Abstract
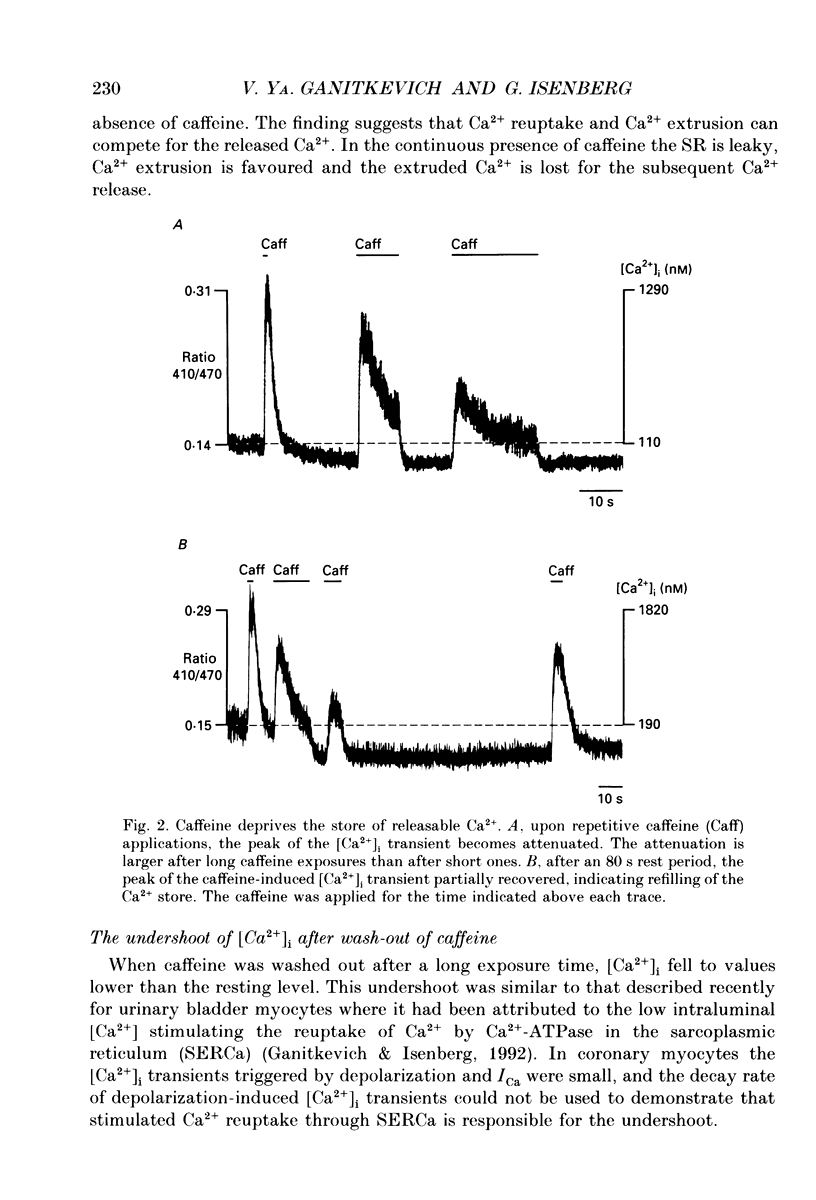
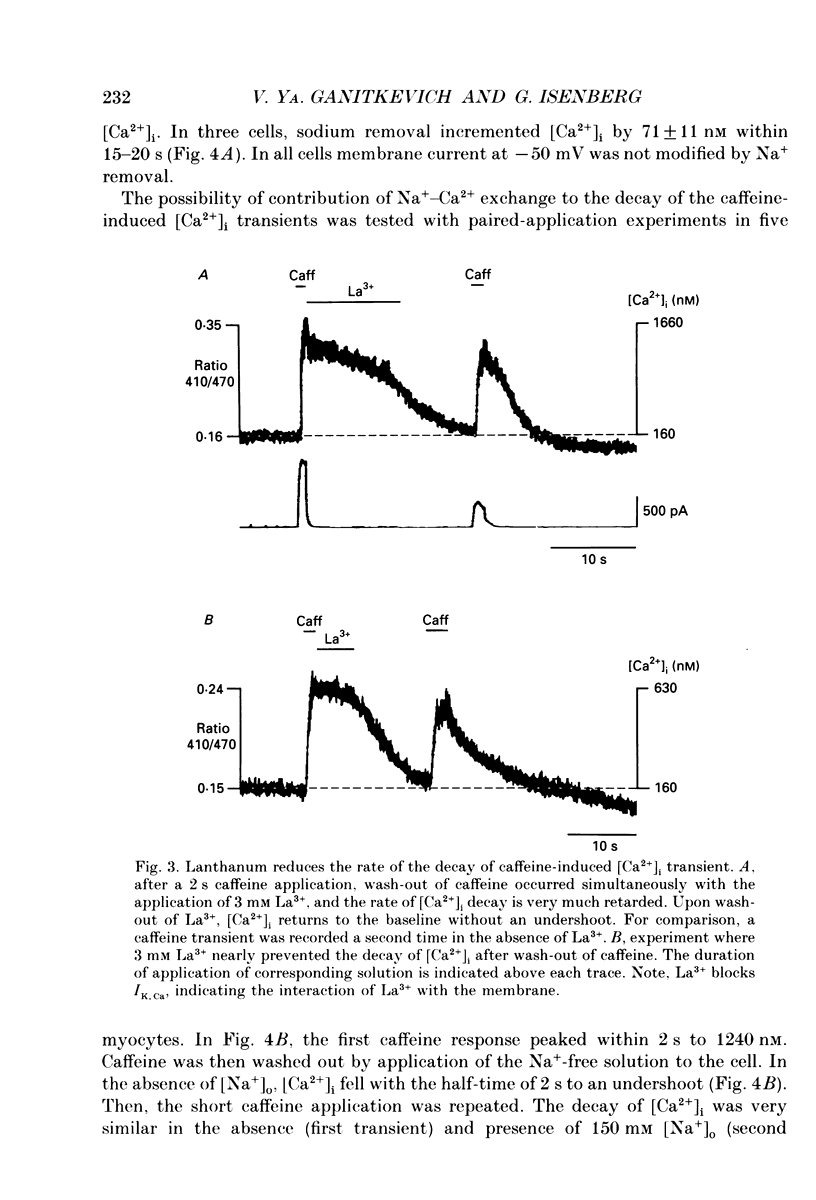
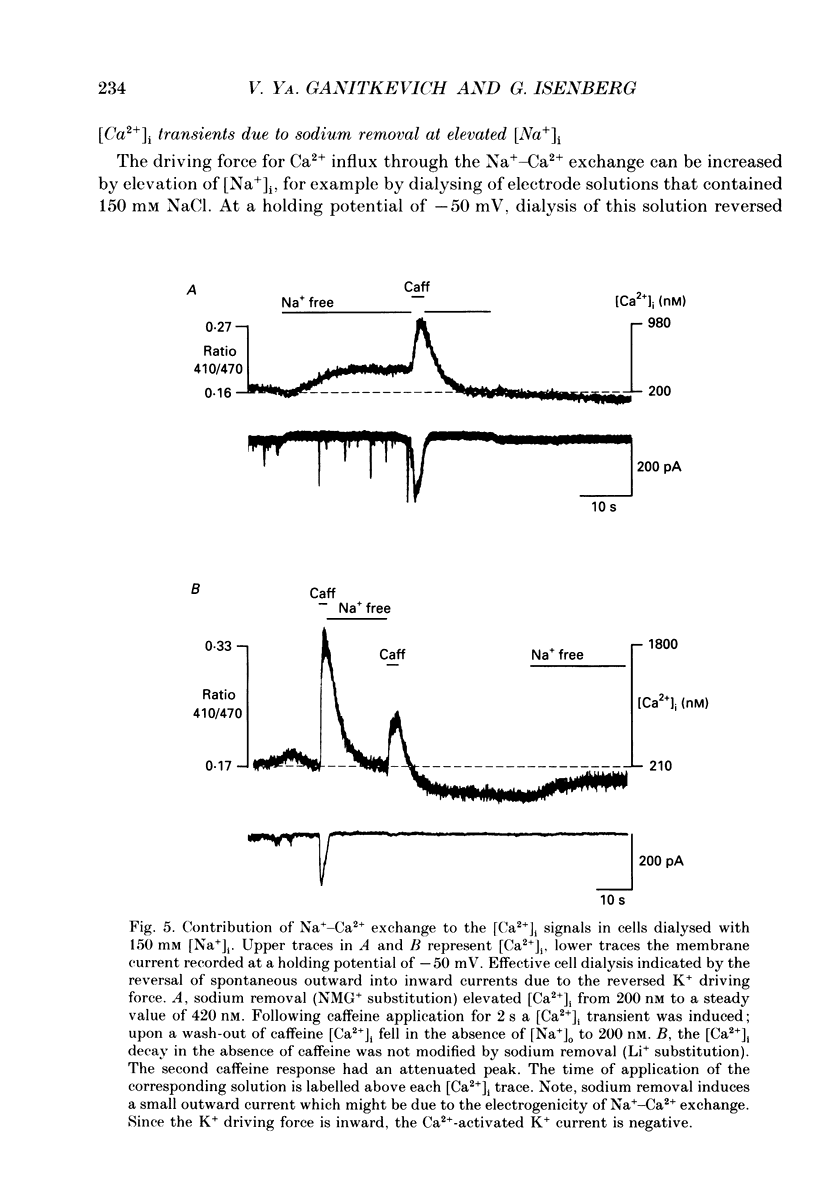
1. The ionized cytosolic calcium concentration ([Ca2+]i) was monitored in voltage-clamped coronary myocytes at 36 degrees C and 2.5 mM [Ca2+]o using the Ca2+ indicator indo-1. [Ca2+]i was transiently increased by fast application of 10 mM caffeine, and the mechanisms involved in decay of [Ca2+]i were analysed. 2. Resting [Ca2+]i was 166 +/- 62 nM (mean +/- S.D.). Caffeine increased [Ca2+]i within 1-2 s to 1618 +/- 490 nM. In the continuous presence of caffeine [Ca2+]i fell close to resting values with a half-decay time of 5.0 +/- 1.6 s. Wash-out of caffeine induced an undershoot of [Ca2+]i to 105 +/- 30 nM. When caffeine was applied repetitively the [Ca2+]i transients were of reduced amplitude indicating that the store had lost a part of releasable Ca2+. 3. After a 1 s caffeine application [Ca2+]i decayed with a half-time of 2.3 +/- 0.8 s to the undershoot of 112 +/- 57 nM. The decay of [Ca2+]i was largely prevented by 3 mM [La3+]o; after wash-out of La3+ [Ca2+]i fell to the resting value without an undershoot. The results demonstrate that La(3+)-sensitive Ca2+ extrusion contributes to the decay of the [Ca2+]i transient and to the undershoot. 4. With 10 mM [Na+]i, sodium removal from the bath incremented [Ca2+]i in three out of ten cells by 71 +/- 11 nM; in the other cells [Ca2+]i did not change. In the absence of extracellular sodium the decay of [Ca2+]i after wash-out of caffeine was not retarded. 5. To stimulate Na(+)-Ca2+ exchange, cells were dialysed with pipette solution containing 150 mM NaCl. Elevation of [Na+]i had no significant effect on the resting [Ca2+]i (180 +/- 47 nM) or on the caffeine-induced [Ca2+]i transients (peak 1614 +/- 530 nM, half-time of decay 3 s, undershoot 107 +/- 40 nM). 6. With 150 mM [Na+]i, sodium removal resulted in an increase of [Ca2+]i, although responses varied in amplitude (from 130 to 2300 nM) and rate of rise. In the absence of sodium [Ca2+]i remained elevated. After a 1 s caffeine application the undershoot of [Ca2+]i was abolished in sodium-free solution. When caffeine was applied in sodium-free solution, the [Ca2+]i transient decayed to a sustained level and the following caffeine response was attenuated. 7. With 150 mM [Na+]i, the effects of sodium removal were strongly suppressed by a preceding depletion of the Ca2+ stores with caffeine. Ryanodine pretreatment abolished the caffeine-induced [Ca2+]i transients and reduced [Ca2+]i response due to sodium removal.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





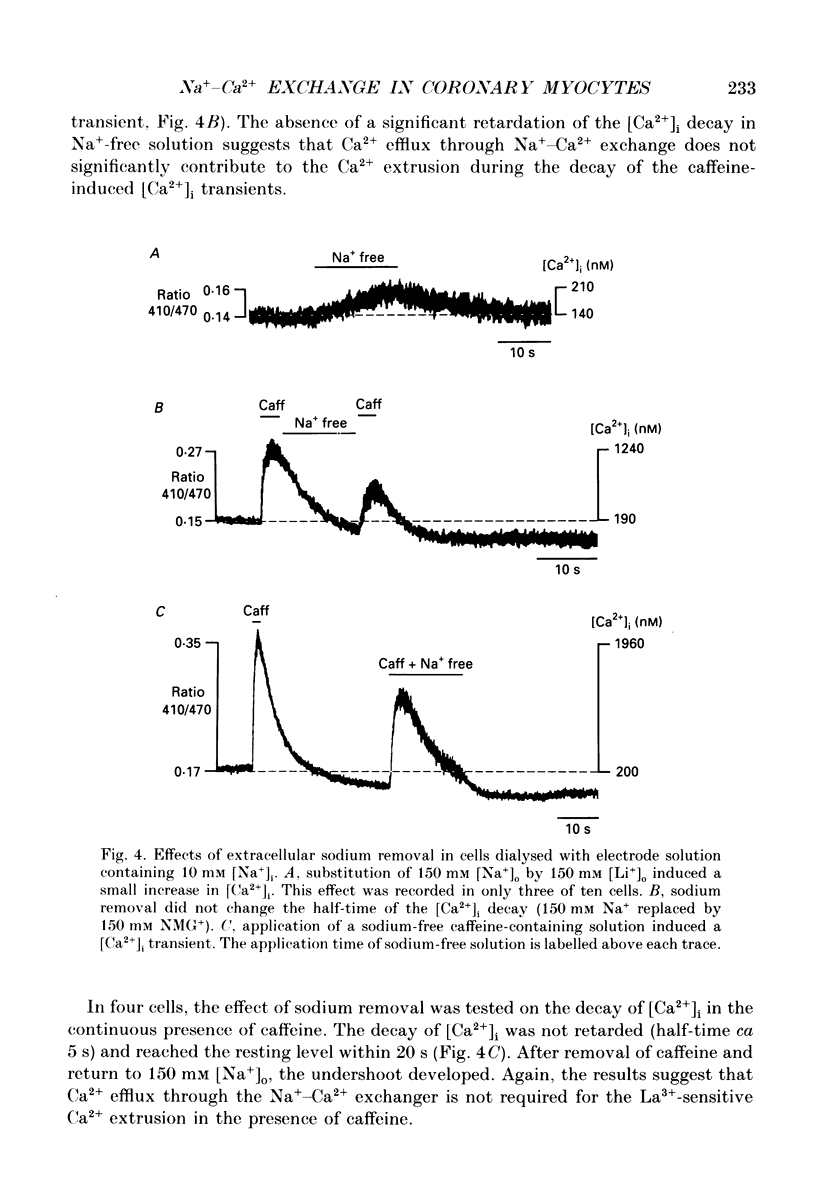







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
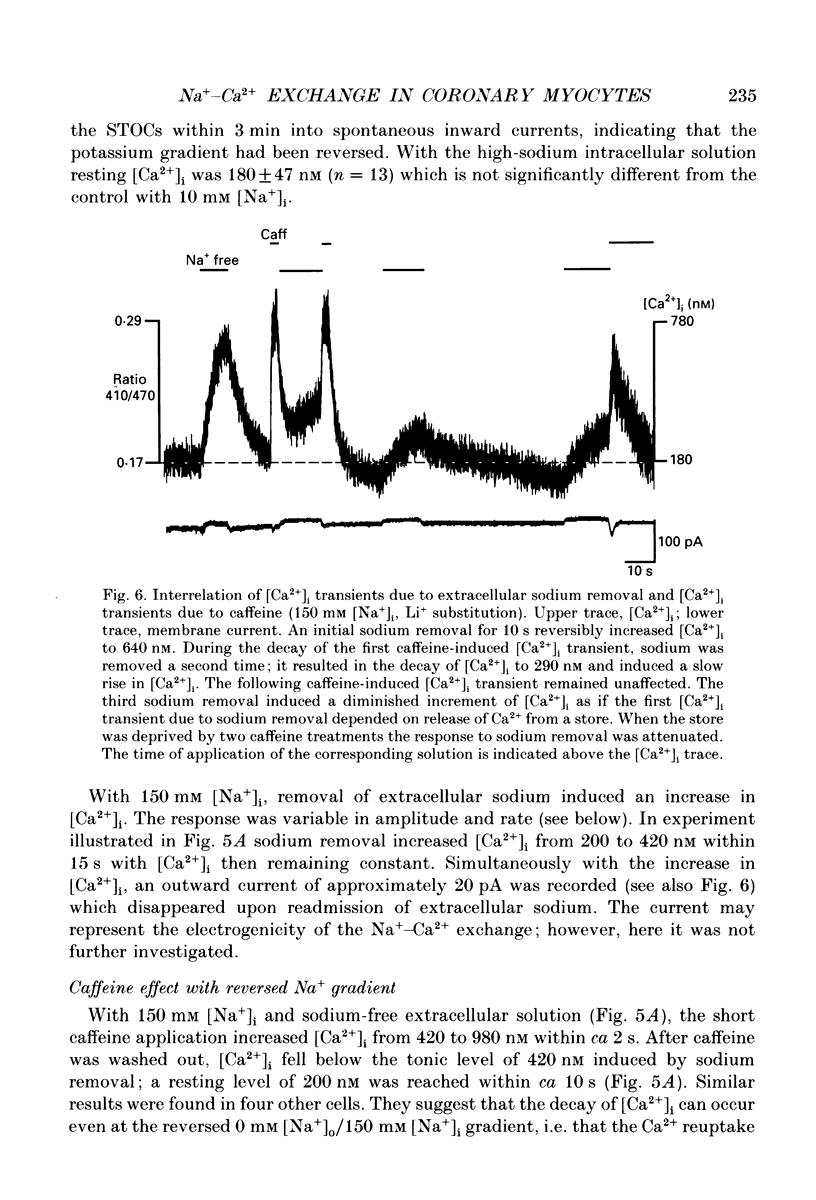
- Aaronson P. I., Benham C. D. Alterations in [Ca2+]i mediated by sodium-calcium exchange in smooth muscle cells isolated from the guinea-pig ureter. J Physiol. 1989 Sep;416:1–18. doi: 10.1113/jphysiol.1989.sp017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
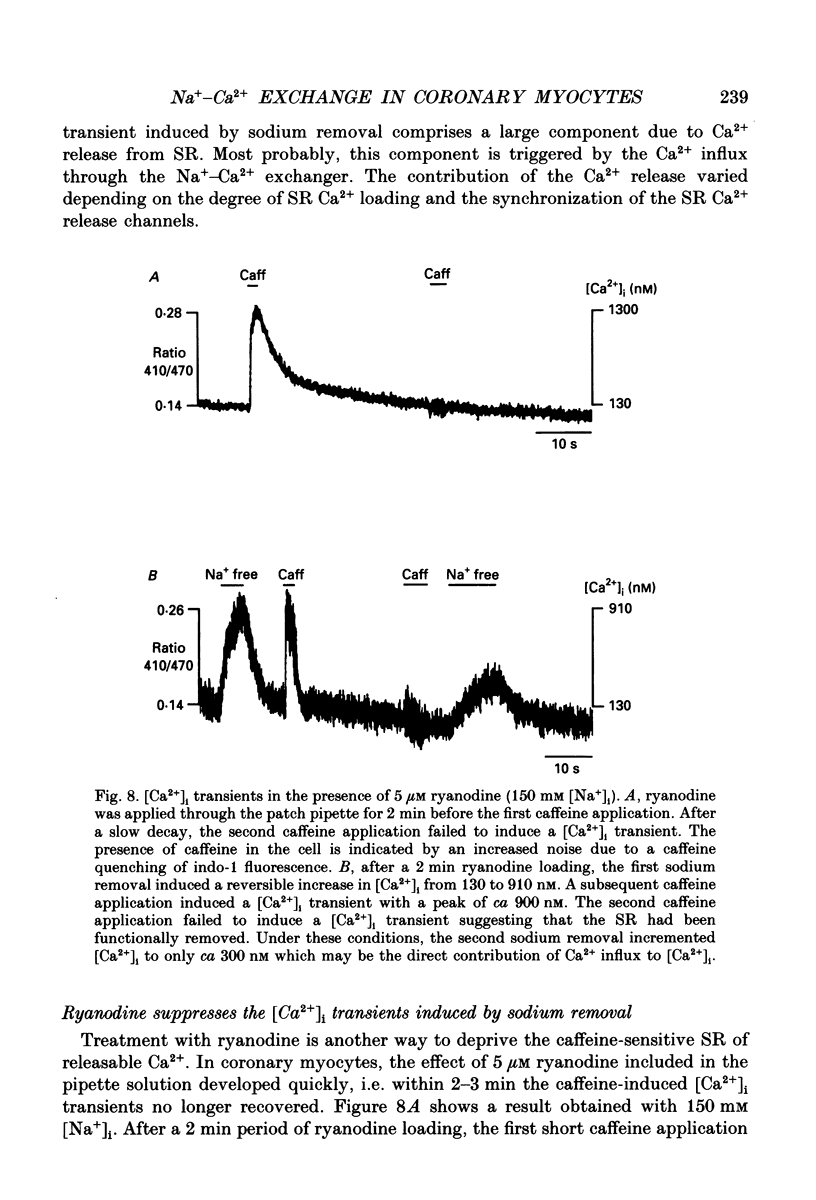
- Ashida T., Blaustein M. P. Regulation of cell calcium and contractility in mammalian arterial smooth muscle: the role of sodium-calcium exchange. J Physiol. 1987 Nov;392:617–635. doi: 10.1113/jphysiol.1987.sp016800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989 Dec;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman W. F., Fontana G., Krueger B. K., Santiago E. M., Steele T. D., Weiss D. N., Yarowsky P. J. Physiological roles of the sodium-calcium exchanger in nerve and muscle. Ann N Y Acad Sci. 1991;639:254–274. doi: 10.1111/j.1749-6632.1991.tb17315.x. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V Y. a., Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J Physiol. 1991 Apr;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Caffeine-induced release and reuptake of Ca2+ by Ca2+ stores in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:99–117. doi: 10.1113/jphysiol.1992.sp019408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A. K., Samson S. E. Pig coronary artery smooth muscle: substrate and pH dependence of the two calcium pumps. Am J Physiol. 1986 Oct;251(4 Pt 1):C529–C534. doi: 10.1152/ajpcell.1986.251.4.C529. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Inesi G., de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989 Apr 5;264(10):5929–5936. [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Kuriyama H. Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol. 1979 Aug;293:119–133. doi: 10.1113/jphysiol.1979.sp012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., Raeymaekers L., De Smedt H., Droogmans G., Declerck I., Casteels R. Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores. Pharmacol Ther. 1991;50(2):191–232. doi: 10.1016/0163-7258(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Aalkjaer C., Jensen P. E. Sodium-calcium exchange in vascular smooth muscle. Ann N Y Acad Sci. 1991;639:498–504. doi: 10.1111/j.1749-6632.1991.tb17343.x. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Richard H., Chen X. L. Na(+)-Ca2+ exchange, myoplasmic Ca2+ concentration, and contraction of arterial smooth muscle. Hypertension. 1992 Apr;19(4):308–313. doi: 10.1161/01.hyp.19.4.308. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Lyu R. M., Smith L. Sodium-calcium exchange in aortic myocytes and renal epithelial cells. Dependence on metabolic energy and intracellular sodium. Ann N Y Acad Sci. 1991;639:505–520. doi: 10.1111/j.1749-6632.1991.tb17344.x. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]
- Vigne P., Breittmayer J. P., Duval D., Frelin C., Lazdunski M. The Na+/Ca2+ antiporter in aortic smooth muscle cells. Characterization and demonstration of an activation by phorbol esters. J Biol Chem. 1988 Jun 15;263(17):8078–8083. [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


