Abstract
We previously reported that herpes simplex virus type 1 (HSV-1) can activate the stress-activated protein kinases (SAPKs) p38 and JNK. In the present study, we undertook a comprehensive and comparative analysis of the requirements for viral protein synthesis in the activation of JNK and p38. Infection with the UL36 mutant tsB7 or with UV-irradiated virus indicated that both JNK and p38 activation required viral gene expression. Cycloheximide reversal or phosphonoacetic acid treatment of wild-type virus-infected cells as well as infection with the ICP4 mutant vi13 indicated that only the immediate-early class of viral proteins were required for SAPK activation. Infection with ICP4, ICP27, or ICP0 mutant viruses indicated that only ICP27 was necessary. Additionally, we determined that in the context of virus infection ICP27 was sufficient for SAPK activation and activation of the p38 targets Mnk1 and MK2 by infecting with mutants deleted for various combinations of immediate-early proteins. Specifically, the d100 (0−/4−) and d103 (4−/22−/47−) mutants activated p38 and JNK, while the d106 (4−/22−/27−/47−) and d107 (4−/27−) mutants did not. Finally, infections with a series of ICP27 mutants demonstrated that the functional domain of ICP27 required for activation was located in the region encompassing amino acids 20 to 65 near the N terminus of the protein and that the C-terminal transactivation activity of ICP27 was not necessary.
The stress-activated protein kinases (SAPKs) p38 and JNK are part of a larger family of serine/threonine terminal kinases termed mitogen-activated protein kinases (MAPK), which includes ERK1 and -2. SAPK pathways are normally activated by UV irradiation, anoxia, and engagement of proinflammatory cytokines or Fas ligand by their cognate receptors. Following ligand binding to a cognate receptor, signaling is initiated through receptor-associated kinases to a MAPKKK (MAPK kinase kinase). MAPKKKs capable of initiating the p38 or JNK pathway include MEKK1, -2, -3, and -4, ASK-1, TAK-1, and TAO. These in turn activate dual-specificity MAPK kinase kinases (MAPKKs) MKK3/6 and MKK4/7, which directly bind and phosphorylate p38 and JNK, respectively, on both tyrosine and threonine, resulting in their activation (for a review, see reference 78).
All three subfamilies of human herpesviruses activate one or more of the MAPKs during infection. The betaherpesvirus cytomegalovirus activates both p38 and ERK by a mechanism dependent on viral gene expression (14, 39, 76) and the upstream kinases MKK3/6 and MKK1/2, respectively, (39, 40). ERK activates the early gene UL112-113 promoter (76) and phosphorylates immediate-early 86 and 72 proteins to alter their transactivation activity (40), while p38 phosphorylates retinoblastoma protein and HSP27 (39). ERK and p38 activation are required for cytomegalovirus DNA replication (16). The gammaherpesvirus Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor activates ERK and p38, causing increased vascular endothelial growth factor expression via phosphorylation of hypoxia-inducible factor 1α (86). The gammaherpesvirus Epstein-Barr virus activates p38 and JNK (1, 21). The immediate-early proteins BZLF1 and BRLF1 induce increased phosphorylation of p38 and JNK and activation of the ATF-2 transcription factor (1). BZLF1 expression during BRLF1-driven virus reactivation requires p38 activity (1). Among the alphaherpesviruses, HSV-2 has been reported to activate ERK through ICP10-PK binding to RAS-GAP, leading to further expression of ICP10 and prevention of apoptosis (68).
Two previous reports documented the activation of the p38 and JNK (SAPK) pathways by HSV-1. Increased JNK and p38 kinase activity was observed as early as 3 hours postinfection and remained active throughout the course of infection (56, 97). SAPKs were activated downstream of Ras, as infection with a dominant-negative form of Ras did not affect activation of the transcription factors ATF-2 and c-Jun, downstream targets of p38 and JNK, respectively (56). Furthermore, activation was specific for SAPKs, as ERK was not activated during infection (56). Overexpression of JIP-1, a JNK scaffold protein, suppressed nuclear import of JNK and reduced virus yield (56). Infection in the presence of small-molecule inhibitors for p38 (SB203580) and JNK (SP600125) resulted in an 85 to 91% drop in virus yield depending on cell type (43), suggesting that activation of the SAPK pathways was essential for efficient viral replication. Besides a role for the MAPKK MKK4 in JNK activation (97), little is understood about the mechanism of SAPK activation in HSV-1-infected cells.
We hypothesize that JNK and p38 are activated by the same mechanism due to the similarity in their activation kinetics and the signaling pathways themselves. The delayed kinetics of JNK and p38 activation (56) suggest that their activation is dependent on viral gene expression. Among the most likely candidates are one or more immediate-early proteins, since significant amounts of these proteins accumulate by 3 hours postinfection, well before the accumulation of significant amounts of delayed-early (DE) or late (L) proteins. Two series of experiments indicated that neither virus binding per se nor the presence of virion components in the cell initiated events leading to JNK activation. In the first, antibody-neutralized virus, capable of binding to but not entering cells, failed to activate JNK (56). In the second, infection with the UL36 mutant tsB7 failed to induce JNK activation (56). By contrast, the ICP4 mutant vi13 activated JNK more efficiently than wild-type virus, consistent with the ability of this mutant to express higher levels of the immediate-early proteins than wild-type virus (85).
To further explore the mechanisms of HSV-induced activation of the SAPK pathways, we conducted experiments to identify virus-encoded effector proteins. In this report, we demonstrate that immediate-early gene expression is sufficient for the activation of both p38 and JNK. In the context of virus infection, ICP27 was sufficient and an ICP27 N-terminal functional domain previously shown to be involved in nuclear export was necessary for SAPK activation.
MATERIALS AND METHODS
Cells and viruses.
CV-1 cells were originally obtained from Saul Silverstein (Columbia University) and were grown in Dulbecco's modified Eagle's medium supplemented with 5% bovine calf serum, 100 U/ml penicillin, 1% streptomycin, and 1% l-glutamine (all from Gibco). Cells were seeded into 100-mm dishes at a density of 2 × 106 cells per plate. Both mock infection and infection with virus were conducted in spent medium for 1 hour at 37°C. The inoculum was then replaced with virus-free spent medium.
The KOS 1.1 strain of HSV-1 was used in all experiments unless otherwise noted. A stock of UV-irradiated virus was prepared and the residual titer was determined by plaque assay on Vero cells. The resulting titer was reduced 2.94 × 103-fold, and therefore when cells were subsequently infected with an amount of virus corresponding to a multiplicity of infection (MOI) of 5 of the original stock, the actual (residual) MOI was 0.0017. The mutant tsB7 (9, 47), the parental HFEM strain of HSV-1, and the KOS ICP27 deletion mutant d27-1 (74) were provided by David Knipe (Harvard University). The ICP4 mutants vi13, n12, and d120 (18, 19, 85) were provided by Neal DeLuca (University of Pittsburgh). The IE multiple mutants d100 (0−/4−), d103 (4−/22−/47−), d106 (4−/22−/27−/47−), d107 (4−/27−), and d109 (0−/4−/22−/27−/47−) were also provided by Neal DeLuca (80). The ICP6 mutant ICP6Δ (27) was provided by Sandra Weller (University of Connecticut). The ICP0 mutant 7134 (11) was provided by Priscilla Schaffer (Harvard Medical School). The ICP27 C-terminal truncation mutants n59r and n504r (74), the in-frame deletion mutants dLeu (51), d1-5, d5-6 (59), d1-2 (71), d2-3, d6-7 (6), d3-4, and d4-5 (58) and the point mutant m11 (70) were all provided by Stephen Rice (University of Minnesota). The temperature-sensitive ICP27 mutant vBSLG4 and a partial revertant, vBS3-3 (83, 87), were provided by Saul Silverstein (Columbia University). A fuller description of these mutants is provided below.
Inhibitor treatment.
Viral gene expression was limited to the immediate-early phase by treating cells for 30 min prior to infection with 0.5 μg per ml of cycloheximide. The same concentration of drug was present in the inoculum and replacement medium. At 3 hours postinfection, monolayers were rinsed with spent medium and then treated with actinomycin D at a concentration of 4 μg per ml of spent medium. To prevent expression of true-late (γ2) viral genes, the viral DNA replication inhibitor phosphonoacetic acid (Sigma) was present in the virus inoculum and replacement medium at a concentration of 400 μg per ml (36).
Preparation of whole-cell lysates and immunoblotting.
At the time of harvest, cells were placed on ice to prevent artifactual induction of stress responses. The medium was removed and the monolayers were rinsed with ice-cold Dulbecco's phosphate-buffered saline. Cells were scraped directly into 1× sodium dodecyl sulfate (SDS) sample buffer (3.85 mM Tris base, pH 6.8, 9.1% β-mercaptoethanol, 1.82% SDS, 4.6% glycerol, and 0.023% bromophenol blue in 100% ethanol) and proteins were denatured by boiling. Cell-equivalent amounts of lysate were separated by electrophoresis on 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) or 15% N,N′-diallyltartardiamide (DATD)-cross-linked gels (Mnk1 and MK2 probes only). Proteins were transferred to PolyScreen polyvinylidene difluoride membranes (PerkinElmer Life Sciences) followed by blocking in TBST (150 mM NaCl, 20 mM Tris [pH 7.6], 0.05% Tween 20) with 5% milk. All probing and washing of membranes were done in TBST. Antibodies for p38 (9212), phospho-p38 (9211), JNK (9252), phospho-JNK (9251), phospho-MK2 (3041), and phospho-Mnk1 (2111) were purchased from Cell Signaling Technology and used at a 1:1,000 dilution overnight at 4°C per manufacturer's instructions. Polyclonal antibody for Mnk1 (sc-6965) was purchased from Santa Cruz Biotechnology and used at a 1:500 dilution. Monoclonal antibody for α-tubulin (B-5-1-2, Sigma) was used at a 1:20,000 dilution. Monoclonal antibodies for the viral proteins ICP0 (1112), ICP4 (1101), and ICP27 (1113 and 1119) were purchased from the Rumbaugh-Goodwin Institute for Cancer Research and used at 1:800, 1:800, 1:800, and 1:4,000 dilutions, respectively. Polyclonal antibody for VP16 (Clonetech) was used at a 1:5,000 dilution. Polyclonal antibody against ICP8 (3-83) was a generous gift from David Knipe and was used at a 1:20,000 dilution. Polyclonal antibody against gC (R47), used at 1:5,000, was a generous gift of Gary Cohen and Roselyn Eisenberg (University of Pennsylvania).
Goat anti-rabbit and anti-mouse immunoglobulin secondary antibodies were purchased from Amersham Biosciences and used at a 1:5,000 dilution. Secondary antibody was detected using SuperSignal West Pico chemiluminescent substrate agent (Pierce). Films were scanned and images were stored as eight-bit grayscale .jpg files for quantification. The density of each band was determined using Image J (National Institutes of Health). Relative density values were corrected for average background by subtracting the density of a blank portion of the film. From the corrected values, a phosphorylation-specific to total ratio was calculated for the SAPKs. These ratios were then used to calculate either average fold increase (sample value/mock-infected cells value ± standard deviation) or average percent of control sample levels from independent experiments, using Microsoft Excel. For viral genes, background-corrected values were used to calculate average percent of wild-type KOS sample levels from independent experiments using Microsoft Excel.
RESULTS
HSV gene expression is required for p38 activation.
While we previously established the requirement of viral gene expression for JNK activation (56), the requirements for p38 activation were assessed here. We first determined whether the mechanisms of p38 activation relied either on virion binding to a cell surface receptor or on delivery of tegument proteins to the infected cell. The mutation in HFEM tsB7 maps to UL36, and this virus expresses a temperature-sensitive VP1/2, a tegument protein required for release of the viral genome from the capsid and its delivery into the nucleus (9, 57). Thus, at the nonpermissive temperature, mechanisms of binding and entry are normal, but viral gene expression is prevented (47). Notably, tsB7 delivers the tegument protein VP16 to the nucleus at the nonpermissive temperature (9). We also monitored the activation of JNK in these experiments as a positive control in order to more closely correlate the present findings with our previously published results.
Parallel cultures of CV-1 cells were mock infected or infected with the parental wild-type HFEM strain of HSV or the tsB7 mutant at an MOI of 5 at 33, 37, or 39°C, and whole-cell extracts were harvested at 8 hours postinfection. We chose this time because in our previous studies we observed maximal JNK activity and phosphorylated p38 after 7 h postinfection (56). To evaluate activation of the JNK and p38 pathways, proteins were separated by SDS-PAGE and Western blotted for phosphorylated forms of JNK and p38. We monitored for total levels of JNK and p38 protein to ensure that any change in phosphorylated protein levels seen was an actual measure of activation. Levels of α-tubulin were monitored for equal protein loading on gels.
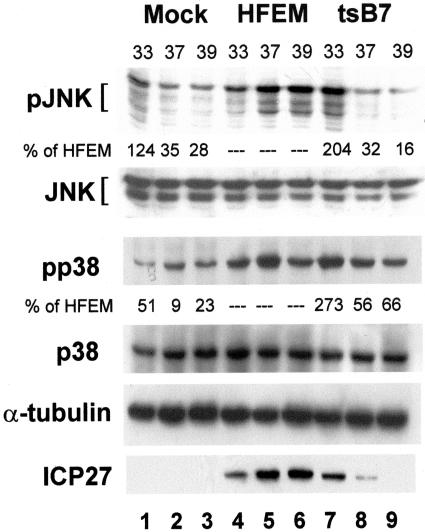
As a measure of viral protein synthesis, the level of ICP27 remained high in HFEM infections at all temperatures (Fig. 1, lanes 4 to 6), but steadily decreased in tsB7 samples and was not detectable in tsB7 infections at 39°C (Fig. 1, lanes 7 to 9). We observed a slight activation of p38 in the mock-infected samples as a function of increasing temperature (lanes 1 to 3). We corrected for the difference during quantification of the image bands by normalizing for total p38 or JNK and for protein loading (α-tubulin) before calculating the reduction in activation during mutant infection. The results of the quantification can be found under the corresponding band in Fig. 1.
FIG. 1.
SAPK activation is dependent on viral gene expression. Replicate CV-1 cultures were mock infected or infected with HFEM or tsB7 at 33, 37, or 39°C as indicated, and lysates were prepared at 8 h postinfection. Western blot analysis of proteins was as described in Materials and Methods. A representative of three independent experiments is shown. Band intensities of phospho-JNK (pJNK) and phospho-p38 (pp38) were quantified using Image J as described in Materials and Methods, and results are presented as a percentage of wild-type parental virus (HFEM) values below the corresponding tsB7 lanes (7-9).
Consistent with the phenotype of tsB7 and a role for viral gene expression for JNK activation, we observed decreased amounts of activated SAPKs, relative to the parental virus HFEM, in mutant virus infections performed at increasingly higher temperatures (Fig. 1, compare lanes 7 to 9). Three distinct JNK species were observed: a minor rapidly migrating species and two relatively abundant slower-mobility bands. Increased phosphorylation of all three forms was observed at all temperatures in HFEM but only at 33° in tsB7 infection. Despite correcting for the increase in basal activation of p38, significant though decreasing activation of p38 was observed as the temperature of infection was raised from 33 to 39°C. We repeated the above experiment at an MOI of 10 (data not shown) and observed similar results.
We evaluated p38 and JNK activation by a second method. As with tsB7, infection with UV-irradiated wild-type virus should still account for all the events prior to viral gene expression, including delivery of tegument proteins, but without the further complication that a change in temperature may introduce into the experimental design. We either mock infected or infected CV-1 cells with wild-type KOS at an MOI of 5, with an equivalent amount of UV-irradiated virus, calculated to have a residual MOI of 0.0017 (see Materials and Methods), or with unirradiated KOS at an MOI of 0.0017, and compared levels of JNK and p38 activation at 8 h postinfection (Fig. 2).
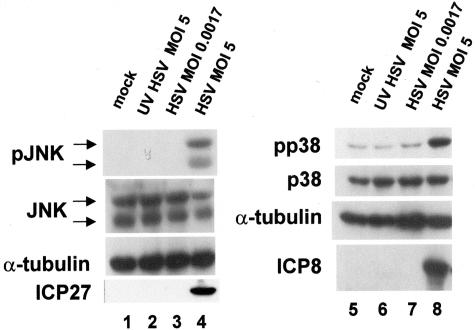
FIG. 2.
Infection with UV-irradiated HSV does not result in SAPK activation. Replicate cultures of CV-1 cells were mock infected, infected with wild-type HSV-1 at the indicated MOIs, or infected with UV-irradiated HSV as described in the text. Lysates were prepared at 8 h postinfection, and levels of the indicated proteins were determined by Western blot.
We confirmed the phenotype of each infection by probing for either IE ICP27 or DE ICP8. We also detected VP16 in both KOS and UV-irradiated KOS (MOI = 5)-infected cells (data not shown), demonstrating that tegument proteins and the viral genome were being released into the host cell during infection. No detectable activation of JNK was observed in the lysates from mock-infected, UV-irradiated virus or KOS infection at the residual MOI (lanes 1 to 3), while the lysate from cells infected with wild-type KOS at an MOI of 5 contained activated JNK (lane 4). The same results were observed for the activation of p38 (lanes 5 to 8). The activation seen in normal wild-type infection was directly correlated with the onset of viral gene expression, indicating through two independent methods that the involvement of VP16 (97) in the activation of either p38 or JNK represents its ability to activate immediate-early gene expression. These data were also consistent with our previously reported findings that neither JNK nor p38 was activated at any time during the first 60 min of infection (56).
Immediate-early gene expression is sufficient for SAPK activation.
The previous experiments could not determine whether the same viral proteins were involved in activating JNK and p38 during infection. To address this question, we first conducted a series of infections to determine which kinetic classes of proteins, IE, DE, or L, were necessary for the activation of the SAPKs. Previously, we demonstrated that JNK and p38 activation occurred within 3 hours postinfection, with maximal activation occurring 6 to 8 hours postinfection (56). Therefore, HSV initiates SAPK activation early in infection, when predominantly immediate-early proteins are expressed, though maximal activation correlates with the onset of early and some late gene expression. We hypothesized that SAPK activation was initiated by one or more IE proteins but may require the expression of DE or L proteins to achieve maximal activation.
Treatment with the protein synthesis inhibitor cycloheximide allows accumulation of IE mRNA, since transcription of viral mRNA does not require de novo viral protein synthesis for expression (35). If the block to protein synthesis is then reversed in the presence of a DNA-dependent transcription inhibitor, actinomycin D, to prevent IE proteins from activating DE or L gene expression, only IE proteins are expressed. Treatment with phosphonoacetic acid, a viral DNA synthesis inhibitor, allows expression of IE, DE, and the γ1 class of L protein but no γ2 gene expression (36).
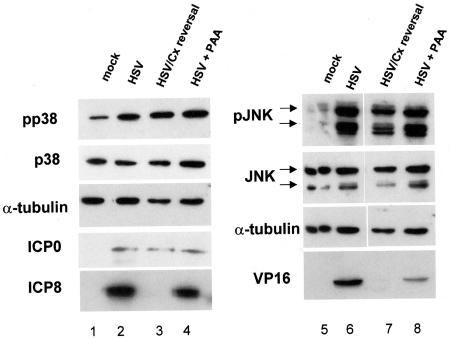
Whole-cell lysates were prepared in three separate experiments and analyzed by Western blot for expression of each class of viral protein. Representative Western blots can be found in Fig. 3. Mock-infected cells expressed no viral proteins (Fig. 3, lanes 1 and 5), while wild-type KOS-infected untreated cells expressed ICP0, ICP8, and VP16 (Fig. 3, lanes 2 and 6). Cells treated with cycloheximide and then reversed in the presence of actinomycin D expressed ICP0 but no detectable ICP8 or VP16 (Fig. 3, lane 3 and 7). The levels of ICP0 were similar in untreated wild-type, cycloheximide reversal, and phosphonoacetic acid-treated infected cells. Phosphonoacetic acid-treated cells accumulated both ICP0 and ICP8 but only low levels of VP16 (Fig. 3, lanes 4 and 8), as expected for a γ1 protein. JNK and p38 activation observed in the infected samples was increased over that of the mock-infected samples regardless of treatment in three independent experiments. Additionally, when the ratio of phosphorylated to total JNK or p38 was determined by Image J (Materials and Methods), we found no difference between untreated and drug-treated infected cells. Subjecting uninfected cells to cycloheximide reversal or phosphonoacetic acid treatment did not induce activation of either pathway (data not shown). Therefore, we concluded that activation of both JNK and p38 was due to expression of one or more immediate-early proteins.
FIG. 3.
Immediate-early gene expression is sufficient for SAPK activation. Replicate cultures of CV-1 cells were mock infected or infected with HSV under the conditions indicated and described in Materials and Methods. Lysates were prepared at 8 h postinfection, and accumulation of the indicated proteins was determined by Western blot. HSV/Cx reversal, infection of cells in the presence of cycloheximide followed by removal of cycloheximide and addition of actinomycin D; HSV + PAA, infection of cells in the presence of phosphonoacetic acid.
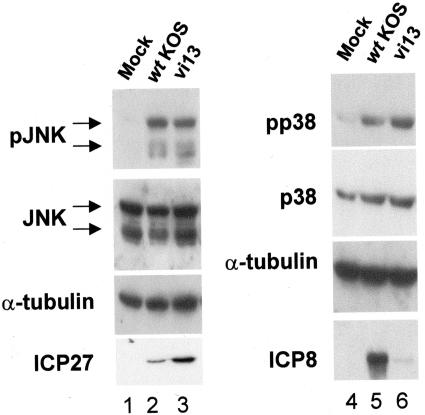
To confirm this observation, we infected cultures of CV-1 cells in triplicate with either wild-type KOS or the ICP4 mutant vi13. An insertion mutation at amino acid 338 and a nonsense mutation at amino acid 774 disrupt the DNA binding activity of ICP4, resulting in the overexpression of IE proteins and no DE or L (85). At 8 h postinfection, we observed accumulation of ICP27 in both wild-type KOS-infected and vi13-infected cells (Fig. 4, lanes 2 to 3), but no detectable ICP8 in vi13-infected cells (Fig. 4, lane 6). The higher accumulation of ICP27 in vi13-infected samples compared to wild-type virus was consistent with other reports using this mutant (56). Significant phosphorylation of JNK and p38 over mock-infected cells was observed in both wild-type KOS- and vi13-infected cells (Fig. 4, lanes 2 to 3 and 5 to 6). We calculated the average activation over wild-type KOS of phospho-p38 (1.32-fold) and JNK (1.01-fold) in vi13-infected cells, normalized to total p38 and JNK levels from triplicate samples. The higher levels of SAPK activation observed in vi13-infected cells correlated with the higher level of ICP27 or other IE proteins (56), consistent with immediate-early protein-dependent SAPK activation during HSV infection.
FIG. 4.
SAPK activation occurs after infection with an ICP4 mutant virus. Replicate cultures of CV-1 cells were mock infected or infected with wild-type KOS or ICP4 mutant vi13. Lysates were prepared at 8 h postinfection, and levels of the indicated proteins were determined by Western blot.
ICP27 is required for p38 and JNK activation.
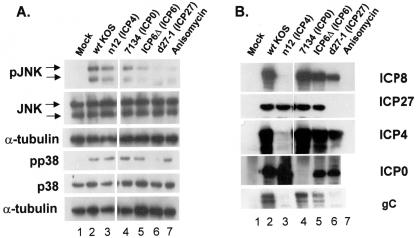
In the previous sections, we demonstrated that IE protein expression was sufficient for activation of p38 and JNK. To identify the IE proteins critical for SAPK activation, CV-1 cells were infected with the following panel of immediate-early mutant viruses: (i) n12, a nonsense mutant which expresses a ≈41-kDa ICP4 protein representing amino acids 1 to 251 (19); (ii) 7134, which contains a lacZ substitution for the ICP0 open reading frame (11); (iii) ICP6Δ, which is deleted for the ICP6 open reading frame (27); and (iv) d27-1, which is deleted for the entire ICP27 gene (74). The n12 mutant was reported to express only IE proteins and the DE protein ICP6 (18, 19).
Following infection with wild-type and mutant viruses at an MOI of 10, whole-cell lysates were prepared at 8 h postinfection, separated by SDS-PAGE, and Western blotted for phospho-specific forms of p38 and JNK. Mock-infected cells exhibited little detectable phosphorylated p38 or JNK (Fig. 5A, lane 1) and, for the purposes of comparison (Table 1, experiment 1), were assigned a value of 1. Levels of total p38 and JNK were comparable in all samples (Fig. 5, lanes 1 to 7). Wild-type infection induced phosphorylation of both JNK and p38 (Fig. 5, lane 2). The n12 and 7134 mutants were also efficient at inducing JNK and p38 activation (Fig. 5, lanes 3 to 4, and Table 1, experiment 1), while ICP6Δ infection resulted in significantly reduced JNK but not p38 activation relative to wild-type virus (Fig. 5, lane 5). Infection with the ICP27 mutant d27-1 failed to activate either stress kinase (Fig. 5, lane 6).
FIG. 5.
ICP27 is necessary for JNK and p38 phosphorylation after HSV infection. (A) Replicate cultures of CV-1 cells were mock infected or infected with the indicated viruses as described in the text. Whole-cell lysates were prepared at 8 h postinfection, and accumulation of phosphorylated forms of p38 and JNK was determined by Western blot. Anisomycin was used as a positive control for p38 activation. Cells were treated with anisomycin (20 μg/ml) for 20 min prior to harvest. (B) Lysates prepared at 8 h postinfection were probed for the indicated viral proteins by Western blot.
TABLE 1.
Activation in wild-type and mutant virus-infected cellsa
| Virus (affected gene) | Relative abundance at time postinfection:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expt 1 (MOI = 10)
|
Expt 2 (MOI = 5)
|
|||||||||
| p38 (8 h) | JNK (8 h) | p38
|
JNK
|
|||||||
| 6 h | 8 h | 12 h | 24 h | 6 h | 8 h | 12 h | 24 h | |||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Wild-type KOS | 2.3 | 87.6 | 3.4 | 5.2 | 11.8 | 14.2 | 11.2 | 10.6 | 7.2 | 8.5 |
| n12 (ICP4) | 3.8 | 57.7 | 1.2 | 5.4 | 7.6 | 11.1 | 0.7 | 1.3 | 5.2 | 5.4 |
| 7134 (ICP0) | 2.9 | 35.0 | 3.1 | 4.6 | 7.1 | 12.5 | 0.0 | 0.8 | 3.2 | 6.2 |
| ICP6Δ (ICP6) | 1.6 | 14.4 | 2.1 | 2.3 | 7.1 | 5.4 | 0.0 | 0.6 | 2.4 | 6.8 |
| d27-1 (ICP27) | 0.1 | 0.3 | 1.5 | 0.2 | 0.1 | 10.2 | 0.0 | 1.0 | 1.2 | 2.0 |
Values represent levels of phospho-p38 and phospho-JNK at each time postinfection relative to mock-infected cells. Accumulation of proteins in whole-cell lysates prepared at 6, 8, 12, and 24 h postinfection was determined by Western blot and quantified as described in Materials and Methods.
Previous reports concerning the ICP6Δ mutant indicated a general depression in viral protein expression and delayed kinetics of infection compared to wild-type virus (26). To determine if the lower JNK activation after ICP6Δ infection was due to a delay in kinetics, we conducted a time course experiment with the same panel of immediate-early mutants this time at an MOI of 5, harvesting samples at 6, 8, and 12 h postinfection and analyzing for phospho-JNK and phospho-p38 by Western blot. The results were quantified as described in Materials and Methods, and the data are presented in Table 1, experiment 2. We observed a continual increase in p38 activation in the wild-type KOS-, n12-, 7134-, and ICP6Δ-infected cells over the time course. However, there was no increase in p38 activation in cells infected with d27-1 except at 24 h postinfection. We suggest that this may be related to a stress response induced by the absence of ICP27 at late times of infection.
While elevated levels of activated JNK were observed throughout the wild-type virus time course, a continual increase in activated JNK was observed in n12-, 7134-, and ICP6Δ-infected cells. By 24 h postinfection, wild-type infection as well as n12,7134, and ICP6Δ infections all activated JNK to similar levels, while d27-1 infection failed to significantly activate JNK at any time after infection. We interpreted these results to indicate that the lower activation of JNK observed at 8 h in experiment 1 can be attributed to a delay in viral protein synthesis in the ICP6Δ infection (26) and not to a requirement of ICP6 for the activation of SAPK proteins. Support for this conclusion was obtained by probing for glycoprotein C (gC), a late protein. We observed decreased gC accumulation in ICP6Δ-infected cells compared to wild-type-infected cells (Fig. 5B, compare lanes 2 and 5). Between the two experiments, we observed the same trend in SAPK activation, with the differences in activation between experiments most likely due to differences in MOI and variations from experiment to experiment in the amount of activated SAPK in mock-infected samples.
To confirm the status of viral protein synthesis under conditions of wild-type and mutant virus infection, lysates prepared at 8 h postinfection were also probed for accumulation of ICP27, ICP4, ICP0, and ICP8 (Fig. 5B). All Western blots for viral proteins were intentionally overexposed to ensure that no low-level expression of the corresponding deleted protein was detectable. Shorter exposure times show variable accumulation of ICP27 in some mutant infections (data not shown). The n12 mutant expresses a 35-kDa, truncated form of ICP4 that was not detectable on the 10% SDS-PAGE gel used. Additionally, n12 failed to express ICP8, confirming its expected phenotype. Since d27-1 was the only immediate-early mutant to consistently fail to activate p38 and JNK, we concluded that ICP27 was necessary for activation of the SAPK pathways.
ICP27 is sufficient for SAPK activation.
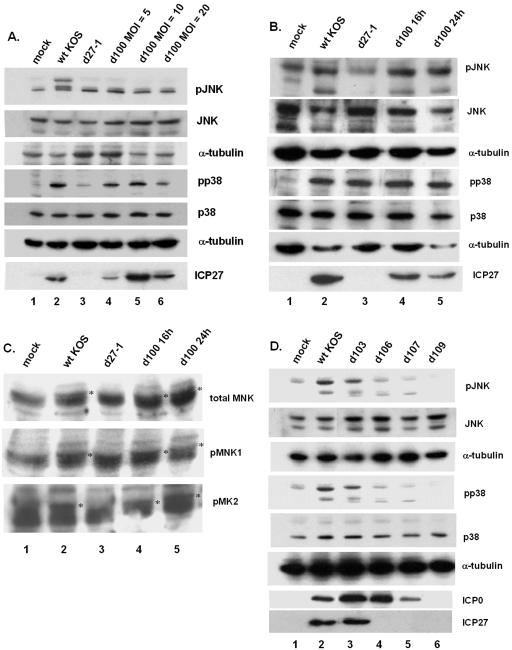
Next, we sought to determine if in the context of virus infection ICP27 was sufficient for the activation of JNK and p38 by taking advantage of a series of multiple-deletion mutants (80) for immediate-early proteins (Table 2). Lysates from infected cells were prepared at 8 h postinfection, separated by SDS-PAGE, and Western blotted for phospho-specific forms of the SAPKs. Figure 6 displays representative data from three independent experiments. The d100 mutant was examined at MOIs of 5, 10, and 20. Since d100 expresses ICP27, ICP22, and ICP47, infections with ICP22 and ICP47 deletion mutants were performed. We observed p38 and JNK activation using either an ICP22 or ICP47 mutant comparable to that of wild-type virus infection (data not shown), suggesting that neither of these IE proteins was involved in SAPK activation. We concluded from this that d100 was an appropriate way of evaluating the sufficiency of ICP27 in SAPK activation in the context of virus infection.
TABLE 2.
IE deletion mutants
FIG. 6.
ICP27 is sufficient for SAPK activation. (A) Replicate cultures of CV-1 cells were mock infected or infected with wild-type KOS (MOI = 5), d27-1 (MOI = 5), or d100 (MOI = 5, 10, and 20). Whole-cell lysates were prepared at 8 h postinfection, and Western blotting was performed to detect phospho-p38, phospho-JNK, or the indicated viral protein. (B) Replicate cultures of CV-1 cells were mock infected or infected with wild-type KOS, d27-1, or d100 at an MOI of 5 and harvested at 16 h postinfection or infected with d100 at an MOI of 5 and harvested at 24 h postinfection. Lysates were prepared and analyzed as in panel A. (C) Samples from the experiment in panel B were separated on 15% DATD-cross-linked gels and probed with phospho-MNK1, MNK1, or phospho-MK2 antibody. ICP27-dependent phosphorylated species are indicated with an asterisk. (D) Replicate cultures of CV-1 cells were mock infected or infected with wild-type KOS, d103, d106, d107, or d109 at an MOI of 5, and lysates were prepared at 8 h postinfection and analyzed as in panel A.
When we evaluated phospho-JNK in these lysates, very low levels of activated JNK were observed in mock-, d27-1-, or d100-infected cells at any MOI used (Fig. 6A, lanes 1 and 3 to 6), while significant activated JNK was seen in the wild-type lysate (Fig. 6A, lane 2). Similarly, we observed very low activation of p38 in mock- and d27-1-infected cells (Fig. 6A, lanes 1 and 3). However, we did observe 62, 78, and 44% of wild-type p38 activation in d100 infections at MOIs of 5, 10, and 20, respectively (compare lane 2 with lanes 4 to 6). Expression of ICP0, ICP4, and ICP27 mRNAs was examined in parallel cultures by reverse transcription-PCR. ICP27 mRNA accumulated after d100 infection, but mRNA for ICP0 and ICP4 did not (data not shown), confirming the genotype of the d100 virus. We also observed that ICP27 mRNA levels were lower in an MOI of 5 with d100 compared to wild-type KOS infection, and this result was confirmed by Western blots for ICP27 (Fig. 6A).
To determine whether longer d100 infections resulted in activated JNK, we compared mock-, wild-type-, d27-1-, and d100-infected cells at 16 h postinfection as well as d100-infected cells at 24 h postinfection, all at an MOI of 5. Increased expression of ICP27 in d100 infections was observed by Western blot at these later times compared to 8 h postinfection (compare Fig. 6A lane 4, with 6B, lanes 4 to 5). We also detected phospho-JNK in wild-type KOS and d100 lysates at both later time points (Fig. 6B, lanes 2, 4, and 5). Mock-infected and d27-1 lysates contained lower levels of phospho-JNK (Fig. 6B, lanes 1 and 3). When we blotted for phospho-p38, no activation in the mock-infected cells (Fig. 6B, lane 1) was observed, but significant activation was evident in all infected samples (Fig. 6B, lanes 2 to 5), including the ICP27 deletion mutant d27-1.
To determine if the activation of p38 seen in d27-1-infected cells at late times postinfection was similar to that of wild-type or d100 infections, we probed for levels of phospho-MNK, a downstream target of p38 known to be activated during infection (92), and a second downstream target MAP kinase-activated protein (MAPKAP) kinase 2 (MK2) (24, 77), which has not previously been evaluated during HSV infection. As seen in Fig. 6C, we detected a series of closely migrating bands, around 45 kDa, in all lanes when probing membranes with either phospho-MNK or total MNK antibody. We detected an additional slower-mobility band in the wild-type KOS- and d100-infected samples at 16 h pi, as well as the d100-infected samples at 24 h pi, indicated by the asterisks (Fig. 6C, lanes 2, 4, and 5). Probing for phospho-MK2, we again observed a series of closely migrating bands, around 48 kDa, in all lanes, with an additional slower-mobility band appearing specifically in the wild-type KOS- and d100-infected lysates (Fig. 6C). This suggests that the mechanism of p38 activation in d27-1-infected cells at late times postinfection was different from the ICP27-induced activation of p38 in wild-type- and d100-infected cells. From these results, we concluded that ICP27 is sufficient for the activation of both JNK and p38.
To confirm that ICP27 was sufficient for SAPK activation, we compared mock-, wild-type-, d103-, d106-, d107-, and d109-infected cells for levels of phospho-p38 and JNK at 8 h postinfection. As seen in Fig. 6C, only wild-type and d103-infected cell lysates contained activated p38 and JNK (lanes 2 and 3), while cells infected with viruses deleted for ICP27 (d106, d107, or d109) contained activated p38 and JNK comparable to mock-infected cell levels (Fig. 6C, compare lane 1 with lanes 4 to 6). We confirmed the gene deletions for each virus by Western blot for ICP27 and ICP0 (Fig. 6C), and ICP4 (data not shown). From these results, we confirmed that ICP27 expression was sufficient for activation of SAPKs.
ICP27 transactivation function is not essential for JNK or p38 activation.
ICP27 has many roles during the course of the HSV-1 replication cycle. In addition to being necessary for transcription of γ1 and γ2 viral genes (79), ICP27 regulates polyadenylation site selection (22, 31, 55), regulates the intranuclear distribution of splicing factors (52, 82), interacts with the export factor Aly/Ref (13), shuttles between the nucleus and cytoplasm (60, 69, 87), and demonstrates RNA binding activity (59). These functions are consistent with a role of ICP27 in suppressing cellular posttranscriptional processing and transport while promoting viral transcription. Indeed, ICP27 may directly stimulate late gene expression through interaction with RNA polymerase II (99). ICP27 also plays a role in controlling the subcellular localization of other viral proteins. Normally, ICP4 and ICP0 are found in both the nucleus and the cytoplasm. Infection with ICP27 mutants, however, results in accumulation of ICP0 and ICP4 exclusively in the nucleus (100-102). Finally, ICP27 is implicated in the posttranslational modifications of ICP0 and ICP4, and it has been suggested that, in the absence of ICP27, ICP4 may not be phosphorylated (65, 73).
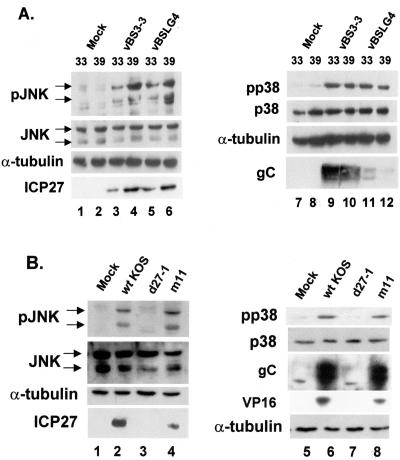
To determine whether stimulation of p38 and JNK by ICP27 was due to ICP27-induced L gene expression, we utilized two mutants of ICP27. The vBSLG4 mutant contains a point mutation, R480H, which at the nonpermissive temperature results in failure to shut off host cell protein synthesis and to activate late gene expression (83, 87). The vBS3-3 revertant has a second-site intragenic mutation, V496I, which allows expression of late genes at the nonpermissive temperature but does not allow shut off of host cell protein synthesis (83, 87). CV-1 cells were mock infected or infected with either vBS3-3 or vBSLG4 at 33 or 39°C and harvested at 8 h postinfection for analysis by Western blot. Both vBS3-3 and vBSLG4 significantly activated both JNK and p38 at both the permissive (33°C) and nonpermissive (39°C) temperatures (Fig. 7A, lanes 3 to 6 and 9 to 12). There were no observable changes in total levels of either SAPK or α-tubulin, suggesting that the vBSLG4 mutant was capable of activating JNK and p38 despite the loss of its transcription activation function. The mutants showed slightly reduced levels of ICP27 at 33°C (Fig. 7A, lanes 3 and 5), likely due to reduced kinetics of infection at this temperature. As previously observed by others, vBSLG4 expressed greatly reduced levels of gC (Fig. 7A, lanes 11 to 12) (79).
FIG. 7.
Late gene activation function of ICP27 is not required for SAPK activation. (A) Replicate cultures of CV-1 cells were mock infected or infected with either vBS3-3 or vBSLG4 at 33 or 39°C as indicated. Whole-cell lysates were prepared at 8 h postinfection, and Western blotting was performed to detect phospho-JNK (left panel) and phospho-p38 (right panel) or accumulation of the indicated viral proteins. (B) Replicate cultures of CV-1 cells were mock infected or infected with wild-type KOS, d27-1, or m11 virus. Whole-cell lysates were prepared at 8 h postinfection, and Western blotting was performed to detect phospho-JNK (left panel) and phospho-p38 (right panel) or accumulation of ICP27, gC, or VP16.
To confirm that the transactivation function of ICP27 was nonessential for SAPK activation, we either mock infected cells or infected cells with wild-type KOS or the ICP27 mutants d27-1 and m11. The last contains two point mutations (R340L and D341E) that prevent the expression of certain γ2 genes (75). At 8 h postinfection, neither mock infection nor infection with d27-1 resulted in detectable activation of JNK or p38, as seen by phospho-specific Western blot (Fig. 7B, lanes 1, 3, 5, and 7). However, infection with either wild-type or m11 virus resulted in significant activation of both JNK and p38 (Fig. 7B, lanes 2, 4, 6, and 8). ICP27 expression in m11-infected cells was detectable, though at a reduced level compared to wild-type KOS (lanes 2 and 4). Late proteins gC and VP16 were detected in wild-type and m11 lysates, although in the latter case at somewhat reduced levels. Neither protein was detectable in the d27-1-infected cell lysate (Fig. 7B, compare lanes 6 to 8). Based on these results, we concluded that the transactivation function of ICP27 was not required for the activation of SAPKs during HSV infection.
Amino acids 12 to 63 in the N terminus of ICP27 are required for SAPK activation.
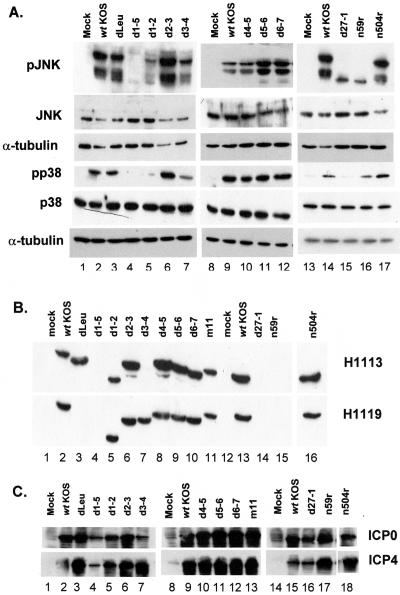
To identify the domains of ICP27 required for the activation of SAPKs, we analyzed a series of ICP27 truncation and in-frame deletion mutants. Table 3 lists the deleted and mutated regions of ICP27. CV-1 cells were mock infected or infected with wild-type HSV-1 or the panel of ICP27 mutants at an MOI of 5. Whole-cell lysates were prepared at 8 h postinfection, separated by SDS-PAGE, and analyzed by Western blot for phospho-specific forms of p38 and JNK (Fig. 8A). The experiment was repeated twice, and the data shown in Fig. 8 were reproducible.
TABLE 3.
ICP27 mutants
| Class and mutant | Regiona affected | Replicationb | Reference(s) |
|---|---|---|---|
| Deletion | |||
| d27-1 | Δ1-512 | D | 74 |
| C-terminal truncation | |||
| n59r | Δ60-512 | D | 74 |
| n504r | Δ505-512 | D | 74 |
| In-frame deletion | |||
| dLeu | Δ6-19 | I | 51 |
| d1-5 | Δ13-153 | D | 59 |
| d1-2 | Δ12-63 | I | 71 |
| d2-3 | Δ64-109 | C | 6 |
| d3-4 | Δ109-138 | C | 59 |
| d4-5 | Δ139-153 | D | 59 |
| d5-6 | Δ154-173 | C | 59 |
| d6-7 | Δ174-200 | C | 6 |
| Point mutation | |||
| m11 | aa340, 341 | D | 75 |
| vBSLG4 | aa480 | Cc | 81, 87 |
FIG. 8.
Amino acids 20 to 63 of ICP27 are required for SAPK activation. (A) Analysis of the null mutant d27-1, the in-frame deletion mutants dLeu, d1-5, d1-2, d2-3, d3-4, d4-5, d5-6, and d6-7, and the C-terminal truncation mutants n59r and n504r. Replicate cultures of CV-1 cells were mock infected or infected with the indicated viruses at an MOI of 5. Whole-cell lysates were prepared at 8 h postinfection, separated on a 12% gel, and transferred to a membrane for Western blot analysis as described in Materials and Methods. Membranes were sequentially probed for phosphorylated and nonphosphorylated forms of p38 and JNK. (B) Accumulation of ICP27 was verified using monoclonal antibodies H1113 (top panel) and H1119 (bottom panel). (C) Analysis of ICP0 and ICP4 accumulation from null, in-frame, and truncation mutants.
Mock-infected cells contained little or no detectable phosphorylated JNK or p38 (Fig. 8A, lanes 1, 8, 13). Wild-type virus-infected cells contained high levels of activated p38 and JNK (Fig. 8A, lanes 2, 9, and 14), as did cells infected with the truncation mutant n504r (Fig. 8A, lane 17) and the in-frame deletion mutants dLeu, d2-3, d4-5, d5-6, and d6-7 (Fig. 8A, lanes 3, 6, and 10 to 12). We consistently observed reduced levels of pJNK and pp38 in d3-4-infected cells (Fig. 8A, lane 7) compared to wild-type lysates. Nevertheless, this was still substantially increased over the mock-infected cell control. Lysates from cells infected with the in-frame deletion mutants d1-5 (Fig. 8A, lane 4) and d1-2 (Fig. 8A, lane 5) and the C-terminal truncation mutant n59r (Fig. 8A, lane 16) contained little to no phosphorylated JNK or p38. In this analysis, in contrast to other experiments (Fig. 5 and 7), we detected a low level of phosphorylated p38 in cells infected with the null mutant d27-1 (Fig. 8A, lane 15). The low level of p38 activation seen in d27-1-infected cells may be a mild stress response to abortive infection. The same stress response was not seen when phospho-JNK levels were evaluated, perhaps indicating a higher propensity for p38 activation during abortive infection. This minor stress response was not observed in other infections with d27-1 (see Fig. 5 to 7) and was deemed to be nonspecific due to its variable nature.
ICP27 mutant infections were typed for expression of ICP0, ICP4, and ICP27 to ensure mutant phenotypes (Fig. 8B and C). All ICP27 mutants expressed ICP0 and ICP4 at fairly comparable levels at an MOI of 5 except d1-5 (Fig. 8C). Similarly, all mutants expressed comparable amounts of ICP27 of the expected molecular weight (Fig. 8B). ICP27 expressed by d3-4 lacks the H1113 epitope and so was only detected by the H1119 monoclonal antibody (Fig. 8B, lane 7). Conversely, dLeu lacks the H1119 epitope and was detected with the H1113 antibody (Fig. 8B, lane 3). Mutant d1-5 failed to activate SAPKs (Fig. 8A, lane 4). However, as can be seen in Fig. 8B, at an MOI of 5 this mutant also failed to express detectable levels of ICP27. Therefore, loss of amino acids 12 through 63 (mutant d1-2) was sufficient to prevent ICP27-dependent activation of p38 and JNK.
DISCUSSION
In this report, we sought to identify the HSV-encoded proteins necessary for inducing activation of the stress-activated protein kinases JNK and p38, previously reported by our group and others. Through the use of the tsB7 mutant and UV-inactivated virus, we demonstrated that SAPK activation during infection required viral gene expression and therefore was not a consequence of cellular stress induced by virus binding or entry. We also demonstrated that only IE gene expression was necessary through the use of the ICP4 vi13 mutant and inhibitors of protein and DNA synthesis.
While p38 activation was observed in cells infected with an ICP27 deletion mutant at late times postinfection, we do not believe that this induction is identical to the ICP27-induced activation of p38. Since JNK is not activated at late times with the d27-1 mutant, nor are the downstream targets of p38, MNK, and MK2, we conclude that the p38 activation observed in d27-1-infected cells at 16 h postinfection is a generalized stress response, possibly induced by the onset of apoptosis. This idea is consistent with the observation that ICP27 deletion mutants induce apoptosis in other cell types (4, 5, 28).
We demonstrated that ICP27 expression was sufficient, in the context of viral infection, for SAPK activation. Specifically, viruses expressing ICP27 but not ICP4 (d103) or expressing ICP27 but neither ICP4 nor ICP0 (d100) induced SAPK activation, while viruses that did not express ICP27 and ICP4 (d107) or only expressed ICP0 (d106), failed to induce SAPK activation. Two other immediate-early proteins, ICP47 and ICP22, are responsible for blocking the function of the transporter associated with antigen processing (TAP) (32) and modifying the C-terminal domain of RNA polymerase II (72), respectively. Analysis of ICP22 and ICP47 null mutants revealed that they were as efficient as wild-type virus in activating SAPKs (McLean and Bachenheimer, unpublished observations).
Distinct functional domains of ICP27 have been identified. The N terminus of ICP27 includes a leucine-rich domain important for nuclear export, an RGG box RNA binding site (59), a nuclear localization sequence, and a casein kinase 2 interaction site (91). The C terminus contains the transcriptional activation and repression domains, the amino acid residues important in spliceosome inhibition, and a zinc finger. ICP27 is posttranslationally modified in several ways. ICP27 is nucleotidylated (10) and methylated in vivo (59) and also phosphorylated at multiple sites (98). It is an in vivo substrate for casein kinase 2 (48), and ICP27 participates in the redistribution of casein kinase 2 in the cytoplasm of the infected cell (48).
A region of the amino terminus of ICP27 that includes the acidic region but not the nearby leucine-rich motif was shown to be required for SAPK activation. This conclusion was based on analysis of a series of in-frame deletion mutants. The d1-2 region has also been identified as necessary for the suppression of apoptosis in infected Hep-2 cells (6). Conversely, the C-terminal transcription activation domain of ICP27 was shown to be nonessential for SAPK activation, based on the properties of viruses expressing amino acid substitution mutants of ICP27. Two ICP27 truncation mutants, n263r and n406r (74), are deleted for the region involved in the transcriptional regulation of late genes. The n263r mutant is not stably expressed in infected cells (S. Rice, personal communication, and data not shown). The n406r mutant does not shuttle efficiently between the cytoplasm and the nucleus because of its inability to bind Aly/Ref (R. Sandri-Goldin, personal communication). We concluded that the properties of these mutants would complicate any interpretation of their phenotype with respect to SAPK activation, and they were not analyzed.
The role of SAPK activation in HSV infection is not well understood. Inhibition of JNK localization to the nucleus and inhibition of JNK and p38 activity by specific inhibitors result in significantly reduced virus yields (56; Hargett, McLean, and Bachenheimer, unpublished observations). A number of functional targets of p38 and JNK might be expected to contribute to efficient virus replication. In the nucleus, MAPKAP kinase and transcription factors ATF-2 and Elk-1 are activated by p38 (24, 77, 88, 90, 94, 95). In the cytoplasm, MNK1, a serine/threonine kinase related to MAPKAP kinase, is phosphorylated by p38 (25) and, once activated, phosphorylates eIF4E, the cap-binding component of the eIF4F multiprotein complex (20, 46, 61). Mitogen- and stress-activated kinase (MSK), which phosphorylates the chromatin-associated proteins histone H3 and high-mobility-group 14 (HMG-14) to promote gene expression, is also activated by p38 (17, 64). JNK activates a number of transcription factors, including c-Jun, p53, ATF-2, NFAT, ELK-1, and SAP-1 (2, 15, 30, 37, 38, 96).
Apoptosis is also regulated by JNK-mediated phosphorylation of Bcl-2 family members, p53, and c-Myc (2, 37, 45, 63). Interestingly, Bcl-2 is extremely unstable in ICP27 mutant-infected cells (97), serving as preliminary evidence that JNK activation in HSV-infected cells may contribute to apoptosis suppression. NF-κB is translocated to the nucleus during infection, accompanied by degradation of IκBα (3, 66). Infection with an ICP27 mutant failed to activate the NF-κB pathway (28, 33, 66). Of interest in this regard is that NF-κB activation can also occur through cross talk from the MEKK1/p38 (93) or by a p38/casein kinase 2-dependent C-terminal phosphorylation of IKKα and IKKβ (44). Activation of the SAPK pathways under certain conditions causes cell cycle arrest. Arrest of cells in G1 is associated with p38 regulation of cyclin D1 levels, as p38 targets cyclin D1 for proteosomal degradation (12) and negatively regulates the cyclin D1 promoter (50). JNK is also reported to block cell cycle progression at G2/M by phosphorylating Cdc25, the phosphatase necessary for activating cyclin B/CDK2 complexes (29). Therefore, JNK and p38 activation may be sufficient for suppression of both the cell cycle and apoptosis during HSV infection.
Though induction of certain SAPK-induced transcription factors, ATF-2, c-Jun, and AP-1, during HSV infection has been reported (56, 97), the impact of these activations on cellular and viral gene expression has not been fully described. Inhibition of p38 kinase activity by treatment of infected cells with the specific inhibitor SB203580 did not affect the viral transcription program (43). Many of the downstream effectors of SAPK activation are currently under investigation to gain a clearer model for the importance of ICP27-dependent phosphorylation of p38 and JNK in the virus life cycle. A recent report identified a p38-dependent activation of MK2 through the Kaposi's sarcoma-associated herpesvirus latency protein kaposin B, which plays a role in the stabilization of cytoplasmic mRNA to allow increased translation of messages with AU-rich elements (53). Interleukin-6 and granulocyte-macrophage colony-stimulating factor, which contain AU-rich elements in their mRNAs, are expressed to higher levels in cells expressing kaposin B (53). Since we here identified p38-dependent MK2 phosphorylation in HSV infection, mRNA stabilization through AU-rich elements may prove to be another posttranscriptional control of viral protein expression by ICP27.
Both DNA and RNA viruses activate MAPK pathways during infection. Apart from the Herpesvirdae family, human immunodeficiency virus, simian immunodeficiency virus, influenza A virus, murine hepatitis virus, encephalomyocarditis virus, respiratory syncytial virus, murine coronavirus, and hepatitis B and C viruses all activate one or more MAPK pathway to enhance viral replication, inflammation, or pathogenesis (8, 23, 41, 42, 49, 54, 62, 84, 89). The activation of p38 during infection also stimulates cellular and viral protein synthesis. Murine coronavirus stimulates cellular and viral translation through a p38-dependent increase in eIF4E phosphorylation (7). Encephalomyocarditis virus RNA translation is suppressed in the presence of p38 inhibitor (34), and respiratory syncytial virus-induced RANTES production is translationally regulated by p38 (67). Efficiency of translation in HSV-1-infected cells is also reported to be regulated by p38-dependent eIF4E phosphorylation (92). While this effect was reported to depend on functional ICP0, the results reported here and other results (Hargett and Bachenheimer, unpublished observations) suggest the direct requirement for ICP27. One of our future efforts will be directed at resolving this discrepancy.
Acknowledgments
This work was supported by National Institutes of Health grant AI43314.
We thank colleagues, especially Stephen Rice and Anna Strain, for provision of viral mutants and Devon Gregory for technical advice.
REFERENCES
- 1.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, V., M. R. Pincus, T. Minamoto, S. Y. Fuchs, M. J. Bluth, P. W. Brandt-Rauf, F. K. Friedman, R. C. Robinson, J. M. Chen, X. W. Wang, C. C. Harris, and Z. Ronai. 1997. Conformation-dependent phosphorylation of p53. Proc. Natl. Acad. Sci. USA 94:1686-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 4.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert, M., J. O'Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber, S. A., J. L. Uhrlaub, J. B. DeWitt, P. M. Tarwater, and M. C. Zink. 2004. Dysregulation of mitogen-activated protein kinase signaling pathways in simian immunodeficiency virus encephalitis. Am. J. Pathol. 164:355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batterson, W., D. Furlong, and B. Roizman. 1983. Molecular genetics of herpes simplex virus. VIII. further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J. Virol. 45:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaho, J. A., C. Mitchell, and B. Roizman. 1993. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J. Virol. 67:3891-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, W. Z., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63:4579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casanovas, O., F. Miro, J. M. Estanyol, E. Itarte, N. Agell, and O. Bachs. 2000. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J. Biol. Chem. 275:35091-35097. [DOI] [PubMed] [Google Scholar]
- 13.Chen, I.-H. B., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, J., and M. F. Stinski. 2002. Role of regulatory elements and the MAPK/ERK or p38 MAPK pathways for activation of human cytomegalovirus gene expression. J. Virol. 76:4873-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow, C. W., M. Rincon, J. Cavanagh, M. Dickens, and R. J. Davis. 1997. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 278:1638-1641. [DOI] [PubMed] [Google Scholar]
- 16.Cinatl, J., Jr., S. Margraf, J.-U. Vogel, M. Scholz, J. Cinatl, and H. W. Doerr. 2001. Human cytomegalovirus circumvents NF-κB dependence in retinal pigment epithelial cells. J. Immunol. 167:1900-1908. [DOI] [PubMed] [Google Scholar]
- 17.Deak, M., A. D. Clifton, L. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLuca, N. A., and P. A. Schaffer. 1988. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J. Virol. 62:732-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyer, J. R., and W. S. Sossin. 2000. Regulation of eukaryotic initiation factor 4E phosphorylation in the nervous system of Aplysia californica. J. Neurochem. 75:872-881. [DOI] [PubMed] [Google Scholar]
- 21.Eliopoulos, A. G., S. M. S. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellison, K. S., S. A. Rice, R. Verity, and J. R. Smiley. 2000. Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants. J. Virol. 74:7307-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erhardt, A., M. Hassan, T. Heintges, and D. Haussinger. 2002. Hepatitis C virus core protein induces cell proliferation and activates ERK, JNK, and p38 MAP kinases together with the MAP kinase phosphatase MKP-1 in a HepG2 Tet-Off cell line. Virology 292:272-284. [DOI] [PubMed] [Google Scholar]
- 24.Freshney, N. W., L. Rawlinson, F. Guesdon, E. Jones, S. Cowley, J. Hsuan, and J. Saklatvala. 1994. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78:1039-1049. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein, D. J., and S. K. Weller. 1988. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology 166:41-51. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 62:196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodkin, M. L., A. T. Ting, and J. A. Blaho. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 77:7261-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goss, V. L., J. V. Cross, K. Ma, Y. Qian, P. W. Mola, and D. J. Templeton. 2003. SAPK/JNK regulates cdc2/cyclin B kinase through phosphorylation and inhibition of cdc25c. Cell Signal. 15:709-718. [DOI] [PubMed] [Google Scholar]
- 30.Gupta, S., D. Campbell, B. Derijard, and R. J. Davis. 1995. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267:389-393. [DOI] [PubMed] [Google Scholar]
- 31.Hann, L. E., W. J. Cook, S. L. Uprichard, D. M. Knipe, and D. M. Coen. 1998. The role of herpes simplex virus ICP27 in the regulation of UL24 gene expression by differential polyadenylation. J. Virol. 72:7709-7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 33.Hilton, M. J., D. Mounghane, T. McLean, N. V. Contractor, J. O'Neil, K. Carpenter, and S. L. Bachenheimer. 1995. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology 213:624-638. [DOI] [PubMed] [Google Scholar]
- 34.Hirasawa, K., A. Kim, H.-S. Han, J. Han, H.-S. Jun, and J.-W. Yoon. 2003. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 77:5649-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honess, R. W., E. Cassai, and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:640-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honess, R. W., and D. H. Watson. 1977. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J. Virol. 21:584-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu, M. C., W. R. Qiu, and Y. P. Wang. 1997. JNK1, JNK2 and JNK3 are p53 N-terminal serine 34 kinases. Oncogene 15:2277-2287. [DOI] [PubMed] [Google Scholar]
- 38.Janknecht, R., and T. Hunter. 1997. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 16:1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson, R. A., S.-M. Huong, and E.-S. Huang. 2000. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J. Virol. 74:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson, R. A., X.-L. Ma, A. D. Yurochko, and E.-S. Huang. 2001. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 82:493-497. [DOI] [PubMed] [Google Scholar]
- 41.Kan, H., Z. Xie, and M. S. Finkel. 2000. HIV gp120 enhances NO production by cardiac myocytes through p38 MAP kinase-mediated NF-kappa B activation. Am. J. Physiol. Heart Circ. Physiol. 279:H3138-3143. [DOI] [PubMed] [Google Scholar]
- 42.Kan, H., Z. Xie, and M. S. Finkel. 2004. p38 MAP kinase-mediated negative inotropic effect of HIV gp120 on cardiac myocytes. Am. J. Physiol. Cell. Physiol. 286:C1-7. [DOI] [PubMed] [Google Scholar]
- 43.Karaca, G., D. Hargett, T. I. McLean, J. S. Aguilar, P. Ghazal, E. K. Wagner, and S. L. Bachenheimer. 2004. Inhibition of the stress-activated kinase, p38, does not affect the virus transcriptional program of herpes simplex virus type 1. Virology 329:142-156. [DOI] [PubMed] [Google Scholar]
- 44.Kato, T., Jr., M. Delhase, A. Hoffmann, and M. Karin. 2003. CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol. Cell 12:829-839. [DOI] [PubMed] [Google Scholar]
- 45.Kharbanda, S., S. Saxena, K. Yoshida, P. Pandey, M. Kaneki, Q. Wang, K. Cheng, Y. N. Chen, A. Campbell, T. Sudha, Z. M. Yuan, J. Narula, R. Weichselbaum, C. Nalin, and D. Kufe. 2000. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J. Biol. Chem. 275:322-327. [DOI] [PubMed] [Google Scholar]
- 46.Knauf, U., C. Tschopp, and H. Gram. 2001. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol. Cell. Biol. 21:5500-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knipe, D. M., W. Batterson, C. Nosal, B. Roizman, and A. Buchan. 1981. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J. Virol. 38:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koffa, M. D., J. Kean, G. Zachos, S. A. Rice, and J. B. Clements. 2003. CK2 protein kinase is stimulated and redistributed by functional herpes simplex virus ICP27 protein. J. Virol. 77:4315-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kujime, K., S. Hashimoto, Y. Gon, K. Shimizu, and T. Horie. 2000. p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 164:3222-3228. [DOI] [PubMed] [Google Scholar]
- 50.Lavoie, J. N., G. L'Allemain, A. Brunet, R. Muller, and J. Pouyssegur. 1996. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271:20608-20616. [DOI] [PubMed] [Google Scholar]
- 51.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG Box RNA-binding domain. J. Virol. 76:11866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindberg, A., and J. P. Kreivi. 2002. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology 294:189-198. [DOI] [PubMed] [Google Scholar]
- 53.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739-741. [DOI] [PubMed] [Google Scholar]
- 54.McGilvray, I. D., Z. Lu, A. C. Wei, A. P. Dackiw, J. C. Marshall, A. Kapus, G. Levy, and O. D. Rotstein. 1998. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J. Biol. Chem. 273:32222-32229. [DOI] [PubMed] [Google Scholar]
- 55.McGregor, F., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J. Virol. 70:1931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of c-Jun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNabb, D. S., and R. J. Courtney. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 66:7581-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mears, W., V. Lam, and S. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mears, W., and S. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 61.Minich, W. B., M. L. Balasta, D. J. Goss, and R. E. Rhoads. 1994. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc. Natl. Acad. Sci. USA 91:7668-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori, I., F. Goshima, T. Koshizuka, N. Koide, T. Sugiyama, T. Yoshida, T. Yokochi, Y. Nishiyama, and Y. Kimura. 2003. Differential activation of the c-Jun N-terminal kinase/stress-activated protein kinase and p38 mitogen-activated protein kinase signal transduction pathways in the mouse brain upon infection with neurovirulent influenza A virus. J. Gen. Virol. 84:2401-2408. [DOI] [PubMed] [Google Scholar]
- 63.Noguchi, K., C. Kitanaka, H. Yamana, A. Kokubu, T. Mochizuki, and Y. Kuchino. 1999. Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. J. Biol. Chem. 274:32580-32587. [DOI] [PubMed] [Google Scholar]
- 64.Nomura, M., A. Kaji, W.-Y. Ma, S. Zhong, G. Liu, G. T. Bowden, K.-i. Miyamoto, and Z. Dong. 2001. Mitogen- and stress-activated protein kinase 1 mediates activation of Akt by ultraviolet B irradiation. J. Biol. Chem. 276:25558-25567. [DOI] [PubMed] [Google Scholar]
- 65.Panagiotidis, C. A., E. K. Lium, and S. J. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 71:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 67.Pazdrak, K., B. Olszewska-Pazdrak, T. Liu, R. Takizawa, A. R. Brasier, R. P. Garofalo, and A. Casola. 2002. MAPK activation is involved in posttranscriptional regulation of RSV-induced RANTES gene expression. Am. J. Physiol Lung Cell Mol. Physiol. 283:L364-372. [DOI] [PubMed] [Google Scholar]
- 68.Perkins, D., E. F. R. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phelan, A., J. Dunlop, and J. B. Clements. 1996. Herpes simplex virus type 1 protein IE63 affects the nuclear export of virus intron-containing transcripts. J. Virol. 70:5255-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rice, S., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rice, S., V. Lam, and D. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rice, S., M. Long, V. Lam, P. Schaffer, and C. Spencer. 1995. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J. Virol. 69:5550-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodems, S. M., and D. H. Spector. 1998. Extracellular signal-Regulated kinase activity is sustained early during human cytomegalovirus infection. J. Virol. 72:9173-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 78.Roux, P. P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68:320-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samaniego, L. A., L. Neiderhiser, and N. A. DeLuca. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 72:3307-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sandri-Goldin, R. M., A. L. Goldin, M. Levine, and J. C. Glorioso. 1981. High-frequency transfer of cloned herpes simplex virus type 1 sequences to mammalian cells by protoplast fusion. Mol. Cell. Biol. 1:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sandri-Goldin, R. M., M. K. Hibbard, and M. A. Hardwicke. 1995. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J. Virol. 69:6063-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandri-Goldin, R. M., M. Levine, and J. C. Glorioso. 1981. Method for induction of mutations in physically defined regions of the herpes simplex virus genome. J. Virol. 38:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shapiro, L., K. A. Heidenreich, M. K. Meintzer, and C. A. Dinarello. 1998. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc. Natl. Acad. Sci. USA 95:7422-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shepard, A. A., and N. A. DeLuca. 1991. Activities of heterodimers composed of DNA-binding- and transactivation-deficient subunits of the herpes simplex virus regulatory protein ICP4. J. Virol. 65:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 87.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan, Y., J. Rouse, A. Zhang, S. Cariati, P. Cohen, and M. J. Comb. 1996. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 15:4629-4642. [PMC free article] [PubMed] [Google Scholar]
- 89.Tarn, C., L. Zou, R. L. Hullinger, and O. M. Andrisani. 2002. Hepatitis B virus X protein activates the p38 mitogen-activated protein kinase pathway in dedifferentiated hepatocytes. J. Virol. 76:9763-9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Dam, H., D. Wilhelm, I. Herr, A. Steffen, P. Herrlich, and P. Angel. 1995. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 14:1798-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wadd, S., H. Bryant, O. Filhol, J. E. Scott, T. Y. Hsieh, R. D. Everett, and J. B. Clements. 1999. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem. 274:28991-28998. [DOI] [PubMed] [Google Scholar]
- 92.Walsh, D., and I. Mohr. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18:660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang, D., and A. Richmond. 2001. Nuclear factor-kappa B activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 276:3650-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitmarsh, A. J., S. H. Yang, M. S. Su, A. D. Sharrocks, and R. J. Davis. 1997. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol. Cell. Biol. 17:2360-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang, S. H., A. Galanis, and A. D. Sharrocks. 1999. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol. Cell. Biol. 19:4028-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang, S. H., A. J. Whitmarsh, R. J. Davis, and A. D. Sharrocks. 1998. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 17:1740-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 98.Zhi, Y., and R. M. Sandri-Goldin. 1999. Analysis of the phosphorylation sites of herpes simplex virus type 1 regulatory protein ICP27. J. Virol. 73:3246-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou, C., and D. M. Knipe. 2002. Association of Herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu, Z., W. Cai, and P. A. Schaffer. 1994. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J. Virol. 68:3027-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu, Z., N. A. DeLuca, and P. A. Schaffer. 1996. Overexpression of the herpes simplex virus type 1 immediate-early regulatory protein, ICP27, is responsible for the aberrant localization of ICP0 and mutant forms of ICP4 in ICP4 mutant virus-infected cells. J. Virol. 70:5346-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu, Z., and P. A. Schaffer. 1995. Intracellular localization of the herpes simplex virus type 1 major transcriptional regulatory protein, ICP4, is affected by ICP27. J. Virol. 69:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]