Abstract
Background:
Among the currently approved antiobesity medications, the glucagon-like-peptide-1 receptor-agonists (GLP-1 RAs) liraglutide and semaglutide, and the dual glucose-dependent-insulinotropic-polypeptide (GIP)/GLP-1 RA tirzepatide have been suggested to reduce cardiovascular-risk in overweight or obesity without diabetes.
Objectives:
The objective of this study was to evaluate the cardio- and neuroprotective potential of these novel agents in the nondiabetic overweight/obese adult population.
Data sources and methods:
A systematic review and meta-analysis of randomized-controlled clinical trials (RCTs) was performed to estimate the risk of major adverse cardiovascular events (MACE), all-cause and cardiovascular mortality in overweight or obese adults without diabetes treated with GLP-1 or GIP/GLP-1 RAs (vs placebo). Secondary outcomes included the risk of myocardial infarction (MI) and stroke.
Results:
Sixteen RCTs (13 and 3 on GLP-1 RAs and tirzepatide, respectively) comprising 28,168 participants were included. GLP-1 or GIP/GLP-1 RAs reduced MACE (odds ratio (OR): 0.79; 95% confidence interval (CI): 0.71–0.89; p < 0.01; I2 = 0) and all-cause mortality (OR: 0.80; 95% CI: 0.70–0.92; p < 0.01; I2 = 0), while there was a trend toward lower cardiovascular-mortality (OR: 0.84; 95% CI: 0.71–1.01; p = 0.06; I2 = 0%) compared to placebo. Additionally, GLP-1 or GIP/GLP-1 RAs reduced the odds of MI (OR: 0.72; 95% CI: 0.61–0.86; p < 0.01; I2 = 0%) and nonfatal-MI (OR: 0.72; 95% CI: 0.61–0.85; p < 0.01; I2 = 0%); while no associations between antiobesity treatment and fatal-MI, stroke, nonfatal, or fatal stroke were uncovered.
Conclusion:
GLP-1 and GIP/GLP-1 RAs reduce cardiovascular-risk and all-cause mortality in overweight or obese adults without diabetes. Additionally, GLP-1 RAs and GIP/GLP-1 RAs attenuate the risk of MI. Since data on stroke are still limited, future RCTs are warranted to evaluate the neuroprotective potential of these novel antiobesity agents.
Trial registration:
PROSPERO CRD42024515966.
Keywords: GLP-1, GIP/GLP-1 receptor agonist, MACE, stroke, tirzepatide
Introduction
With the prevalence of overweight and adiposity rising exponentially worldwide, 1 emerging therapeutic strategies with novel antiobesity agents are gaining increasing traction. Overweight and obesity rank among the leading causes of death globally, conferring increased risk for disability and comorbid chronic diseases, most notably diabetes, metabolic syndrome, cardiovascular disease, and cancer.2,3 While geographic and racial/ethnic disparities still exist, epidemiological models forecast that by 2030, the global prevalence of overweight and obesity will amount to 2.16 and 1.12 billion cases, accounting for 38% and 20% of the world’s adult population. 1 By this time, if secular trends continue unabated, obesity and severe obesity in the United States are expected to affect nearly 1 in 2 and 1 in 4 adults, respectively. 4 The growing health and economic burden of obesity has rendered antiobesity interventions a top-priority on the global health agenda, 5 with antiobesity drugs currently racing from bench to bedside.
As per clinical practice guidelines, pharmacotherapy is recommended as an adjunct to lifestyle interventions for obese or overweight adults with a body mass index (BMI) ⩾ 30 kg/m2 or a BMI ⩾ 27 kg/m2 with at least one cardiovascular risk factor (e.g., prediabetes, diabetes, hypertension, dyslipidemia, elevated waist circumference) or obesity-related comorbidity (e.g., nonalcoholic fatty liver disease, obstructive sleep apnea).6–8 Among the currently Food Drug Administration approved antiobesity medications, two glucagon-like peptide-1 receptor agonists (GLP-1 RAs), liraglutide and semaglutide, and a novel dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist, tirzepatide, are indicated for treatment of nonsyndromic obesity. 9 GLP-1 RAs exert antiobesity effects by targeting peripheral and central pathways that increase insulin and inhibit glucagon secretion, while inducing satiety.9,10 GIP/GLP-1 RAs additionally act on peripheral tissues and islets, improving pancreatic beta cell function and augmenting energy expenditure. 10
Experimental and clinical evidence from randomized-controlled clinical trials (RCT) have previously established that GLP-1 RAs and GIP/GLP-1 RAs exert pleiotropic cardioprotective effects, attenuating cardiovascular risk in type-2 diabetes (T2DM).11–13 Importantly, meta-analyses of RCTs have established that GLP-1 RAs exert highly potent glucose-regulating effects in T2DM, significantly reducing hemoglobin A1c (HbA1c) levels and body weight in a dose-dependent manner compared to placebo. 14 Additionally, tirzepatide has demonstrated even greater effects on glycemic control and weight loss, suggesting potentially superior efficacy compared to other antidiabetic agents. 15 Beyond diabetes, phase III RCTs have provided first evidence on the efficacy and safety of GLP-1 and GIP/GLP-1 RAs for treatment of overweight and obesity in nondiabetic individuals.16,17 This evidence was recently reinforced by the publication of the SELECT trial results, 18 the first cardiovascular outcome trial (CVOT) assessing the cardiovascular efficacy of semaglutide, which demonstrated significant reduction of major adverse cardiovascular events (MACE) in patients with preexisting cardiovascular disease and overweight or obesity without diabetes.
In light of the rapidly evolving landscape of obesity trials and the availability of new RCT data, the aim of the present systematic review and meta-analysis was to evaluate the cardio- and neuroprotective potential of GLP-1 RAs and GIP/GLP-1 RAs assessing the risk of MACE, including stroke, all-cause, and cardiovascular mortality in overweight or obese adults without diabetes.
Methods
Standard protocol approvals and registrations
Reporting adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 19 No Ethical Committee approval was required as per study design (systematic review and meta-analysis). The study protocol, comprising predetermined PICOS (Population, Intervention, Comparison, Outcome, and Study) framework, was a priori designed and registered at the PROSPERO database (CRD42024515966). All supporting data are available within the article and its Supplemental Files.
Data sources and searches
Two independent reviewers (M.-I.S., L.P.) searched for published randomized placebo-controlled trials testing GLP-1 RAs or GIP/GLP-1 RA in adults with overweight or obesity without diabetes. Eligible RCTs were identified by systematic search in MEDLINE (via PubMed) and Scopus databases. The combination of search strings for all database queries included combined search terms: “glucagon-like peptide-1 receptor agonist,” “dual GLP-1/GIP receptor agonists,” “semaglutide,” “lixisenatide,” “exenatide,” “albiglutide,” “liraglutide,” “dulaglutide,” “tirzepatide,” “randomized controlled trial,” “placebo,” “MACE,” “major adverse cardiovascular events,” or “stroke.” The full search algorithms used in MEDLINE and SCOPUS databases are provided in the Supplemental Material. Our search was restricted to RCTs, while no language restrictions were applied. The search spanned from each electronic database’s inception to 10 February 2024. Manual search of bibliographies of articles meeting study inclusion criteria was additionally performed to ensure the comprehensiveness of the literature.
Placebo-controlled RCTs that reported on MACE in adults with overweight or obesity without diabetes treated with GLP-1 RAs or GIP/GLP-1 RAs were eligible for inclusion. Exclusion criteria comprised: (1) RCTs that were not placebo-controlled; (2) RCTs not including overweight or obese populations without diabetes; (3) RCTs investigating compounds of GLP-1 RAs or GIP/GLP-1 RA combined with other drugs; (4) study population of <300 participants12,20; (5) reported outcomes not aligned with our inclusion criteria; (6) observational studies, narrative, and systematic reviews, case-series or case-reports, commentaries, pre-prints or nonpeer reviewed studies, and conference abstracts. Given the fact that inclusion of individual studies that are underpowered to detect differences in rare outcomes (such as MACE or mortality) introduces significant sampling errors and statistical/methodological biases that cannot be mitigated using meta-analytical approaches, we excluded small RCTs with <300 participants in line with a previously published meta-analysis by our group. 12 In case of studies with overlapping data, the study with the largest dataset was retained. All retrieved studies were independently assessed by two reviewers (M.-I.S., L.P.) and disagreements were resolved by consensus after discussion with a third tie-breaking evaluator (G.T.).
Quality control, bias assessment, and data extraction
For relevant domains of each included study, the risk of bias was assessed using the Cochrane Collaboration risk of bias tool. 21 Three independent reviewers (M.-I.S., L.P., A.T.) performed quality control and bias assessment, and in case of disagreement consensus after discussion with the corresponding author (G.T.) was reached. Data including first author name, publication year, study design and duration, patient population, sample size, and event type (i.e., MACE, all-cause mortality, cardiovascular mortality, stroke, and myocardial infarction (MI)) were extracted from individual studies in structured reports.
Publication bias across individual studies was evaluated for all primary outcomes graphically using funnel plots, 22 while Egger’s linear regression test was used for funnel plot asymmetry assessment, 23 and the threshold of the statistical significance was set at p < 0.10.
Outcomes
An aggregate data meta-analysis was performed including all identified placebo-controlled RCTs. The predefined primary outcomes of interest were threefold: (i) the incidence of MACE; (ii) all-cause; and (iii) cardiovascular mortality. Secondary outcomes included the incidence of (i) stroke, (ii) fatal, and (iii) nonfatal stroke; (iv) MI, (v) fatal, and (vi) nonfatal MI. Subgroup analysis was performed based on type of treatment either with GLP-1 RAs or GIP/GLP-1 RAs.
Statistical analysis
R-software version 3.5.0 (packages: meta and metafor) was used for meta-analysis (R Foundation for Statistical Computing, Vienna, Austria; URL: http://www.R-project.org/). All intended outcomes of interest were handled as dichotomous variables, and all the associations evaluating the effect of GLP-1 RAs or GIP/GLP-1 RAs with different outcomes are reported as odds ratios (ORs) with their corresponding 95% confidence intervals (CIs). The random-effects model of meta-analysis (DerSimonian and Laird) was utilized for estimation of the pooled estimates. We used the Q test to assess subgroup differences. The I2 and Cochran Q statistics were employed for heterogeneity assessment. With respect to qualitative heterogeneity interpretation, I2 values >50% and values >75% were considered to represent substantial or considerable heterogeneity, respectively. The significance level was set at 0.1 for the Q statistic, 24 while the equivalent z test with a two-tailed p value < 0.05 was considered statistically significant for each pooled estimate.
Results
Literature search and included studies
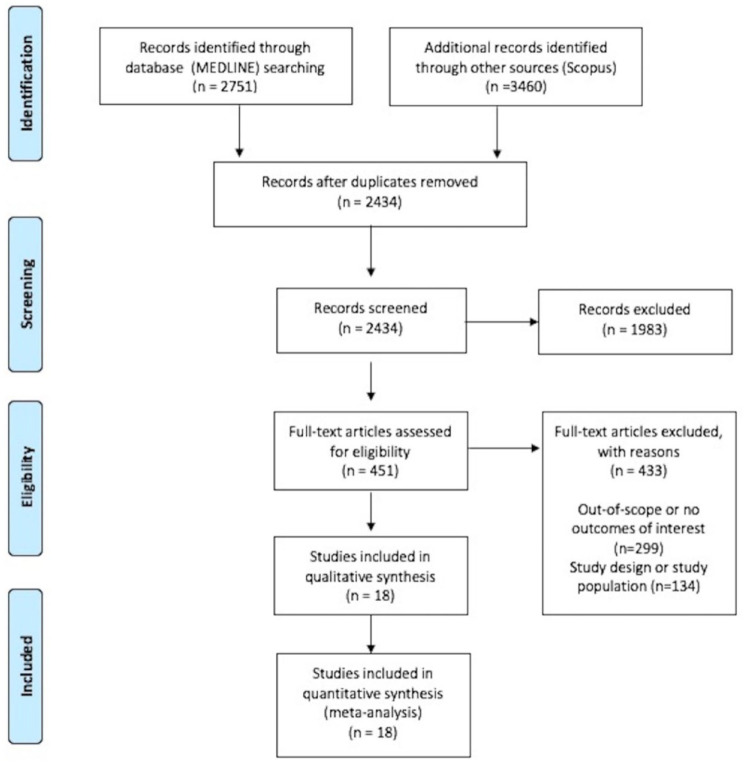
The systematic database search yielded 2751 records from MEDLINE and 3460 records from SCOPUS databases. After exclusion of duplicates and articles that were out-of-scope, 451 records were considered eligible for inclusion and were assessed in full. After reading the full-text articles, 433 were further excluded (Supplemental Material). Finally, we identified 18 eligible studies for inclusion reporting on 16 RCTs (13 RCTs on GLP-1 RAs,18,25–36 1 study reporting on 3-year assessment of a previous RCT on GLP-1 RA, 37 1 post hoc analysis, 38 3 RCTs on tirzepatide16,17,39), comprising a total of 28,168 participants. All original studies were placebo-controlled RCTs, and Table 1 summarizes their main characteristics. In Figure 1, the PRISMA flowchart of the meta-analysis is presented.
Table 1.
Main characteristics of randomized-controlled trials (n = 16) included in the meta-analysis and reported outcomes of interest.
| Trial name | Year | Medication/type | Study design | Population | Study period | Total population (n) | Active (n) | MACE | All-cause mortality | CV mortality | Stroke | Fatal stroke | Nonfatal stroke | MI | Fatal MI | Nonfatal MI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCALE Maintenance25,38 |
2013 | Liraglutide 3.0 mg per day or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 56 weeks; plus 12-week follow-up | 422 | 212 | + | + | + | + | + | ||||
| SCALE Obesity and Prediabetes26,37 |
2015 | Liraglutide 3.0 mg per day or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 56 weeks; plus 12-week follow-up | 3731 | 2487 | + | + | + | + | + | + | + | + | + |
| SCALE Sleep Apnea 27 |
2016 | Liraglutide 3.0 mg per day or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese participants (⩾18 years, BMI ⩾30 kg/m2) with moderate or severe obstructive sleep apnea without diabetes | 32 weeks; plus 12-week follow-up |
359 | 180 | + | + | +++ | + | + | + | + | + | |
| O‘Neil et al.
28
NCT02453711 |
2018 | Semaglutide (0.05, 0.1, 0.2, 0.3, or 0.4 mg) or liraglutide (3.0 mg) per day or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo, and active controlled | Obese participants (⩾18 years, BMI ⩾30 kg/m2) without diabetes | 52 weeks; plus 7-week follow-up | 957 | 821 | + | + | + | + | + | + | + | ||
| STEP 1 29 | 2021 | Semaglutide 2.4 mg once a week or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 68 weeks; plus 7-week follow-up | 1961 | 1306 | + | + | |||||||
| STEP 3 30 | 2021 | Semaglutide 2.4 mg once a week or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 68 weeks; plus 7-week follow-up | 611 | 407 | + | + | + | + | + | + | |||
| STEP 4 35 | 2021 | Semaglutide 2.4 mg once a week or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled withdrawal trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 48 weeks; plus 7-week follow-up | 803 | 535 | + | + | + | + | + | ||||
| STEP 5 34 | 2022 | Semaglutide 2.4 mg once a week or placebo SC/GLP-1 RA |
Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 104 weeks; plus 7-week follow-up | 304 | 152 | + | + | + | + | + | + | |||
| STEP 6 32 | 2022 | Semaglutide 1.7 or 2.4 mg once a week or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, double-dummy, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾35 kg/m2 with ⩾1 comorbidity or BMI ⩾27 kg/m2 with ⩾1 comorbidities); only subgroup without diabetes included in the current meta-analysis | 68 weeks; plus 7-week follow-up | 401 | 300 | + | + | + | + | + | + | |||
| STEP 8 31 | 2022 | Semaglutide 2.4 mg once a week or liraglutide (3.0 mg) per day or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, open-label, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 68 weeks; plus 7-week follow-up | 338 | 253 | + | + | + | + | + | + | |||
| OASIS 1 36 | 2023 | Semaglutide 50 mg once per day orally/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 68 weeks; plus 7-week follow-up | 667 | 334 | + | + | + | + | + | + | |||
| SELECT 18 | 2023 | Semaglutide 2.4 mg once a week or placebo SC/GLP-1 RA | Multicenter, double-blind, randomized, placebo-controlled, event-driven superiority trial | Obese/overweight participants (⩾45 years, BMI ⩾27 kg/m2 with established cardiovascular disease: previous myocardial infarction, previous stroke, or symptomatic peripheral arterial disease) without diabetes | Mean (±SD) 34.2 ± 13.7 months | 17,604 | 8803 | + | + | + | + | + | + | + | ||
| STEP 7 33 | 2024 | Semaglutide 2.4 mg once a week or placebo SC/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾35 kg/m2 with ⩾1 comorbidity or BMI ⩾27 kg/m2 with ⩾1 comorbidities); only subgroup without diabetes included in the current meta-analysis | 44 weeks | 375 | 249 | + | + | + | + | + | + | |||
| SURMOUNT-1 39 | 2022 | Tirzepatide (5, 10, or 15 mg) once a week or placebo SC/GIP/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 72 weeks; plus 4-week follow-up | 2539 | 1896 | + | + | + | + | + | + | + | ||
| SURMOUNT-3 17 | 2023 | Tirzepatide (10 or 15 mg) once a week or placebo SC/GIP/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 72 weeks; plus 4-week follow-up | 579 | 287 | + | + | + | + | + | + | + | + | + |
| SURMOUNT-4 16 | 2024 | Tirzepatide (10 or 15 mg) once a week or placebo SC/GIP/GLP-1 RA | Multicenter, randomized, parallel-arm, double-blind, placebo-controlled withdrawal trial | Obese/overweight participants (⩾18 years, BMI ⩾30 or ⩾27 kg/m2 with comorbidities) without diabetes | 72 weeks; preceded by 36-week, open-label tirzepatide lead-in period | 670 | 335 | + | + | + | + | + | + |
BMI, body mass index; CV, cardiovascular; GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; MACE, major adverse cardiovascular event; MI, myocardial infarction; SC, subcutaneous; SD, standard deviation.
Figure 1.
PRISMA flowchart diagram presenting the selection of eligible studies.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Quality control and publication bias of included studies
The risk of bias of studies included in the present meta-analysis is presented in Supplemental Figure S1. The risk of bias was considered low in all the included RCTs.
Funnel plot symmetry inspection and Egger statistical testing were performed for outcomes involving ⩾4 studies. 22 Accordingly, no asymmetry was revealed for assessment of publication bias among trials reporting MACE (p = 0.0795; Supplemental Figure S2), cardiovascular mortality (p = 0.939; Supplemental Figure S3), and all-cause mortality (p = 0.8691; Supplemental Figure S4) between treatment with GLP-1 RAs or GIP/GLP-1 RAs and placebo.
Primary and secondary outcomes
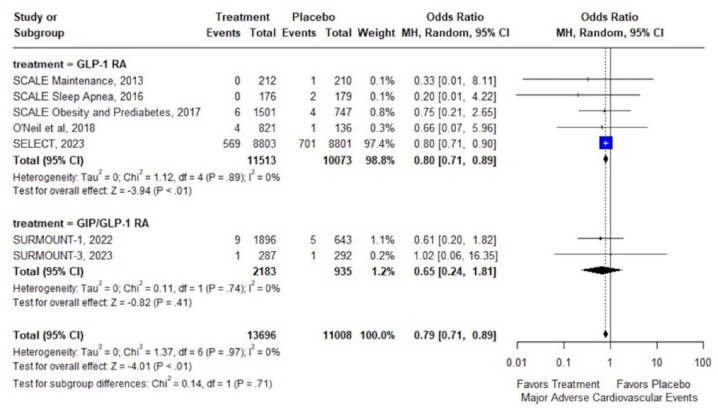
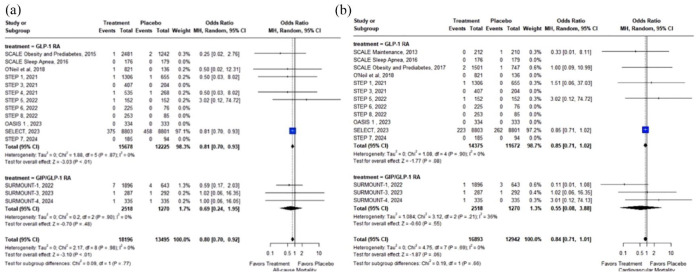
In overweight or obese adults without diabetes, treatment with GLP-1 RAs or GIP/GLP-1 RAs was associated with significant reduction of MACE (7 RCTs; OR: 0.79; 95% CI: 0.71–0.89; p < 0.01; I2 = 0%; Figure 2) and all-cause mortality (15 RCTs; OR: 0.80; 95% CI: 0.70–0.92; p < 0.01; I2 = 0%; Figure 3(a)). In addition, there was a trend toward reduced cardiovascular mortality (15 RCTs; OR: 0.84; 95% CI: 0.71–1.01; p = 0.06; I2 = 0%; Figure 3(b)), which did not reach statistical significance (summarized outcomes in Table 2).
Figure 2.
Forest plot comparing the risk of MACE in overweight or obese adults without diabetes treated with GLP-1 or GIP/GLP-1 RAs versus placebo.
GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RA, glucagon-like peptide-1 receptor agonists; MACE, major adverse cardiovascular event.
Figure 3.
Forest plot comparing the risk of all-cause mortality (a) and cardiovascular mortality (b) in overweight or obese adults without diabetes treated with GLP-1 or GIP/GLP-1 RAs versus placebo.
GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RA, glucagon-like peptide-1 receptor agonists.
Table 2.
Overview of vascular outcomes among overweight or obese adults without diabetes treated with GLP-1 or GIP/GLP-1 RAs versus placebo.
| Clinical outcome | Number of RCTs | OR (95% CI) | p Value | Heterogeneity (I2, p for Cochran Q) |
|---|---|---|---|---|
| MACE | 7 | 0.79 (0.71–0.89) | <0.01 | 0%, 0.97 |
| All-cause mortality | 15 | 0.80 (0.70–0.92) | <0.01 | 0%, 0.98 |
| Cardiovascular mortality | 15 | 0.84 (0.71–1.01) | 0.06 | 0%, 0.66 |
| Stroke | 14 | 0.92 (0.74–1.14) | 0.48 | 0%, 0.45 |
| Fatal stroke | 13 | 0.34 (0.02–5.42) | 0.44 | 0%, NA |
| Nonfatal stroke | 3 | 0.92 (0.74–1.15) | 0.49 | 0%, 0.53 |
| MI | 15 | 0.72 (0.61–0.86) | <0.01 | 0%, 0.84 |
| Fatal MI | 14 | 1.62 (0.20–13.23) | 0.65 | 0%, 0.61 |
| Nonfatal MI | 5 | 0.72 (0.61–0.85) | <0.01 | 0%, 0.74 |
CI, confidence interval; GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; MACE, major adverse cardiovascular event; MI, myocardial infarction; OR, odds ratio; RCTs, randomized controlled trials.
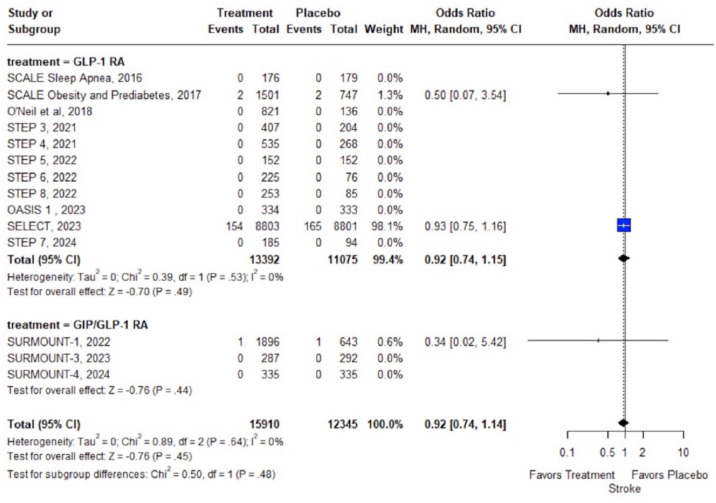
Concerning secondary outcomes, no association was uncovered between GLP-1 RA or GIP/GLP-1 RA treatment and the risk of stroke (14 RCTs; OR: 0.92; 95% CI: 0.74–1.14; p = 0.48; I2 = 0%; Figure 4); fatal (13 RCTs; OR: 0.34; 95% CI: 0.02–5.42; p = 0.44; I2 = NA; Supplemental Figure S5); or nonfatal stroke (3 RCTs; OR: 0.92; 95% CI: 0.74–1.15; p = 0.49; I2 = 0%; Supplemental Figure S6). Conversely, GLP-1 RA or GIP/GLP-1 RA treatment was associated with significant reduction of MI (15 RCTs; OR: 0.72; 95% CI: 0.61–0.86; p < 0.01; I2 = 0%; Supplemental Figure S7) and nonfatal MI (5 RCTs; OR: 0.72; 95% CI: 0.61–0.85; p < 0.01; I2 = 0%; Supplemental Figure S8); while no association between fatal MI and GLP-1 RA or GIP/GLP-1 RA treatment was disclosed (14 RCTs; OR: 1.62; 95% CI: 0.20–13.23; p = 0.65; I2 = 0%; Supplemental Figure S9). With respect to stroke subtypes, no meta-analysis could be performed for hemorrhagic stroke (only 1 RCT 17 reported 0 events in the treatment and placebo arms), while no association between ischemic stroke (2 RCTs; OR: 0.50; 95% CI: 0.02–12.31; p = 0.67; I2 = NA%; Supplemental Figure S10) and transient ischemic attack (2 RCTs; OR: 0.83; 95% CI: 0.04–17.44; p = 0.91; I2 = NA%; Supplemental Figure S11) and GLP-1 RA or GIP/GLP-1 RA treatment was uncovered. Subgroup analyses on GLP-1 RA versus GIP/GLP-1 RA treatment, revealed no significant subgroup effects on any of the primary or secondary outcomes.
Figure 4.
Forest plot comparing the risk of stroke in overweight or obese adults without diabetes treated with GLP-1 or GIP/GLP-1 RAs versus placebo.
GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RAs, glucagon-like peptide-1 receptor agonists.
Discussion
In the present systematic review and meta-analysis, treatment with GLP-1 RAs or GIP/GLP-1 RAs for overweight or obesity in the absence of diabetes was associated with significant reduction of MACE. In addition, GLP-1 RA or GIP/GLP-1 RA treatment was associated with significant reduction of all-cause mortality, while there was a trend toward reduced cardiovascular mortality, which did not reach statistical significance. Regarding secondary outcomes, treatment with GLP-1 or GIP/GLP-1 RAs was associated with significant reduction of MI and nonfatal MI compared to placebo, whereas no association between GLP-1 or GIP/GLP-1 RAs and fatal MI, stroke, fatal stroke, and nonfatal stroke was uncovered.
These findings align with results of prior meta-analyses from our group and others that have demonstrated potent cardioprotective effects of GLP-1 RAs or GIP/GLP-1 RAs in T2DM.12,13,40 In addition, the current meta-analysis expands on our previous findings, documenting consistent cardiovascular benefits in the non-diabetic overweight/obese adult population. Considering the global prevalence of overweight and obesity, these findings could have critical public health implications. Currently, the accruing evidence on GLP-1 RAs and GIP/GLP-1 RAs, particularly the compelling recent data from the SELECT trial, highlights a shift in the clinical approach to managing overweight and obesity. Notably, prior research on atherosclerotic cardiovascular disease (ASCVD) prevention primarily relied on observational data, which showed limited efficacy of pharmacological or bariatric-induced body weight loss for secondary ASCVD prevention. 41 It should thus be emphasized that the striking results of the SELECT trial, along with emerging evidence from ongoing RCTs on GLP-1 and GIP/GLP-1 RAs, are expected to reshape future treatment guidelines, especially for managing patients with obesity and preexisting ASCVD. 41
With respect to cardiovascular mortality, which showed a nonsignificant trend toward reduction with treatment, several reasons may have contributed to an underestimation of observed treatment effects, including the limited number of events and thus statistical power, adjudication biases, along with substantial heterogeneity in definitions of cardiovascular mortality and safety outcomes in included RCTs. Notably, several mechanisms have been implicated in GLP-1 and GIP/GLP-1 RAs-induced cardiovascular risk reduction, comprising (i) mechanisms directly related to weight loss effects, including improved glucose and arterial blood pressure regulation, lipid metabolism, myocardial, and endothelial function; and (ii) weight loss independent effects, including anti-inflammatory, 42 and nephroprotective 43 actions. In addition, GLP-1 and GIP/GLP-1 RAs have shown promising efficacy in reducing liver fat, improving hepatic enzymes, and promoting the resolution of metabolic dysfunction-associated steatotic liver disease and steatohepatitis through mechanisms such as enhanced insulin sensitivity, decreased lipogenesis, weight loss, and attenuation of hepatic inflammation. 44 Clinical trials have demonstrated these agents’ potential not only in improving liver histology but also in conferring cardiovascular and metabolic benefits.45,46 The pleiotropic effects of GLP-1 and GIP/GLP-1 RAs may synergistically account for the observed attenuation of all-cause mortality, extending beyond reduction of cardiovascular risk.
Certain methodological nuances must be considered for an accurate interpretation of the current findings. First, as evidenced by the individual study weights of the present meta-analyses, the pooled effects were mainly driven by the effects of GLP-1 RA treatment in the SELECT trial. 18 SELECT was a phase III RCT investigating the cardiovascular efficacy and safety of once-weekly subcutaneous semaglutide at a dose of 2.4 mg at a mean follow-up of 39.8 ± 9.4 months including 8803 and 8801 nondiabetic individuals with preexisting cardiovascular disease and a BMI ⩾27 kg/m2 allocated to treatment or placebo groups, respectively. As per CVOT design, outcome events in SELECT exceeded by far the events documented in other included RCTs, which largely excluded overweight or obese individuals at high cardiovascular risk, had limited sample sizes, and short follow-up periods. Although these trials were underpowered to detect cardiovascular efficacy, the included data from 10,564 overweight or obese participants (in addition to the SELECT trial population) from 15 phase III RCTs on GLP-1 RAs and GIP/GLP-1 RAs significantly strengthen the findings of our meta-analysis and expand on the safety profile of these novel agents, demonstrating lower all-cause mortality and tendentially lower cardiovascular mortality compared to placebo. Second, it should be noted that the incidence of fatal stroke and fatal MI was not reported in SELECT; thus, data were unavailable for meta-analysis. Third, regarding the nonsignificant association of GLP-1 or GIP/GLP-1 RA treatment with stroke, it should be noted that these results stand in contrast to the well-established reduction of stroke risk and particularly ischemic stroke with GLP-1 RAs in T2DM.12,13 Several concerns regarding SELECT’s design should be expressed at this point: (i) as per study design, only patients with preexisting cardiovascular disease were included; however, stroke was clearly underrepresented, with the ratio of prior MI to prior stroke being 4:1 (i.e., corresponding to 68% and 18% of included patients, respectively); (ii) the vast majority of patients were under treatment with lipid-lowering medications (90%) and platelet-aggregation inhibitors (86%); (iii) patients at high risk for stroke were excluded (i.e., patients could not be enrolled within 2 months after a cardiovascular or neurologic event or if they planned to undergo coronary, carotid, or peripheral revascularization). Notably, experimental evidence also suggests robust neuroprotective effects from GLP-1 RAs, particularly in ischemic stroke. 47 Given the limited number of outcome events in the present meta-analysis (326 cumulative stroke events) and the limited follow-up, type II errors cannot be excluded and larger well-designed RCTs with adequate sample sizes and trial periods are warranted to evaluate GLP-1 RAs and GIP/GLP-1 RAs efficacy for primary and secondary stroke prevention in overweight and obese adults without diabetes.
Concerning differential effects of GLP-1 RA versus GIP/GLP-1 RA treatment, subgroup analyses revealed no significant subgroup effects on any of the primary or secondary outcomes. Nonetheless, due to limited data availability on the novel dual GIP/GLP-1 RA tirzepatide and the lack of dedicated CVOTs, we caution that the lack of observed associations should not be interpreted as evidence of comparable efficacy of these agents in the overweight or obese nondiabetic population. The available evidence on tirzepatide primarily stems from the SURMOUNT clinical development program, which aimed to evaluate the safety and efficacy of tirzepatide as an adjunct to lifestyle intervention, compared to placebo, for chronic weight management in adults with a BMI ⩾27 kg/m², with or without T2DM. Notably, among the seminal SURMOUNT trials, SURMOUNT-2 has not been included in the present meta-analysis due to inclusion of patients with obesity and established T2DM. 48 SURMOUNT-1 was a phase III, double-blind RCT involving 2539 adults with a BMI ⩾30 kg/m² (or ⩾27 kg/m² with at least one weight-related complication, excluding T2DM), which demonstrated that weekly administration of tirzepatide at doses of 5, 10, and 15 mg significantly reduced body weight by up to 20.9% compared to 3.1% with placebo over 72 weeks. 39 This weight reduction was accompanied by improvements in cardiometabolic risk factors, including reductions in systolic and diastolic blood pressure, fasting insulin levels, waist circumference, and lipid levels. The most common adverse events associated with tirzepatide were gastrointestinal, primarily occurring during dose escalation. SURMOUNT-3 was a phase III, double-blind RCT that included 579 adults with a BMI of ⩾30, or ⩾27 kg/m² with at least one weight-related complication, including those with T2DM. Participants achieved a ⩾5.0% weight reduction following a 12-week intensive lifestyle intervention and were subsequently randomized to receive the maximum tolerated dose of tirzepatide (10 or 15 mg) or placebo once weekly for 72 weeks. 17 The tirzepatide group experienced significant, dose-dependent weight loss of up to 18.4%, while those in the placebo group experienced a 2.5% weight regain. Additionally, tirzepatide improved HbA1c levels, lipid profiles, waist circumference, fasting glucose, fasting insulin, and blood pressure, with a safety profile similar to that observed in SURMOUNT-1. SURMOUNT-4 was a phase III, double-blind RCT involving 670 adults with a BMI of ⩾30, or ⩾27 kg/m² with at least one weight-related complication, excluding those with T2DM. 16 The trial comprised a 36-week open-label lead-in period where all participants received tirzepatide (10 or 15 mg), followed by a 52-week double-blind phase, where participants were randomized to continue on tirzepatide or switch to placebo. Those continuing on tirzepatide achieved a total weight loss of 25%, with significant improvements in waist circumference, lipid profiles, and blood pressure. From week 36 to week 88, the mean percent weight change was −5.5% with tirzepatide versus a 14.0% weight regain in the placebo group, demonstrating a substantial regain of lost weight upon tirzepatide withdrawal.
In fact, emerging data indicate that the effects of tirzepatide on glycemic control and weight loss may supersede those of GLP-1 RAs,49,50 with the growing body of real-world evidence and upcoming head-to-head trials (e.g., SURMOUNT-5) expected to shed more light on potential differential effects of novel antiobesity medications in the near future. At present, it should be emphasized however, that based on the robust design of the SELECT trial, the so-far available data favor the use of the GLP-1 RA semaglutide for cardiovascular risk reduction in nondiabetic overweight or obese adults with established cardiovascular disease or at high cardiovascular risk.
The following limitations of the current meta-analysis need to be acknowledged. First, due to lack of individual participant data and the reliance on aggregate data, meta-analyses of participant characteristics in association with the risk of MACE, MI, or stroke could not be performed. Second, regarding stroke subtypes, the interpretability of our findings was significantly limited by the extremely low number of reported outcome events. Consequently, no reliable inferences regarding potential associations between GLP-1 RAs or GIP/GLP-1 RAs and stroke in overweight or obese populations can be drawn based on the so-far available evidence. Third, the generalizability of the current findings is limited by the fact that RCTs on GLP-1 RAs or GIP/GLP-1 RAs—with the exception of SELECT—included overweight or obese adults without cardiovascular comorbidities; thus, real-word data and future CVOTs are warranted to corroborate our findings. Despite these limitations, a significant number of RCTs comprising a total population of 34,575 participants contributed data to the pooled primary outcomes analyses, with the exceptionally low heterogeneity of reported cardiovascular outcomes from included RCTs supporting the robustness of the present results. Finally, to the best of our knowledge, this is the first to date meta-analysis evaluating the comparative efficacy of GLP-1 versus GIP/GLP-1 RAs and the largest assessing the cardiovascular safety and efficacy of these novel medications in the nondiabetic overweight or obese adult population.
Conclusion
In conclusion, the findings of the present systematic review and meta-analysis indicate that GLP-1 RAs and GIP/GLP-1 RAs for treatment of overweight and obesity in the absence of diabetes significantly attenuate the risk of MACE. Moreover, our findings suggest a favorable safety profile with significant survival benefits, reinforcing the utility of these agents in obesity management strategies. With respect to individual components of MACE, our meta-analysis demonstrates a significant reduction of the risk of MI with anti-obesity treatment, while further well-designed RCTs are needed to firmly ascertain the role of GLP-1 RAs and GIP/GLP-1 RAs for stroke prevention in overweight or obese individuals without diabetes.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241281903 for Risk of major adverse cardiovascular events and all-cause mortality under treatment with GLP-1 RAs or the dual GIP/GLP-1 receptor agonist tirzepatide in overweight or obese adults without diabetes: a systematic review and meta-analysis by Maria-Ioanna Stefanou, Lina Palaiodimou, Aikaterini Theodorou, Apostolos Safouris, Urs Fischer, Peter J. Kelly, Jesse Dawson, Mira Katan, Aristeidis H. Katsanos, Vaia Lambadiari, Sotirios Giannopoulos, Andrei V. Alexandrov, Gerasimos Siasos and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864241281903 for Risk of major adverse cardiovascular events and all-cause mortality under treatment with GLP-1 RAs or the dual GIP/GLP-1 receptor agonist tirzepatide in overweight or obese adults without diabetes: a systematic review and meta-analysis by Maria-Ioanna Stefanou, Lina Palaiodimou, Aikaterini Theodorou, Apostolos Safouris, Urs Fischer, Peter J. Kelly, Jesse Dawson, Mira Katan, Aristeidis H. Katsanos, Vaia Lambadiari, Sotirios Giannopoulos, Andrei V. Alexandrov, Gerasimos Siasos and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iDs: Maria-Ioanna Stefanou  https://orcid.org/0000-0002-2305-6627
https://orcid.org/0000-0002-2305-6627
Lina Palaiodimou  https://orcid.org/0000-0001-7757-609X
https://orcid.org/0000-0001-7757-609X
Aikaterini Theodorou  https://orcid.org/0000-0001-7229-2610
https://orcid.org/0000-0001-7229-2610
Apostolos Safouris  https://orcid.org/0000-0002-9630-6949
https://orcid.org/0000-0002-9630-6949
Sotirios Giannopoulos  https://orcid.org/0000-0001-7443-5179
https://orcid.org/0000-0001-7443-5179
Andrei V. Alexandrov  https://orcid.org/0000-0001-8871-1023
https://orcid.org/0000-0001-8871-1023
Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maria-Ioanna Stefanou, Second Department of Neurology, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Lina Palaiodimou, Second Department of Neurology, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Aikaterini Theodorou, Second Department of Neurology, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Apostolos Safouris, Second Department of Neurology, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece; Stroke Unit, Metropolitan Hospital, Piraeus, Greece.
Urs Fischer, Department of Neurology, University Hospital Basel, University of Basel, Basel, Switzerland.
Peter J. Kelly, Stroke Service, Mater University Hospital and University College Dublin, Dublin, Ireland
Jesse Dawson, School of Cardiovascular and Metabolic Health, College of Medical, Veterinary and Life Sciences, Queen Elizabeth University Hospital, Glasgow, UK.
Mira Katan, Department of Neurology, University Hospital Basel, University of Basel, Basel, Switzerland.
Aristeidis H. Katsanos, Division of Neurology, McMaster University/Population Health Research Institute, Hamilton, ON, Canada
Vaia Lambadiari, Second Department of Internal Medicine, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Sotirios Giannopoulos, Second Department of Neurology, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Andrei V. Alexandrov, Department of Neurology, University of Tennessee Health Science Center, Memphis, USA
Gerasimos Siasos, Third Department of Cardiology, Sotiria Thoracic Diseases General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Georgios Tsivgoulis, Second Department of Neurology, “Attikon” University Hospital, School of Medicine, National and Kapodistrian University of Athens, Rimini 1, Chaidari, Athens 12462, Greece.
Declarations
Ethics approval and consent to participate: This study did not require an ethical board approval or written informed consent by the patients according to the study design (systematic review and meta-analysis).
Consent for publication: Not applicable.
Author contributions: Maria-Ioanna Stefanou: Conceptualization; Data curation; Formal analysis; Investigation; Validation; Writing – original draft.
Lina Palaiodimou: Data curation; Methodology; Software; Validation.
Aikaterini Theodorou: Formal analysis; Investigation; Software; Writing – review & editing.
Apostolos Safouris: Investigation; Validation; Writing – review & editing.
Urs Fischer: Formal analysis; Methodology; Validation; Writing – review & editing.
Peter J. Kelly: Methodology; Validation; Writing – review & editing.
Jesse Dawson: Methodology; Validation; Writing – review & editing.
Mira Katan: Methodology; Validation; Writing – review & editing.
Aristeidis H. Katsanos: Methodology; Writing – review & editing.
Vaia Lambadiari: Investigation; Validation; Writing – review & editing.
Sotirios Giannopoulos: Methodology; Writing – review & editing.
Andrei V. Alexandrov: Methodology; Writing – review & editing.
Gerasimos Siasos: Methodology; Validation; Writing – review & editing.
Georgios Tsivgoulis: Conceptualization; Formal analysis; Methodology; Supervision; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: G.T. is Associate Editor of Therapeutic Advances in Neurological Disorders, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Availability of data and materials: The authors confirm that the data supporting the findings of this study are available within the article and its Supplemental Materials.
References
- 1. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Safaei M, Sundararajan EA, Driss M, et al. A systematic literature review on obesity: understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med 2021; 136: 104754. [DOI] [PubMed] [Google Scholar]
- 3. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019; 15: 288–298. [DOI] [PubMed] [Google Scholar]
- 4. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 2019; 381: 2440–2450. [DOI] [PubMed] [Google Scholar]
- 5. Wang YC, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011; 378: 815–825. [DOI] [PubMed] [Google Scholar]
- 6. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts 2015; 8: 402–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Semlitsch T, Stigler FL, Jeitler K, et al. Management of overweight and obesity in primary care—a systematic overview of international evidence-based guidelines. Obes Rev 2019; 20: 1218–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen MD, Ryan DH, Apovian CM, et al. 2013. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakhtoura M, Haber R, Ghezzawi M, et al. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine 2023; 58: 101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tschöp M, Nogueiras R, Ahrén B. Gut hormone-based pharmacology: novel formulations and future possibilities for metabolic disease therapy. Diabetologia 2023; 66: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marx N, Husain M, Lehrke M, et al. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation 2022; 146: 1882–1894. [DOI] [PubMed] [Google Scholar]
- 12. Stefanou MI, Theodorou A, Malhotra K, et al. Risk of major adverse cardiovascular events and stroke associated with treatment with GLP-1 or the dual GIP/GLP-1 receptor agonist tirzepatide for type 2 diabetes: a systematic review and meta-analysis. Eur Stroke J 2024; 9: 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malhotra K, Katsanos AH, Lambadiari V, et al. GLP-1 receptor agonists in diabetes for stroke prevention: a systematic review and meta-analysis. J Neurol 2020; 267: 2117–2122. [DOI] [PubMed] [Google Scholar]
- 14. Li A, Su X, Hu S, et al. Efficacy and safety of oral semaglutide in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2023; 198: 110605. [DOI] [PubMed] [Google Scholar]
- 15. Salmen T, Serbanoiu LI, Bica IC, et al. A critical view over the newest antidiabetic molecules in light of efficacy—a systematic review and meta-analysis. Int J Mol Sci 2023; 24: 9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aronne LJ, Sattar N, Horn DB, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA 2024; 331: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med 2023; 29: 2909–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221–2232. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–34. [DOI] [PubMed] [Google Scholar]
- 20. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653–662. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester, UK: John Wiley & Sons, 2019. [Google Scholar]
- 25. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond) 2013; 37: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 26. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 27. Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond) 2016; 40: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018; 392: 637–649. [DOI] [PubMed] [Google Scholar]
- 29. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989–1002. [DOI] [PubMed] [Google Scholar]
- 30. Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 2021; 325: 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA 2022; 327: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol 2022; 10: 193–206. [DOI] [PubMed] [Google Scholar]
- 33. Mu Y, Bao X, Eliaschewitz FG, et al. Efficacy and safety of once weekly semaglutide 2·4 mg for weight management in a predominantly east Asian population with overweight or obesity (STEP 7): a double-blind, multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2024; 12: 184–195. [DOI] [PubMed] [Google Scholar]
- 34. Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 2022; 28: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 2021; 325: 1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knop FK, Aroda VR, do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023; 402: 705–719. [DOI] [PubMed] [Google Scholar]
- 37. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017; 389: 1399–1409. [DOI] [PubMed] [Google Scholar]
- 38. Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab 2018; 20: 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205–216. [DOI] [PubMed] [Google Scholar]
- 40. Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 2022; 28: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muzurović E, Yumuk VD, Rizzo M. GLP-1 and dual GIP/GLP-1 receptor agonists in overweight/obese patients for atherosclerotic cardiovascular disease prevention: where are we now? J Diabetes Complications 2023; 37: 108647. [DOI] [PubMed] [Google Scholar]
- 42. Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm 2016; 2016: 3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mosterd CM, Bjornstad P, van Raalte DH. Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol 2020; 33: 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kokkorakis M, Muzurović E, Volcˇanšek Š, et al. Steatotic liver disease: pathophysiology and emerging pharmacotherapies. Pharmacol Rev 2024; 76: 454–499. [DOI] [PubMed] [Google Scholar]
- 45. Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol 2023; 8: 179–191. [DOI] [PubMed] [Google Scholar]
- 46. Abushamat LA, Shah PA, Eckel RH, et al. The emerging role of glucagon-like peptide-1 receptor agonists for the treatment of metabolic dysfunction-associated steatohepatitis. Clin Gastroenterol Hepatol 2024; 22: 1565–1574. [DOI] [PubMed] [Google Scholar]
- 47. Nizari S, Basalay M, Chapman P, et al. Glucagon-like peptide-1 (GLP-1) receptor activation dilates cerebral arterioles, increases cerebral blood flow, and mediates remote (pre)conditioning neuroprotection against ischaemic stroke. Basic Res Cardiol 2021; 116: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023; 402: 613–626. [DOI] [PubMed] [Google Scholar]
- 49. Nauck MA, D’Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol 2022; 21: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andraos J, Muhar H, Smith SR. Beyond glycemia: comparing tirzepatide to GLP-1 analogues. Rev Endocr Metab Disord 2023; 24: 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241281903 for Risk of major adverse cardiovascular events and all-cause mortality under treatment with GLP-1 RAs or the dual GIP/GLP-1 receptor agonist tirzepatide in overweight or obese adults without diabetes: a systematic review and meta-analysis by Maria-Ioanna Stefanou, Lina Palaiodimou, Aikaterini Theodorou, Apostolos Safouris, Urs Fischer, Peter J. Kelly, Jesse Dawson, Mira Katan, Aristeidis H. Katsanos, Vaia Lambadiari, Sotirios Giannopoulos, Andrei V. Alexandrov, Gerasimos Siasos and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864241281903 for Risk of major adverse cardiovascular events and all-cause mortality under treatment with GLP-1 RAs or the dual GIP/GLP-1 receptor agonist tirzepatide in overweight or obese adults without diabetes: a systematic review and meta-analysis by Maria-Ioanna Stefanou, Lina Palaiodimou, Aikaterini Theodorou, Apostolos Safouris, Urs Fischer, Peter J. Kelly, Jesse Dawson, Mira Katan, Aristeidis H. Katsanos, Vaia Lambadiari, Sotirios Giannopoulos, Andrei V. Alexandrov, Gerasimos Siasos and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders