and 2-5 oligoadenylate synthetase (2-5 OAS) (46), both of which are activated by double-stranded RNA (dsRNA) (35). Activated PKR phosphorylates eukaryotic translation initiation factor eIF2α, leading to cessation of translation initiation. 2-5 OAS activates RNase L, a latent endoribonuclease that degrades rRNA and mRNAs, thereby inhibiting protein synthesis. Since viruses rely upon the host cell for protein synthesis, the net result of PKR and 2-5 OAS activation is the inhibition of viral replication and spread.
Many viruses possess one or more mechanisms to counteract these pathways (35). Vaccinia virus (VV) encodes a dsRNA-binding protein, pE3L, as well a PKR pseudosubstrate, pK3L (reviewed in reference 21). pE3L binds specifically to dsRNA via a dsRNA-binding domain (dsRBD) located at its carboxy terminus (9, 10, 19, 20). Infection with VV from which the E3L gene has been deleted (VVΔE3L) results in PKR and RNaseL activation and consequently shuts off protein synthesis in many cell types (2-4, 26, 27). The dsRBD of pE3L is necessary and sufficient for restoration of the full host cell range of VVΔE3L in cell culture (44), and dsRNA-binding proteins from other viruses have been shown to rescue replication of VVΔE3L in otherwise nonpermissive cells (2, 28).
Previously, we reported that the human cytomegalovirus (HCMV) TRS1 and IRS1 genes decrease levels of phosphorylated eIF2α, inhibit RNase L activation, prevent the shutoff of cellular protein synthesis, and rescue replication of VVΔE3L in human fibroblasts (HF) (11). These findings suggested that, like E3L, the HCMV genes may encode dsRNA-binding proteins. We now report that the TRS1 gene product, pTRS1, does indeed contain a dsRNA-binding domain at its amino terminus. However, unlike E3L, the pTRS1 dsRBD is not sufficient to rescue VVΔE3L replication; a second carboxy-terminal domain is also necessary.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium supplemented with 10% NuSerum (Collaborative Biomedical), penicillin-streptomycin (100 U/ml), and 2 mM l-glutamine. VVΔE3L, the E3L deletion mutant and its parent VV Copenhagen strain (VC-2, vP1080 [2, 49]), obtained from Bertram Jacobs (Arizona State University), and VVeq904, a recombinant derived from VVΔE3L containing TRS1, were propagated as previously described (11). HCMV(GFP Toledo) (strain HV5.111 (23) was obtained from Jeff Vieira (Fred Hutchinson Cancer Research Center).
Stable cell lines were created by transfecting HeLa cells with pEQ879, pEQ876, pEQ979, and pEQ1001 (described below) followed by selection with G418 ([0.6 mg/ml]).
Plasmids.
HCMV Towne strain was PCR amplified using oligonucleotides GCCTCGACGTCGGATCCGTCCGGCGGCCATGGCC (#356) and CATTAAGCTTCTCGTAAGCATGTTGACAACTGCAA (#362) as well as oligonucleotides #356 and CACAGAATTCTCGTAAGCATGTTGACAACTG (#357). The resulting PCR products were cloned into the pcDNA3.1/V5-His TOPO TA expression vector (Invitrogen) to make pEQ876 and pEQ875, respectively, each containing the entire TRS1 open reading frame (1-796), where the amino acid numbers are based on the published HCMV Towne TRS1 sequence, accession no. AC146851 (31). For purposes of translating the His tag present in pcDNA3.1/V5-His TOPO TA, pEQ876 was PCR amplified using oligonucleotides #356 and TTGAGCATTGTAATGGTAGTG (#463) that eliminate only the TRS1 stop codon. The resulting PCR product was cloned into pcDNA3.1/V5-His TOPO TA (as were all PCR products described below) to make pEQ981 (1-795). Carboxy-terminal truncations of TRS1 were made by PCR amplifying pEQ875 using oligonucleotides #356 and GGTCTGTCGAAGCGCCGG and oligonucleotides #356 and GATCAGCGTGTTGTTCGC to make pEQ894 (1-270) and pEQ900 (1-226), respectively. Additional carboxy-terminal mutants were made by PCR amplifying pEQ876 with oligonucleotide #356 along with oligonucleotides CGTCGCGCGCGGGGTGCC to make pEQ909 (1-260), GTTCGAGCGGTACACCCGCAC (#390) to make pEQ910 (1-248), CGCGCCGTGCCGAAACCA to make pEQ927 (1-240), CTGCCCGTGCGGCTCGGGCGC to make pEQ971 (1-550), AGTCAACGGTGGTTCTGT to make pEQ978 (1-648), CTGAGTAGCACCACCCATCAA to make pEQ979 (1-738), and CTCCAAACCACAGCCTAA to make pEQ1001 (1-703).
Amino-terminal truncation mutants were made by PCR amplifying pEQ876 using oligonucleotide #362 and oligonucleotide ACCATGGGTGCAAGTACTGCGGGTTCG to make pEQ903 (45-796), oligonucleotides #357 and TACCAGGCCATGGGCGCCGTG to make pEQ923 (106-796), oligonucleotides #362 and ACCATGGGCGTCCAACGGGTAGAACCC to make pEQ924 (57-796), oligonucleotides 362 and ACCATGGGCAACAATAGCAACTTTTGG (#400) to make pEQ925 (74-796) and oligonucleotides #362 and ACCATGGTGGAGCGGCAGGCGCTG to make pEQ926 (93-796). pEQ982 (74-795), a truncation equivalent to pEQ925 but lacking the TRS1 stop codon, was made by PCR amplifying pEQ876 with oligonucleotides #400 and #463. pEQ1000 (93-795), a truncation equivalent to pEQ926 but lacking the TRS1 stop codon, was made by digesting pEQ926 with BamHI and PpuMI and ligating the resulting insert fragment into the same sites in pEQ981. pEQ931 (74-248) was made by PCR amplifying pEQ876 with oligonucleotides #400 and #390. To delete amino acids 86 to 246, pEQ876 was PCR amplified using oligonucleotides #356 and GCATCGATAGCGCTCCGGACCGTGCCAAAAGTT to make pEQ989 and oligonucleotides GTGTATCGATGCAACCGGCTGGGC along with #463 to make pEQ990. The EcoRV/ClaI insert pEQ990 was inserted into the EcoRV/ClaI sites in pEQ989 to make pEQ993 (Δ86-246).
The plasmid pBJ375 containing the HCMV UL35 gene was obtained from Bonita Biegalke (University of Ohio). pEQ843 contains the VV E3L gene and has been previously described (11). pEQ879, the pcDNA3.1V5-His TOPO TA vector containing a short noncoding insert, was created by digesting pEQ842 (22) with HindIII followed by religation.
In vitro translation of plasmid constructs. Individual plasmids were linearized by digestion with XhoI, XbaI, or EcoRV followed by in vitro transcription (AmpliScribe T7 Transcription kit; Epicentre) according to manufacturer's instructions. The resulting RNA was translated in rabbit reticulocyte lysate (Promega) according to manufacturer's instructions in the presence of 1 mCi/ml [35S]l-methionine (Translabel; MP Biomedicals Inc.).
dsRNA-agarose binding assay.
Poly(I · C)-agarose beads (Amersham) containing approximately 200 μg of poly(I · C) were washed twice in Buffer A (100 mM KCl, 20 mM HEPES [pH 7.5], 10% glycerol [vol/vol], 5 mM MgOAC, 1 mM dithiothreitol [DTT; Sigma], and 1 mM benzamidine [Sigma]). In vitro-translated, radiolabeled protein was added to the dsRNA beads that had been resuspended in 200 μl Buffer A and 50 μl Buffer B (120 mM KCl, 5 mM MgCl2, 1 mM DTT, 10% glycerol, 1 mM benzamidine, and 1% NP-40 [Calbiochem]) and incubated on a rotator at 4°C for 60 min. The dsRNA-agarose was then washed twice in Buffer C (identical to Buffer A, except 200 mM KCl) and twice in Buffer A, each wash being followed by centrifugation at 200 × g at 4°C for 4 min. Proteins were eluted by boiling the dsRNA-agarose in gel loading buffer for 4 min and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), fluorographic enhancement (EN3HANCE [PerkinElmer]), and autoradiography.
To analyze the dsRNA-binding properties of pTRS1 expressed in cells, HF were mock infected or infected with HCMV green fluorescent protein (GFP) (Toledo), VVeq904 (a VVΔE3L recombinant virus expressing pTRS1) (11), or wild-type VV (VC2) at a multiplicity of infection (MOI) of 3. 24 h postinfection (hpi), and cells were washed twice with ice-cold phosphate-buffered saline and then lysed in Buffer B. Equivalent fractions of each lysate (approximately 106 cell equivalents) were then added to poly(I · C)-agarose beads that had been washed as described above, and incubation, washing, and elution of bound protein was performed as described above.
VVΔE3L rescue by transient transfection.
HeLa cells were grown to 80 to 90% confluence and transfected with plasmid DNA using Lipofectamine (Invitrogen) according to manufacturer's instructions. Forty-eight hours after transfection, cells were infected with VVΔE3L at an MOI of 3. Twenty-four hpi, β-galactosidase (β-gal) activity was assayed by adding 4-methylumbelliferyl β-d-galactoside (MUG) ([0.15 mg/ml]) and measuring the fluorescent cleavage present after 1 h using a Fluoroskan Ascent (ThermoLabsystems) plate reader.
Immunoblot analysis.
Detection of pTRS1 in lysates from infected HFs and after elution from the dsRNA-agarose was accomplished by separating protein on a SDS-12% polyacrylamide gel was followed by transfer to a polyvinylidene difluoride membrane by electroblotting. Monoclonal antibodies specific for pTRS1 were obtained from Thomas Shenk (Princeton University) (42), and immunoblot analysis was carried out with the Western-Star chemiluminescent detection system (TROPIX, Inc.) according to manufacturer's recommendations. Expression of protein from each plasmid after transient transfection was determined by washing the transfected HeLa cells in ice-cold phosphate-buffered saline twice, followed by lysis in 100 mM NaCl, 50 mM Na2HPO4 (pH 8.0), 10% glycerol (vol/vol), 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1% NP-40. After removal of nuclei by centrifugation at 1,200 × g for 10 min, the protein concentrations of the cytoplasmic fractions were determined by fluoraldehyde o-phthalaldehyde (Pierce) assay (16) and equivalent amounts of protein were separated on 12% SDS-polyacrylamide gels. Immunoblot analysis was carried out using Penta · His Antibody (QIAGEN) and Western-Star chemiluminescent detection system (TROPIX, Inc.) according to manufacturer's recommendations.
Proteins from the stable HeLa cells lines were separated on a 12% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Immunoblot analysis was performed using anti-phospho-eIF2α polyclonal antisera (Cell Signaling Technology, Inc.) according to manufacturer's instructions. The membrane was then stripped by washing in glycine stripping buffer (0.2 M glycine, 0.1% SDS, 1% polyoxyethylenesorbitan monolaurate [Tween-20; Sigma]), pH 2.4) two times for 30 min each wash at room temperature. Immunoblot analysis was then performed using anti-eIF2α polyclonal antisera (Cell Signaling Technologies, Inc.) according to manufacturer's instructions.
Radiolabeling of virus-infected cells.
At 6 h post-VV infection, HeLa cells were placed in cysteine- and methionine-lacking medium for 30 min and then pulse labeled with [35S]l-methionine (100 μCi/ml; Translabel; MP Biomedicals Inc.) in medium lacking cysteine and methionine for 1 h. Cells were washed, lysed, and centrifuged as described above. Protein synthesis was assessed by separation of equivalent amounts of protein on a 12% SDS-polyacrylamide gel followed by fluorographic enhancement and autoradiography.
RESULTS
pTRS1 expressed by cell-free translation or infection binds dsRNA.
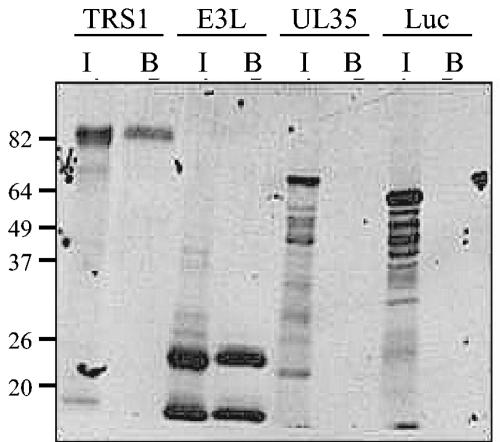
Since previous work determined that TRS1 can complement E3L function in restoring the host cell range of VVΔE3L replication (11), we tested the hypothesis that pTRS1, like pE3L, is a dsRNA-binding protein. In vitro-translated, radiolabeled pTRS1 was incubated with poly(I · C)-agarose beads, and protein binding was detected by SDS-PAGE and autoradiography as described in Materials and Methods. In vitro-translated pE3L was used as a positive control for the experiment along with two negative controls, pUL35, an HCMV structural protein with no known nucleic acid binding properties (30), and firefly luciferase. In vitro translation of TRS1, E3L, UL35, and luciferase yielded proteins of approximately 82 kDa, 20 and 25 kDa (due to initiation of translation at two in-frame AUG codons in the E3L mRNA), 72 kDa, and 61 kDa, respectively, consistent with their predicted sizes (Fig. 1, lanes I). Both forms of the pE3L bound to the dsRNA-agarose, as expected (lane B). pTRS1 also bound to dsRNA, although somewhat less completely than pE3L. Neither UL35 nor luciferase exhibited any binding to the dsRNA-agarose beads, indicating that the conditions used in the experiment resulted in little or no nonspecific binding.
FIG. 1.
In vitro-translated TRS1 binds to dsRNA-agarose. TRS1, E3L, HCMV UL35, and luciferase (luc) mRNAs were translated in rabbit reticulocyte lysate in the presence of [35S] methionine. An aliquot of 7.5 μl of the reactions was incubated with poly(I · C)-agarose beads, and bound protein (lanes B) was analyzed by SDS-PAGE and autoradiography as described in Materials and Methods. An amount of 1.5 μl of each translation reaction mixture was loaded in lanes I. Molecular size markers (in kDa) are indicated on the left.
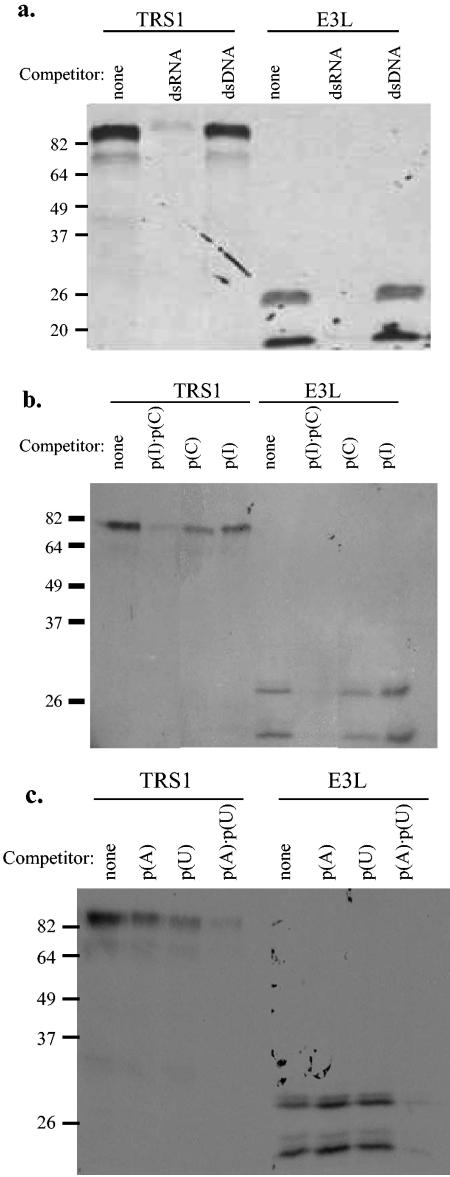
Next, we performed competition experiments to assess the nucleic acid-binding specificity of pTRS1. In vitro-translated pTRS1 or pE3L was incubated with 200 μg (approximately equal to the estimated mass of dsRNA attached to the agarose) of free dsRNA [poly(I · C)] or free dsDNA (salmon sperm DNA) for 30 min prior to performing the dsRNA-agarose binding assay. Only preincubation with dsRNA prevented binding of pTRS1 and pE3L to the agarose beads, indicating that TRS1 binds dsRNA but not double-stranded DNA (dsDNA) (Fig. 2a). Preincubation with free poly(I) or poly(C) alone reduced binding of pTRS1 to the dsRNA agarose but had much less effect than poly(I · C) (Fig. 2b). Similarly, pTRS1 binding to poly(I · C) beads was competed by duplex poly(A · U) while the individual single-stranded homopolymers had much less competitive effect (Fig. 2c). Together, these data demonstrate that in vitro-translated pTRS1 binds preferentially to dsRNA in a sequence-independent manner.
FIG. 2.
Specificity of TRS1 binding to dsRNA. (a) A total of 7.5 μl of in vitro-translated radiolabeled pTRS1 and pE3L was incubated with 200 μg of free dsRNA [poly(I · C)] or free dsDNA (salmon sperm DNA) for 30 min prior to incubation with dsRNA-agarose. (b and c) Competition experiments were performed in the same manner with 200 μg of free poly(I), poly(C), poly(I · C), poly(A), poly(U), and poly(A · U).
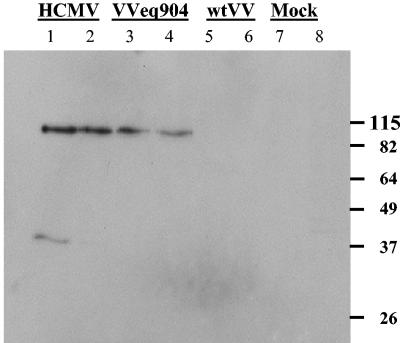
In order to determine whether pTRS1 expressed in mammalian cells by viral infection could also bind to dsRNA, HF were mock infected or infected for 24 h with HCMV GFP (Toledo), a VV recombinant virus expressing TRS1 (VVeq904), or wild-type VV (wtVV). As expected, pTRS1 was detected by immunoblot in lysates from HF infected with HCMV and VVeq904 but not in mock- or wtVV-infected HF (Fig. 3, lanes 1, 3, 5, 7). pTRS1 in HCMV and VVeq904 lysates was pulled down using dsRNA-agarose (Fig. 3, lanes 2, 4). Thus, pTRS1 expressed either by cell-free translation or in cells by viral infection binds to dsRNA.
FIG. 3.
pTRS1 expressed by viral infection binds dsRNA-agarose. Lysates of mock-infected HFs or HFs infected with HCMV(GFP-Toledo), VVeq904 (a VV recombinant expressing pTRS1), or wtVV were prepared 24 hpi. Approximately 106 cell equivalents of each lysate were incubated with dsRNA-agarose. The presence of pTRS1 in unfractionated lysates (lanes 1, 3, 5, 7) and after incubation with dsRNA-agarose (lanes 2, 4, 6, 8) was analyzed by SDS-PAGE and immunoblotting using pTRS1-specific antibodies. A total of 2.5-fold more lysate was used in each binding experiment compared to the amount of total lysate shown in lanes 1, 3, 5, and 7.
The dsRBD is located near the amino terminus of TRS1.
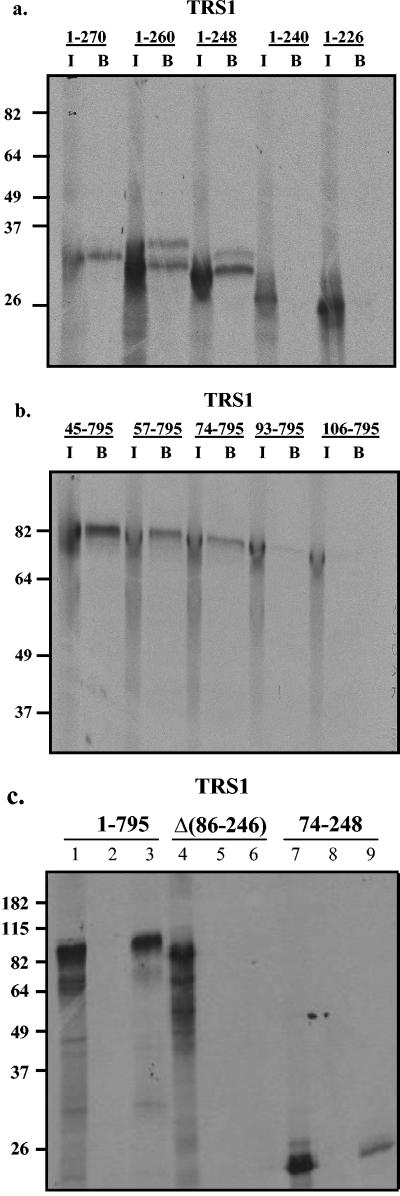
Inspection of the pTRS1 amino acid sequence did not reveal regions of homology to known dsRBDs of other dsRNA-binding proteins (15, 36). Therefore, in order to delineate the domain of TRS1 responsible for binding dsRNA, we made serial truncations of TRS1 from the carboxy terminus and assessed the dsRNA-binding properties of each in vitro-translated mutant using dsRNA-agarose pulldown assays as described above. TRS1 truncations were made by digesting pEQ876 with PpuMI, SgrAI, and MluI, which cut immediately after nucleotides 1707, 854, and 503, respectively, relative to the start of the open reading frame. The protein products of TRS1 cut with PpuMI and SgrAI both bound to dsRNA similarly to full-length TRS1, but the protein product resulting from MluI digestion exhibited no dsRNA-binding activity (data not shown), indicating that the carboxy-terminal boundary of the dsRBD lay in the 117-amino-acid region encoded by sequences between the SgrAI and MluI restriction sites. This boundary of the dsRBD was further defined by PCR-amplifying TRS1 with a 5′ oligonucleotide annealing at the TRS1 start site along with 3′ oligonucleotides designed to divide this 117-amino-acid region. Truncations containing amino acids 1 to 270, 1 to 260, and 1 to 248 all bound dsRNA, while those containing amino acids 1 to 240 and 1 to 226 did not (Fig. 4a). Although we do not know the reason for there being protein doublets evident in the bound fractions of the 1 to 260 and 1 to 248 lanes, these might be a result of partial retention of the final decoding tRNA fused to the proteins following cell-free translation, or they could be due to incomplete plasmid digestion prior to in vitro transcription which would result in addition of a few additional carboxy-terminal polylinker-encoded amino acids. Regardless, these results demonstrate that the carboxy-terminal boundary of the TRS1 dsRBD lies between amino acids 240 and 248.
FIG. 4.
Delineation of the TRS1 dsRNA-binding domain. Truncations of pTRS1 were made by deleting from the (a) carboxy and (b) amino termini, and each in vitro-translated mutant was tested for dsRNA-binding activity using the dsRNA-agarose pulldown assay. Numbers indicate the range of TRS1 amino acids that were contained in each protein. (c) Binding of in vitro-translated proteins (lanes 1, 4, 7) to dsRNA-agarose (lanes 3, 6, 9) and to agarose alone (lanes 2, 5, 8) was assayed for the minimal dsRBD (74-248) and for pTRS1 in which the dsRBD was deleted (Δ86-246).
To define the amino-terminal boundary of the dsRBD, serial amino-terminal truncations, each of which included the full carboxy-terminus of TRS1, were prepared as described in Materials and Methods. Proteins containing amino acids 45 to 795, 57 to 795, and 74 to 795 of TRS1 each bound dsRNA, while those corresponding to amino acids 93 to 795 and 106 to 795 exhibited little or no dsRNA-binding activity (Fig. 4b).
These results suggested that the dsRBD of pTRS1 is located between amino acids 74 and 248. Consistent with this conclusion, in vitro-translated protein consisting solely of amino acids 74 to 248 clearly bound dsRNA, albeit to a lesser extent than full-length pTRS1, while deletion of amino acids 86 to 246 from full-length TRS1 resulted in complete abrogation of dsRNA-binding activity (Fig. 4c, lanes 3, 6, 9). As an additional control in this experiment, we incubated the proteins with agarose that did not have dsRNA attached and detected no nonspecific binding (Fig. 4c, lanes 2, 5, 8).
The dsRBD of pTRS1 is not sufficient for restoration of VVΔE3L host cell range.
The dsRBD of pE3L is necessary and sufficient for rescue of VVΔE3L replication in nonpermissive cell lines such as HeLa cells (44). To determine whether the same is true for TRS1, we transfected HeLa cells with a plasmid containing amino acids 74 to 248, the TRS1 dsRBD defined by the dsRNA-agarose pulldown assay, and infected the cells 48 hpi with VVΔE3L at an MOI of 3. Since VVΔE3L contains a lacZ cassette under the control of a late VV promoter, β-gal measured 24 hpi serves as a marker of viral replication (11). Additionally, the same plasmid was used to create a stable HeLa cell line expressing this region. The dsRBD did not rescue VVΔE3L replication by either method compared to full-length TRS1, although expression of this small protein as determined by anti-His immunoblot was poor in both cases (data not shown). These results raised the possibility that the dsRNA-binding domain of TRS1 may not be sufficient to rescue VVΔE3L replication.
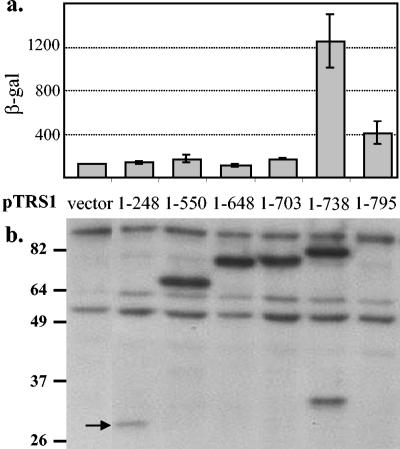
Next, plasmids containing full-length or carboxy-terminal truncations of TRS1 were transfected into HeLa cells in order to assess their ability to rescue VVΔE3L replication. Expression of full-length pTRS1 and of amino acids 1 to 738 increased β-gal expression after VVΔE3L infection, but carboxy-terminal deletions to amino acids 248, 556, 648, and 703 failed to do so (Fig. 5a).
FIG. 5.
The dsRBD is not sufficient for rescue of VVΔE3L host cell range. Plasmids containing an empty vector backbone (pEQ879), full-length TRS1 (1-795), and the indicated carboxy-terminal truncations of TRS1 were transfected into HeLa cells, followed 48 h later by infection with VVΔE3L. (a) β-Gal activity was measured 24 hpi from duplicate transfections as a surrogate marker of VVΔE3L replication. (b) Expression of each protein was determined by immunoblot using anti-His antibodies as described in Materials and Methods.
We examined transgene expression in these cells by immunoblot assays using anti-His antibodies (Fig. 5b). While expression of the shortest protein containing amino acids 1 to 248 (arrow) was not as robust as the other carboxy-terminal truncations, those containing amino acids 1 to 556, 1 to 648, 1 to 703, and 1 to 738 were expressed at similar levels. Although the expression level of the full-length pTRS1 (1 to 795) was difficult to assess since it comigrated with a background band, it did appear to be less abundant than most of the deletion mutants. This observation may explain the relatively weak rescue of VVΔE3L by full-length TRS1 compared to TRS (1 to 738) in this experiment. In other experiments (see, e.g., Fig. 7), expression of the full-length pTRS1 was greater and resulted in stronger rescue of VVΔE3L, highlighting the importance of examining protein expression in transfection experiments. As the majority of the carboxy terminus was necessary for rescuing VVΔE3L replication, these experiments revealed that the dsRBD of pTRS1 is not sufficient for restoration of VVΔE3L replication in HeLa cells.
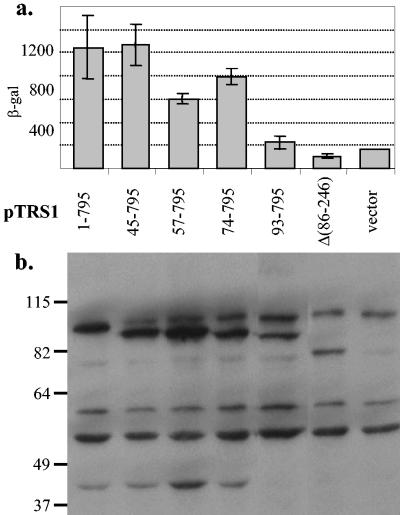
FIG. 7.
The dsRBD is necessary for rescue of VVΔE3L host cell range. Plasmids containing an empty vector (pEQ879), full-length TRS1 (1-795), the indicated amino-terminal truncations of TRS1, and TRS1 in which the dsRBD was deleted (Δ86-246) were transfected into HeLa cells. Forty-eight hours later the cells were infected with VVΔE3L and (a) β-gal activity was measured 24 hpi as a surrogate marker of VVΔE3L replication. (b) Protein expression was determined by immunoblot using anti-His antibodies as described in Materials and Methods.
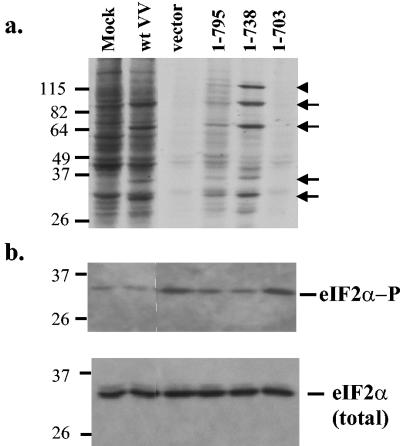
To investigate the role of the pTRS1 carboxy terminus in rescuing VVΔE3L replication further, we established stable HeLa cells lines expressing full-length or carboxy-terminal deletions (1 to 738 and 1 to 703) of pTRS1 as well as a control line containing the empty vector. Analyses of protein synthesis by metabolic labeling revealed a marked reduction after VVΔE3L infection of cells containing the empty vector or pTRS (1 to 703) (Fig. 6a). In contrast, pTRS (1 to 738) and the full-length proteins (1 to 795) enabled continued synthesis of proteins, including viral-specific proteins detectable in cells infected with wild-type VV. The two carboxy-terminal deletions both expressed equal levels of the transgene as judged by immunoblot assays using anti-His tag antiserum (data not shown). The lower abundance of protein synthesis in the cell line containing full-length TRS1 (1 to 795) might be due to lower expression of the protein, but the construct used here did not have a His tag, thus precluding our ability to evaluate its relative expression level.
FIG. 6.
The TRS1 carboxy terminus is necessary to maintain protein synthesis and prevent eIF2α phosphorylation. (a) Stable HeLa cell lines containing the empty vector pEQ879 or expressing full-length pTRS1 (1-795) or carboxy-terminal truncations pTRS (1-738) or pTRS (1-703) were infected with VVΔE3L (10 PFU/cell), and as controls HeLa cells were mock infected or infected with wild-type VV (wtVV; 5 PFU/cell). At 6 h postinfection, cells were labeled for 1 h with [35S]methionine and cell extracts (30 μg) were analyzed by SDS-PAGE and autoradiography. Examples of proteins specific to VV-infected cells (arrows) and β-gal (arrowhead), which is present in VVΔE3L but not wild-type VV, are indicated. (b) Forty micrograms of protein from these same cells was separated by SDS-PAGE and analyzed by immunoblot assays using antibodies specific for phosphorylated eIF2α (top panel) and total eIF2α (bottom panel) as described in Materials and Methods.
We also analyzed eIF2α phosphorylation in these cell lines after VVΔE3L infection. As shown in Fig. 6b, the abundance of phosphorylated eIF2α was elevated in cells containing the empty vector or the 1-to-703 carboxy-terminal deletion compared to the other cell lines. Total eIF2α levels appeared to be similar (Fig. 6b, bottom panel). These data suggest that the carboxy-terminal domain of pTRS1 is needed to prevent dsRNA-mediated eIF2α phosphorylation and the shutoff of protein synthesis.
In order investigate whether the dsRBD is necessary for rescue of VVΔE3L replication, we transfected HeLa cells with plasmids containing amino-terminal truncations of pTRS1. Mutants in which the dsRBD remained intact (45 to 795, 57 to 795, and 74 to 795) rescued VVΔE3L similarly to full-length pTRS1 (1 to 795), whereas mutants in which the dsRBD was disrupted (93 to 795) or deleted (Δ86-246) failed to rescue VVΔE3L replication (Fig. 7a). Analyses of expression of each pTRS1 mutant by anti-His immunoblot (Fig. 7b) revealed that mutants lacking part or all of the dsRBD accumulated to lower levels than those containing the entire dsRBD. Thus, the dsRBD appears to be necessary to rescue VVΔE3L, but these results do not permit clearly distinguishing whether this region is required to enable high levels of protein expression or because of its dsRNA-binding activity.
In our transfection experiments (Fig. 5 and 7), we detected a small immunoreactive protein in extracts from cells transfected with the TRS1 expression constructs that rescued VVΔE3L. Based on its size and immunoreactivity, we suspect that this protein is a degradation product of the longer TRS1 proteins expressed in each of these samples. Expression of the short protein was not dependent on VVΔE3L infection as we detected it in transfected but uninfected cells (data not shown). Although the smaller protein appears to correlate with rescue of VVΔE3L, we found that another plasmid that expresses TRS1 amino acids 86 to 795 also rescued VVΔE3L but did not express the truncated protein (data not shown), suggesting that, regardless of the its origin, it is not essential for rescue of VVΔE3L.
DISCUSSION
Previous studies revealed common as well as distinctive features of the TRS1 and IRS1 genes of HCMV. Both genes are expressed in infected cells from immediate-early until late times after infection, and their protein products are packaged into virions (41). They both augment the stimulatory effect of other HCMV immediate-early proteins on expression of reporter genes controlled by various HCMV promoters (42, 47). Additionally, both genes function in HCMV oriLyt-dependent DNA replication in transient assays, likely by stimulating expression of other viral genes (33, 34). Unlike TRS1, IRS1 encodes a second, smaller protein that inhibits the activation of reporter genes by other immediate early genes (42). TRS1, but not IRS1, is required for efficient HCMV replication in HFs (6, 14, 24). Deletion of the carboxy terminus of TRS1 results in inefficient assembly of virions and reduces viral production by ∼200-fold after a low-MOI infection (6), an effect that may be due in part to inefficient assembly of viral capsids (1).
Recently, we reported that both TRS1 and IRS1 complement the replication defect of VVΔE3L. They both prevent the shutoff of protein synthesis, eIF2α phosphorylation, and RNaseL activation that otherwise occur in most cell types after VVΔE3L infection (11). These results suggested that pTRS1 and pIRS1 might act like pE3L by binding to dsRNA and thereby preventing its ability to activate PKR and 2-5 OAS, the factors that mediate eIF2α phosphorylation and RNaseL activation, respectively. In the current studies, we found that pTRS1 does indeed bind to dsRNA through a domain that is conserved in pIRS1.
dsRNA-binding proteins typically interact with the ribose 2′-OH groups of RNA, thereby conferring specificity for RNA over DNA (5). Consistent with this, in vitro-translated pTRS1 bound to dsRNA but not to dsDNA (Fig. 2a). Similar to most dsRNA-binding proteins (43), pTRS1 apparently binds to dsRNA independent of specific nucleotide sequence, since its binding to dsRNA agarose was inhibited by preincubation with either poly(I · C) or poly(A · U) (Fig. 2b and 2c). However, we did detect a small competitive effect of preincubation with the single-stranded RNAs, especially poly(U), on binding of pTRS1 to dsRNA agarose (Fig. 2c). This observation might result from single-stranded RNA (ssRNA) homopolymers adopting secondary structures as a result of non-Watson-Crick base pairing (48). Alternatively, pTRS1 might bind to some ssRNAs as well as to dsRNA, analogous to the influenza NS1 protein that binds to U6 small nuclear RNA and polyadenlyated mRNA in addition to dsRNA (18, 37-39, 50). Experiments using assays complementary to the dsRNA-agarose pulldown assay will be needed to determine the precise nucleic acid binding properties of TRS1.
Proteins that bind dsRNA are found in a wide variety of organisms, and many contain a conserved core of residues spanning approximately 70 amino acids that define the dsRBD (15, 45). TRS1 does not possess any region with recognizable homology to this characteristic dsRBD. However, other proteins such as the HSV US11 protein and 2-5 OAS bind dsRNA via alternative or unknown motifs (17, 25, 36). Using a dsRNA-agarose pulldown assay, we demonstrated that TRS1 binds to dsRNA through a dsRBD that is located in a 175-amino-acid stretch close to the amino terminus of the protein. This region is considerably larger than the other known dsRBDs, so it may be composed of more than one discontinuous part.
Since the dsRBD of E3L is necessary and sufficient for restoration of VVΔE3L host cell range (44), we were surprised to discover that the dsRBD of pTRS1 was not sufficient for this function. The requirement for carboxy-terminal sequences between codons 703 and 738 (Fig. 5) was particularly unexpected, since this is a region that is divergent between TRS1 and IRS1. Since both genes complement VVΔE3L replication, we had presumed, incorrectly, that the critical domain for this common activity would map to their identical amino-terminal regions, extending up through codon 556. However, the carboxy termini of these two proteins are moderately homologous, with ∼50% of the codons in the interval between codons 703 and 738 of pTRS1 being identical to those in pIRS1. Small stretches of conserved amino acids in the carboxy termini of both proteins may account for the ability of both pTRS1 and pIRS1 to rescue VVΔE3L replication. Alternatively, the carboxy terminus of pTRS1 is relatively charged compared to the rest of the protein, a characteristic which is also present in the carboxy terminus of pIRS1 (47) and perhaps this common feature, and not primary amino acid sequence, is required the TRS1 and IRS1 to rescue VVΔE3L.
Although we have shown that the carboxy-terminal domain of pTRS1 required for rescuing VVΔE3L replication is also required to prevent the shutoff of protein synthesis and the phosphorylation of eIF2α after VVΔE3L infection (Fig. 6), we do not yet know what how the carboxy-terminal domain of pTRS1 functions. It seems unlikely that the reported defect in HCMV capsid assembly resulting from deletion of the TRS1 carboxy terminus (1) is related to its role in rescuing VVΔE3L, since (i) our VVΔE3L complementation assays do not depend on assembly nor does E3L have any known role in VV virion assembly and (ii) the IRS1 carboxy terminus is not required for HCMV assembly (1) but is required for rescue of VVΔE3L (E. Marshall and A. P. Geballe, unpublished data). One hypothesis is that the carboxy-terminal domain is essential for protein oligomerization. Other viral dsRNA-binding proteins, including pE3L and pNS1, form dimers or higher order structures (20, 32). In the case of pE3L, the dimerization domain maps to the same general region as the dsRBD (20). Thus, it is possible that the carboxy terminus of pE3L is sufficient to rescue VVΔE3L host cell range, because it contains both dsRNA-binding and dimerization domains while the two domains of pTRS1 may be separated. Another possibility is that the pTRS1 carboxy terminus is required for interactions with other proteins. The amino-terminal portion of pE3L binds to PKR and is necessary for pathogenesis for inhibiting PKR activity in yeast and for preventing the phosphorylation of eIF2α at late times after VV infection (8, 27, 40). Even though this PKR-interacting domain is not required to rescue VVΔE3L replication by pE3L, such an interaction might be critical for the activity of other double-stranded RNA-binding proteins like pTRS1 that may be less abundant or potent. Similarly, it is possible that carboxy terminus, independent of any protein-protein interaction, is necessary in vivo in order to maintain the pTRS1 structure required for dsRNA-binding.
Like the carboxy terminus, the dsRBD of pTRS1 appears to be necessary to rescue VVΔE3L (Fig. 7). Disruption of the TRS1 dsRBD resulted in lower levels of transgenic protein expression in several but not all experiments (not shown). Thus, our data suggest that the dsRNA-binding activity of the dsRBD is essential, but we cannot completely exclude an indirect role for this domain on pTRS1 accumulation. A more detailed analysis of mutants of the dsRBD will be necessary to determine whether the dsRNA-binding activity is indeed essential for VVΔE3L rescue by pTRS1.
In summary, our results demonstrate that pTRS1 binds dsRNA via an unconventional domain located near its amino terminus, but that in addition the carboxy terminus of the protein is necessary for restoration of VVΔE3L host cell range. Counteracting dsRNA-induced antiviral host defense mechanisms has been shown to be important to the in vivo pathogenesis of several viruses, including herpes simplex virus type 1, influenza virus, and vaccinia virus (7, 8, 12, 13, 29). Therefore, further studies of this dsRNA-binding protein present in HCMV are likely to yield insight towards the understanding of HCMV pathogenesis.
Acknowledgments
We thank Bertram Jacobs (Arizona State University) for VC2 and VVΔE3L, Tom Shenk (Princeton University) for antibodies to TRS1, Jeff Vieira (Fred Hutchinson Cancer Research Center) for HCMV(GFP Toledo), and Bonita Biegalke (University of Ohio) for pBJ375. We also thank Katherine De Niro for technical assistance.
This work was supported by Public Health Service grant AI26672 (A.P.G.) and the Joel Meyers Endowment Fund of the FHCRC, the Roche Postdoctoral Fellowship award of the Infectious Disease Society of America, and NIH AI058089 (M.H.).
REFERENCES
- 1.Adamo, J. E., J. Schroer, and T. Shenk. 2004. Human cytomegalovirus TRS1 protein is required for efficient assembly of DNA-containing capsids. J. Virol. 78:10221-10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. [DOI] [PubMed] [Google Scholar]
- 4.Beattie, E., E. Paoletti, and J. Tartaglia. 1995. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L- and E3L-mutant viruses. Virology 210:254-263. [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua, P. C., and T. R. Cech. 1996. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry 35:9983-9994. [DOI] [PubMed] [Google Scholar]
- 6.Blankenship, C. A., and T. Shenk. 2002. Mutant human cytomegalovirus lacking the immediate-early TRS1 coding region exhibits a late defect. J. Virol. 76:12290-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolovan, C. A., N. M. Sawtell, and R. L. Thompson. 1994. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 68:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, H. W., and B. L. Jacobs. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology 194:537-547. [DOI] [PubMed] [Google Scholar]
- 10.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Child, S. J., M. Hakki, K. L. De Niro, and A. P. Geballe. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 13.Donelan, N. R., C. F. Basler, and A. Garcia-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:13257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fierro-Monti, I., and M. B. Mathews. 2000. Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem. Sci. 25:241-246. [DOI] [PubMed] [Google Scholar]
- 16.Geballe, A. P., and E. S. Mocarski. 1988. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J. Virol. 62:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann, R., J. Justesen, S. N. Sarkar, G. C. Sen, and V. C. Yee. 2003. Crystal structure of the 2′-specific and double-stranded RNA-activated interferon-induced antiviral protein 2′-5′-oligoadenylate synthetase. Mol. Cell 12:1173-1185. [DOI] [PubMed] [Google Scholar]
- 18.Hatada, E., and R. Fukuda. 1992. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73:3325-3329. [DOI] [PubMed] [Google Scholar]
- 19.Ho, C. K., and S. Shuman. 1996. Mutational analysis of the vaccinia virus E3 protein defines amino acid residues involved in E3 binding to double-stranded RNA. J. Virol. 70:2611-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho, C. K., and S. Shuman. 1996. Physical and functional characterization of the double-stranded RNA binding protein encoded by the vaccinia virus E3 gene. Virology 217:272-284. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, B. 2000. Translational control in poxvirus-infected cells, p. 951-971. In H. J. W. B. Sonenberg and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Janzen, D. M., and A. P. Geballe. 2004. The effect of eukaryotic release factor depletion on translation termination in human cell lines. Nucleic Acids Res. 32:4491-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis, M. A., C. E. Wang, H. L. Meyers, P. P. Smith, C. L. Corless, G. J. Henderson, J. Vieira, W. J. Britt, and J. A. Nelson. 1999. Human cytomegalovirus infection of caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J. Virol. 73:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, T. R., and V. P. Muzithras. 1992. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J. Virol. 66:2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoo, D., C. Perez, and I. Mohr. 2002. Characterization of RNA determinants recognized by the arginine- and proline-rich region of us11, a herpes simplex virus type 1-encoded double-stranded RNA binding protein that prevents PKR activation. J. Virol. 76:11971-11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langland, J., and B. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133. [DOI] [PubMed] [Google Scholar]
- 27.Langland, J. O., and B. L. Jacobs. 2004. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology 324:419-429. [DOI] [PubMed] [Google Scholar]
- 28.Langland, J. O., S. Pettiford, B. Jiang, and B. L. Jacobs. 1994. Products of the porcine group C rotavirus NSP3 gene bind specifically to double-stranded RNA and inhibit activation of the interferon-induced protein kinase PKR. J. Virol. 68:3821-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Y., and B. J. Biegalke. 2002. The human cytomegalovirus UL35 gene encodes two proteins with different functions. J. Virol. 76:2460-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemeroff, M. E., X. Y. Qian, and R. M. Krug. 1995. The influenza virus NS1 protein forms multimers in vitro and in vivo. Virology 212:422-428. [DOI] [PubMed] [Google Scholar]
- 33.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pari, G. S., M. A. Kacica, and D. G. Anders. 1993. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67:2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pe'ery, T., and M. B. Mathews. 2000. Viral translational strategies and host defense mechanisms, p. 371-424. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian, X. Y., C. Y. Chien, Y. Lu, G. T. Montelione, and R. M. Krug. 1995. An amino-terminal polypeptide fragment of the influenza virus NS1 protein possesses specific RNA-binding activity and largely helical backbone structure. RNA 1:948-956. [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu, Y., and R. M. Krug. 1994. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A). J. Virol. 68:2425-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu, Y., M. Nemeroff, and R. M. Krug. 1995. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6-U2 and U6-U4 snRNA interactions during splicing. RNA 1:304-316. [PMC free article] [PubMed] [Google Scholar]
- 40.Romano, P. R., F. Zhang, S. L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanowski, M. J., E. Garrido-Guerrero, and T. Shenk. 1997. pIRS1 and pTRS1 are present in human cytomegalovirus virions. J. Virol. 71:5703-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romanowski, M. J., and T. Shenk. 1997. Characterization of the human cytomegalovirus irs1 and trs1 genes: a second immediate-early transcription unit within irs1, whose product antagonizes transcriptional activation. J. Virol. 71:1485-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders, L. R., and G. N. Barber. 2003. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 17:961-983. [DOI] [PubMed] [Google Scholar]
- 44.Shors, T., K. V. Kibler, K. B. Perkins, R. Seidler-Wulff, M. P. Banaszak, and B. L. Jacobs. 1997. Complementation of vaccinia virus deleted of the E3L gene by mutants of E3L. Virology 239:269-276. [DOI] [PubMed] [Google Scholar]
- 45.St. Johnston, D., N. H. Brown, J. G. Gall, and M. Jantsch. 1992. A conserved double-stranded RNA-binding domain. Proc. Natl. Acad. Sci. USA 89:10979-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 47.Stasiak, P. C., and E. S. Mocarski. 1992. Transactivation of the cytomegalovirus ICP36 gene promoter requires the alpha gene product TRS1 in addition to IE1 and IE2. J. Virol. 66:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steitz, T. A. 1999. RNA recognition by proteins. .In R. F. Gesteland, T. R. Cech, and J. F. Atkins (ed.), The RNA world, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davis, J. van der Hoeven, B. Meignier, M. Riviere, et al. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217-232. [DOI] [PubMed] [Google Scholar]
- 50.Wang, W., K. Riedel, P. Lynch, C. Y. Chien, G. T. Montelione, and R. M. Krug. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]