Abstract
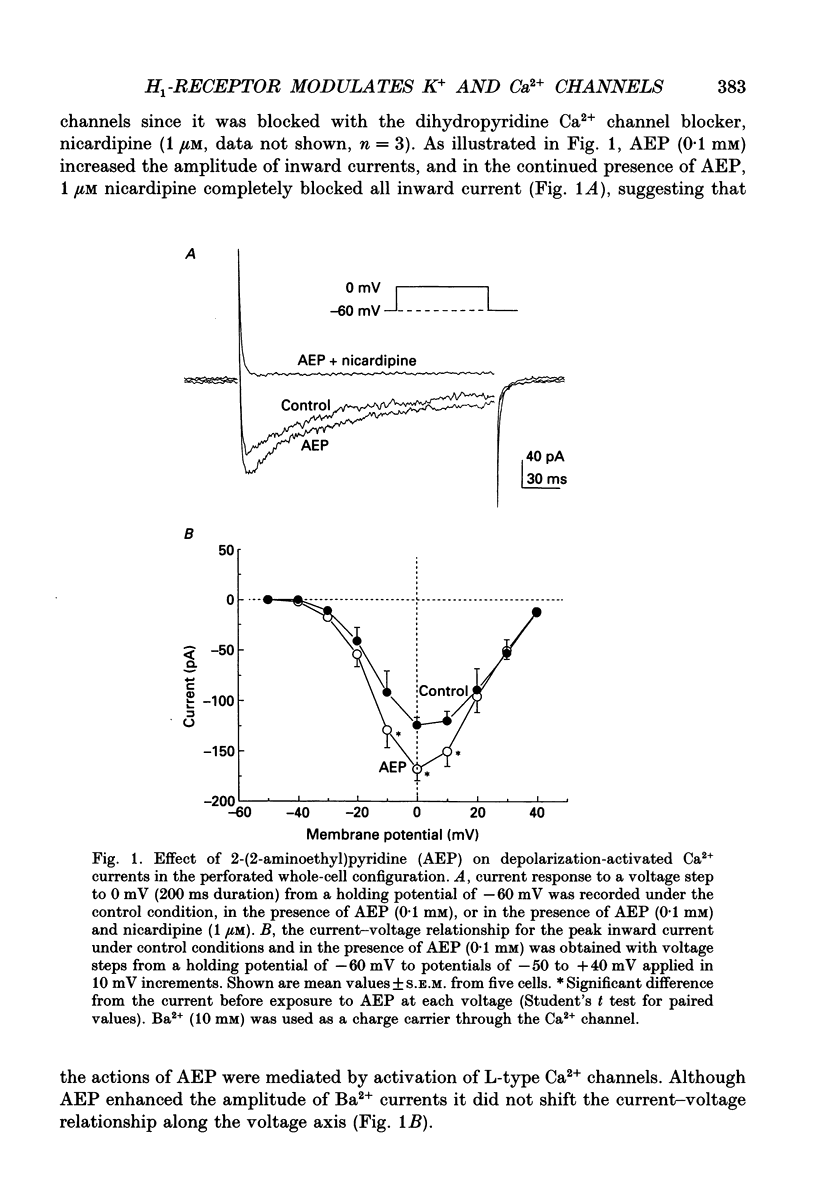
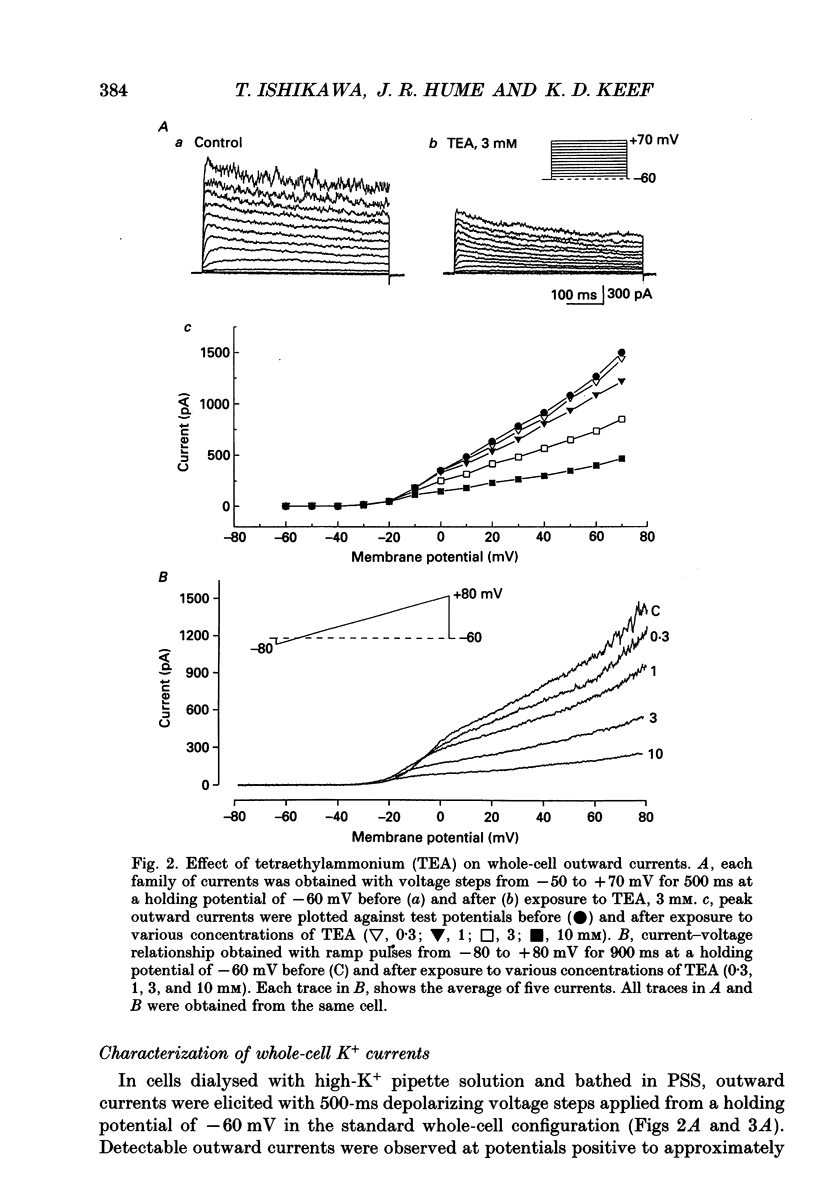
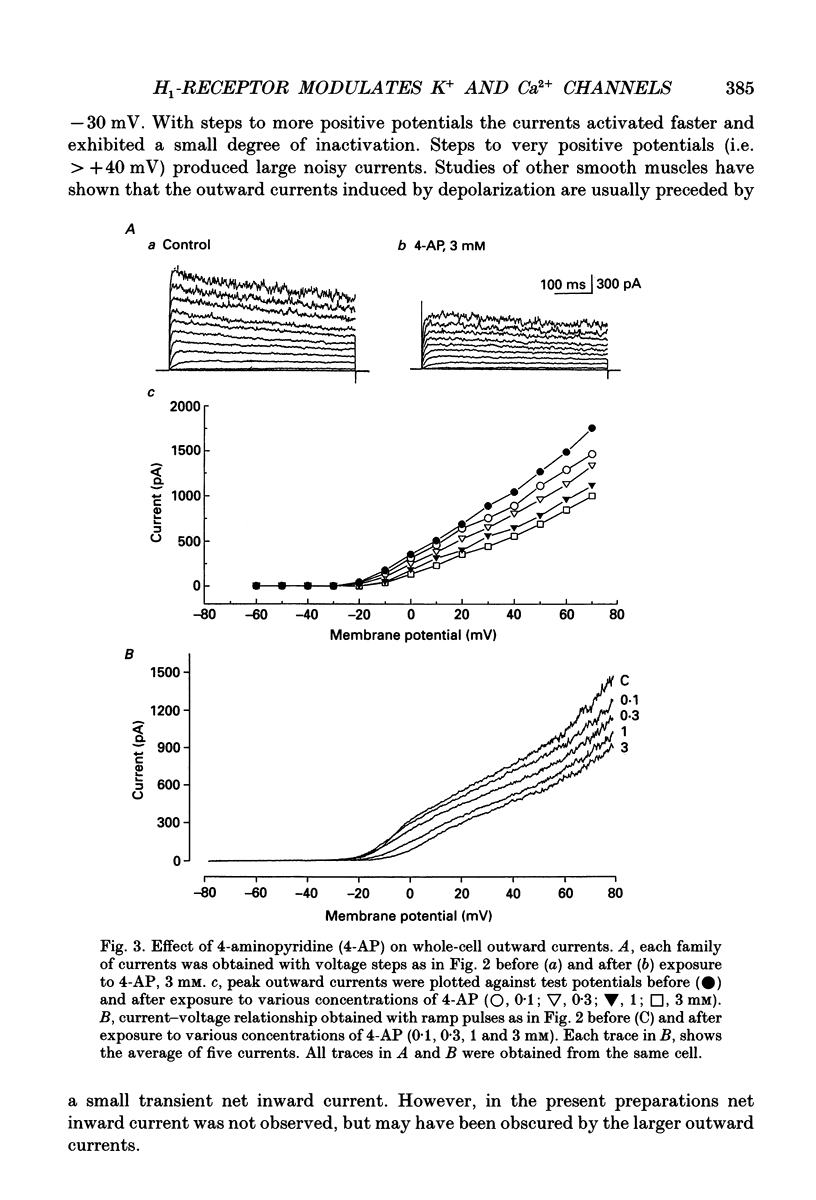
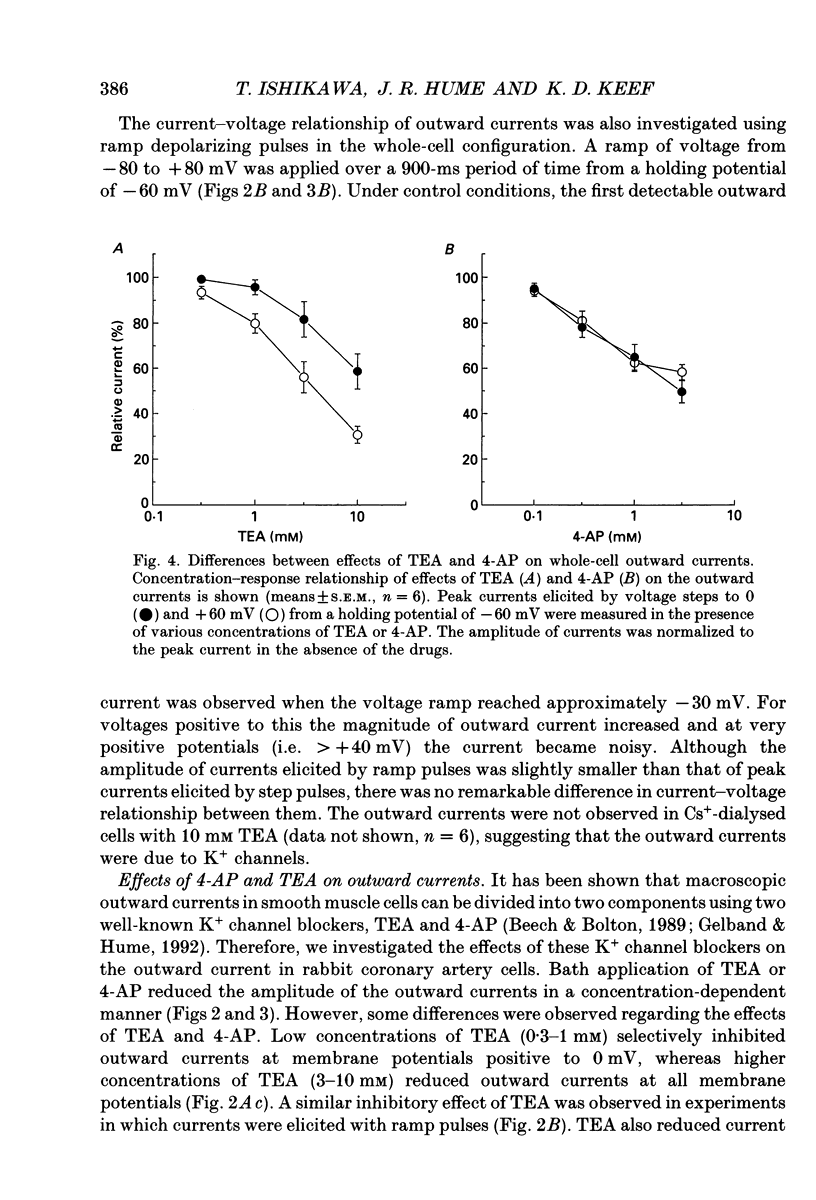
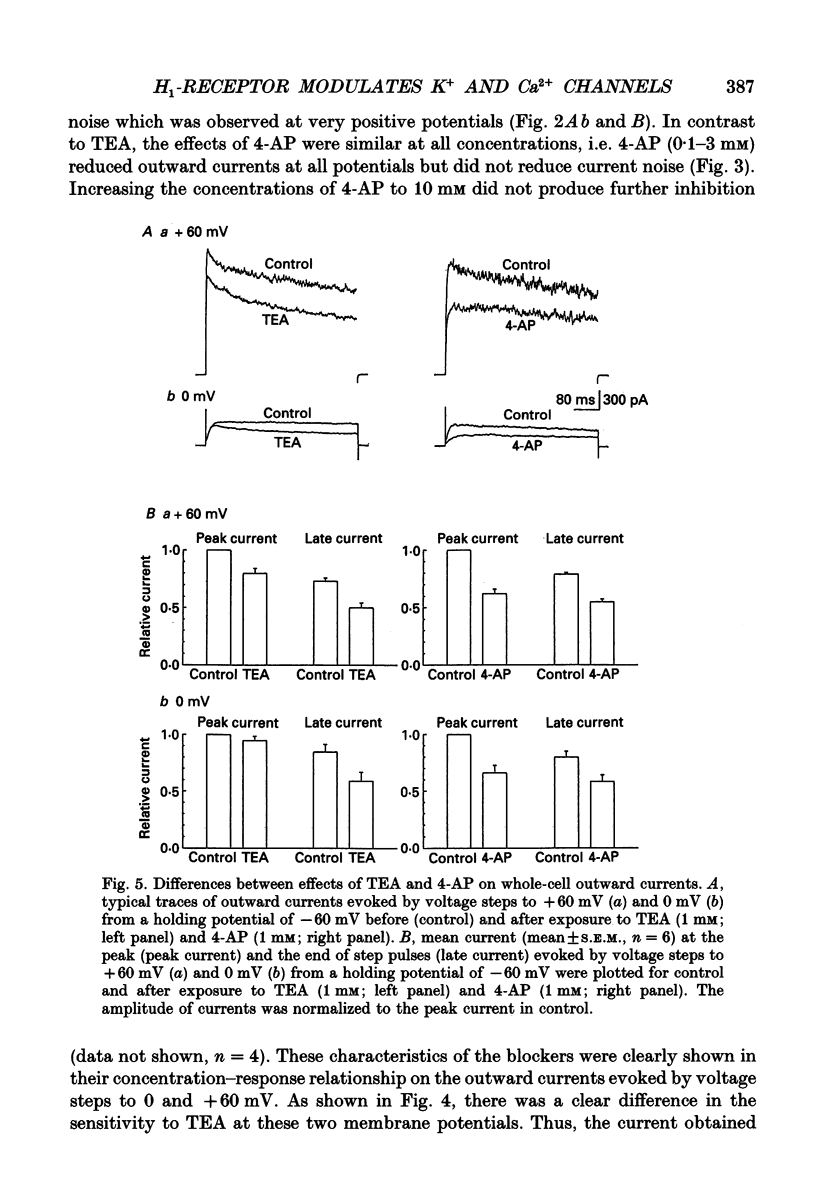
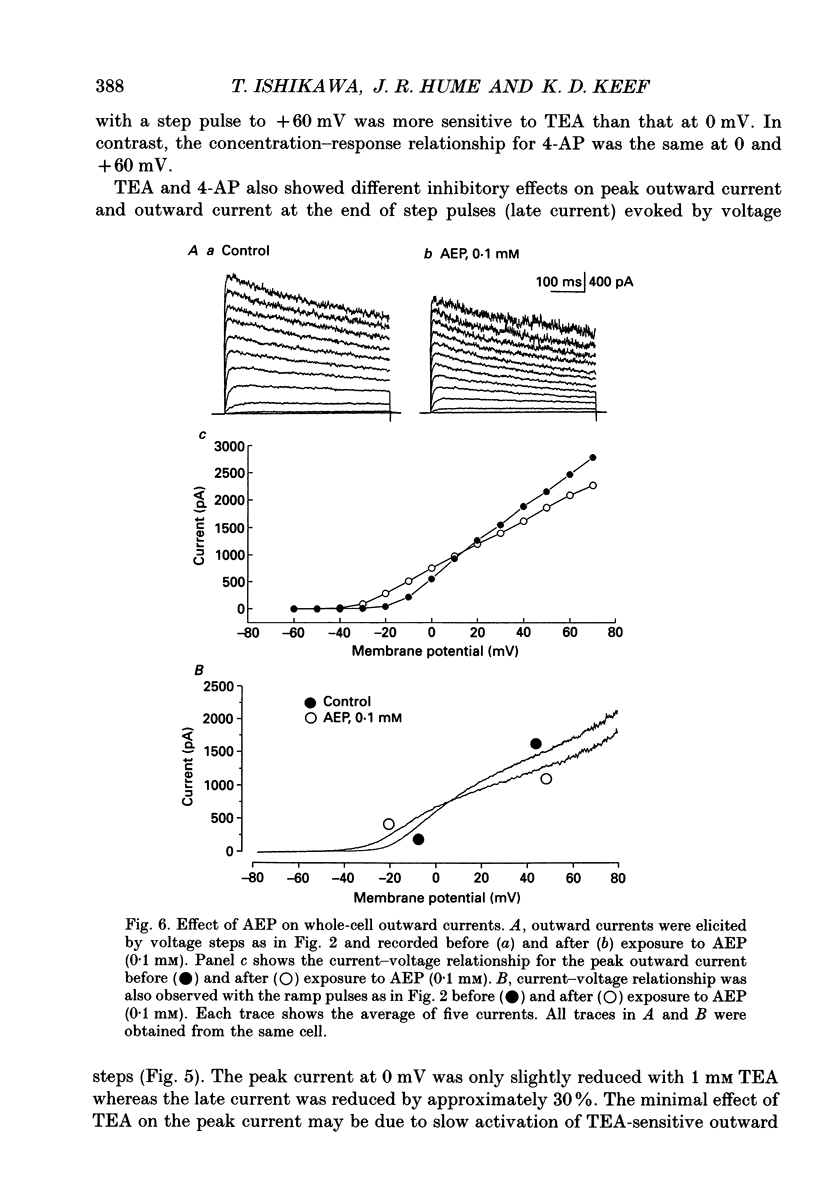
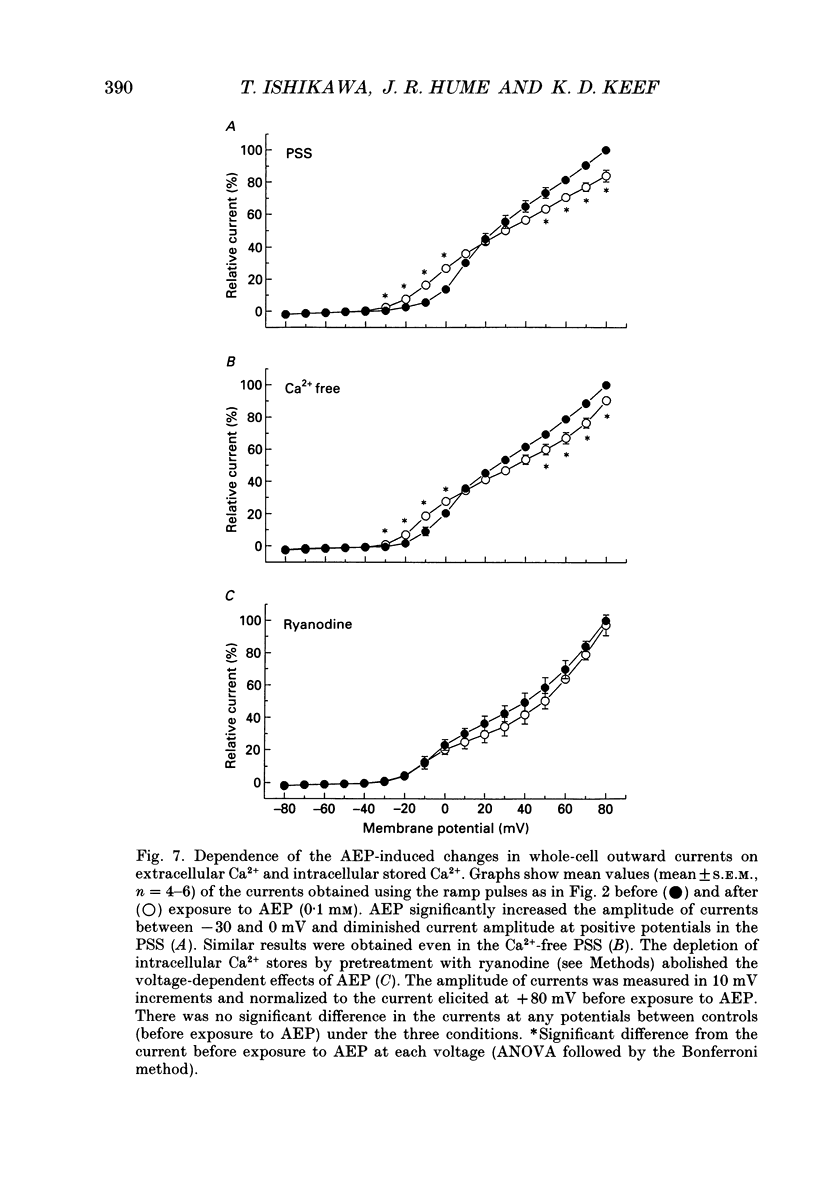
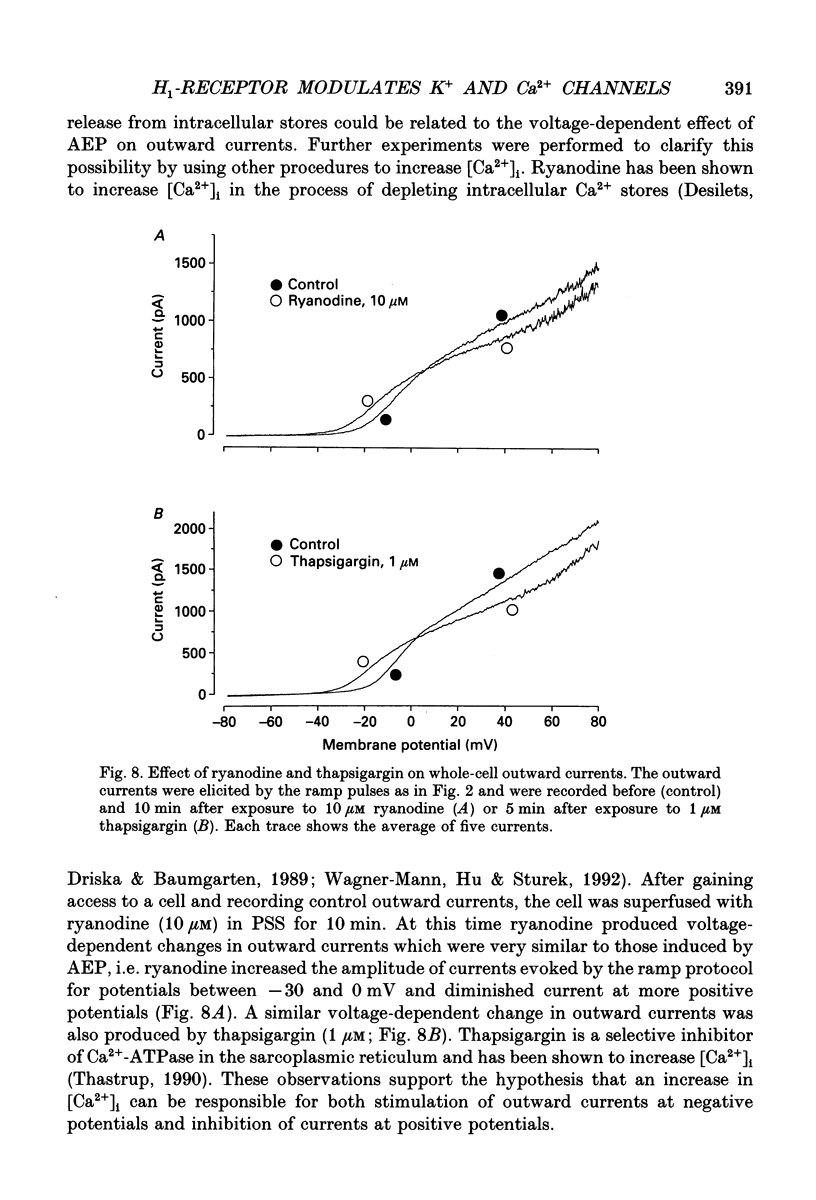
1. The modulation of whole-cell K+ and Ca2+ currents by stimulation of histamine H1-receptors in freshly isolated single smooth muscle cells from the rabbit coronary artery was characterized using the patch-clamp technique at 35 degrees C. Single-channel K+ currents were also analysed using the cell-attached patch configuration. 2. The histamine H1-receptor agonist, 2-(2-aminoethyl)pyridine (AEP) (0.1 mM), increased the amplitude of voltage-activated inward Ba2+ currents, recorded using the perforated-patch recording technique, which could be completely blocked by the dihydropyridine antagonist, nicardipine (1 microM). 3. Whole-cell outward K+ currents in rabbit coronary artery cells could be classified into at least two components: (a) a slowly inactivating, 4-aminopyridine (4-AP)-sensitive low-noise current, and (b) a non-inactivating, tetraethylammonium (TEA)-sensitive high-noise current. 4. AEP (0.1 mM) caused changes in whole-cell outward K+ currents which depended upon membrane voltage. Specifically: (a) AEP enhanced the amplitude of outward currents at voltages between -30 and 0 mV, and (b) AEP decreased the outward currents at more positive potentials. 5. The removal of extracellular Ca2+ caused little inhibition of the effects of AEP on K+ currents, whereas the depletion of intracellular Ca2+ stores by pretreatment with ryanodine and caffeine prevented the effects of AEP on K+ channels. Moreover, acute exposure to ryanodine (10 microM) or thapsigargin (1 microM), a Ca(2+)-ATPase inhibitor, caused voltage-dependent changes in the outward currents similar to those observed with AEP. These results suggest that the voltage-dependent effects of AEP on K+ currents are mainly mediated by release of Ca2+ from intracellular stores. 6. The dual stimulatory and inhibitory effect of AEP on whole-cell K+ currents was shown to be due to a differential effect on two distinct types of K+ channels. The stimulatory effect observed over the voltage range -30 to 0 mV was prevented by pretreatment of cells with low concentrations of TEA (1 mM), whereas the inhibitory effect observed at positive potentials was prevented by pretreatment of cells with 4-AP (3 mM). 7. Single-channel recordings revealed two types of unitary K+ currents with conductances of 225 and 70 pS in the cell-attached configuration with symmetrical K+ solutions (150 mM K+ in pipette-150 mM K+ in bath). Bath application of AEP (0.1 mM) caused a marked increase in the open probability of the large conductance channels.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




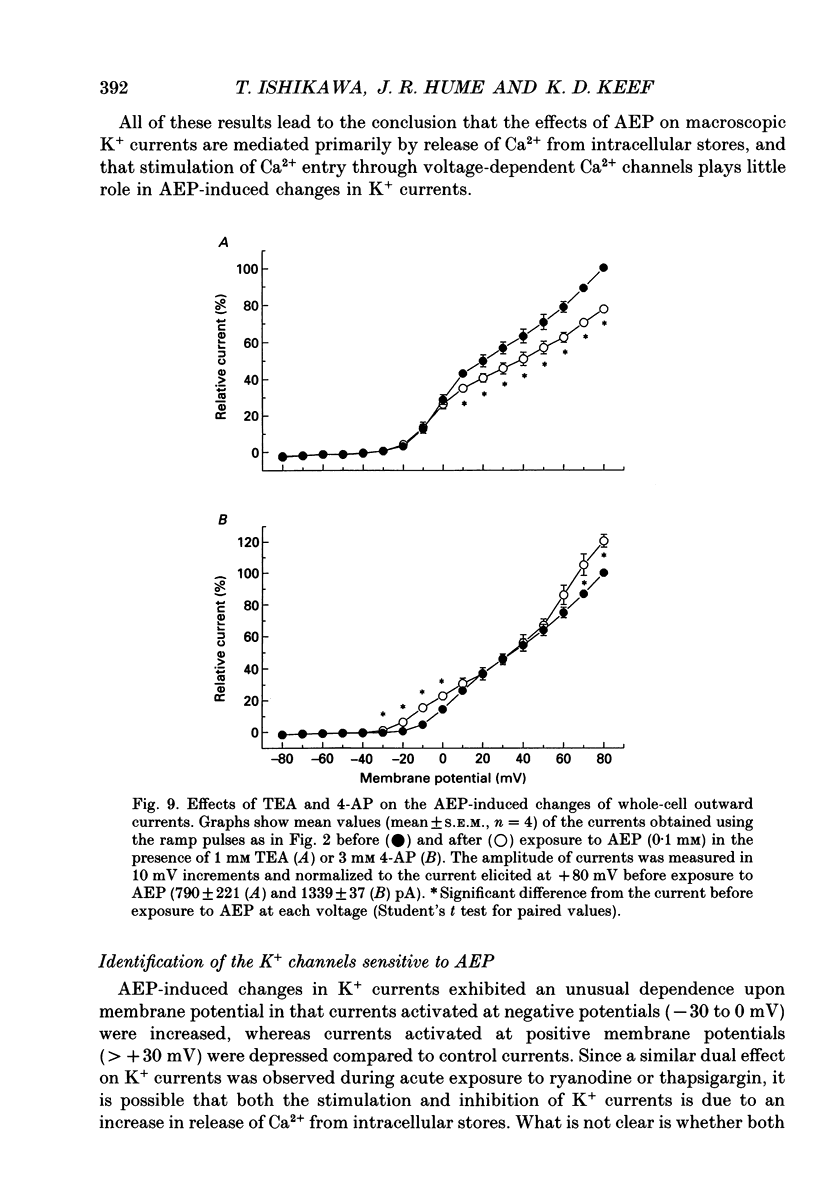
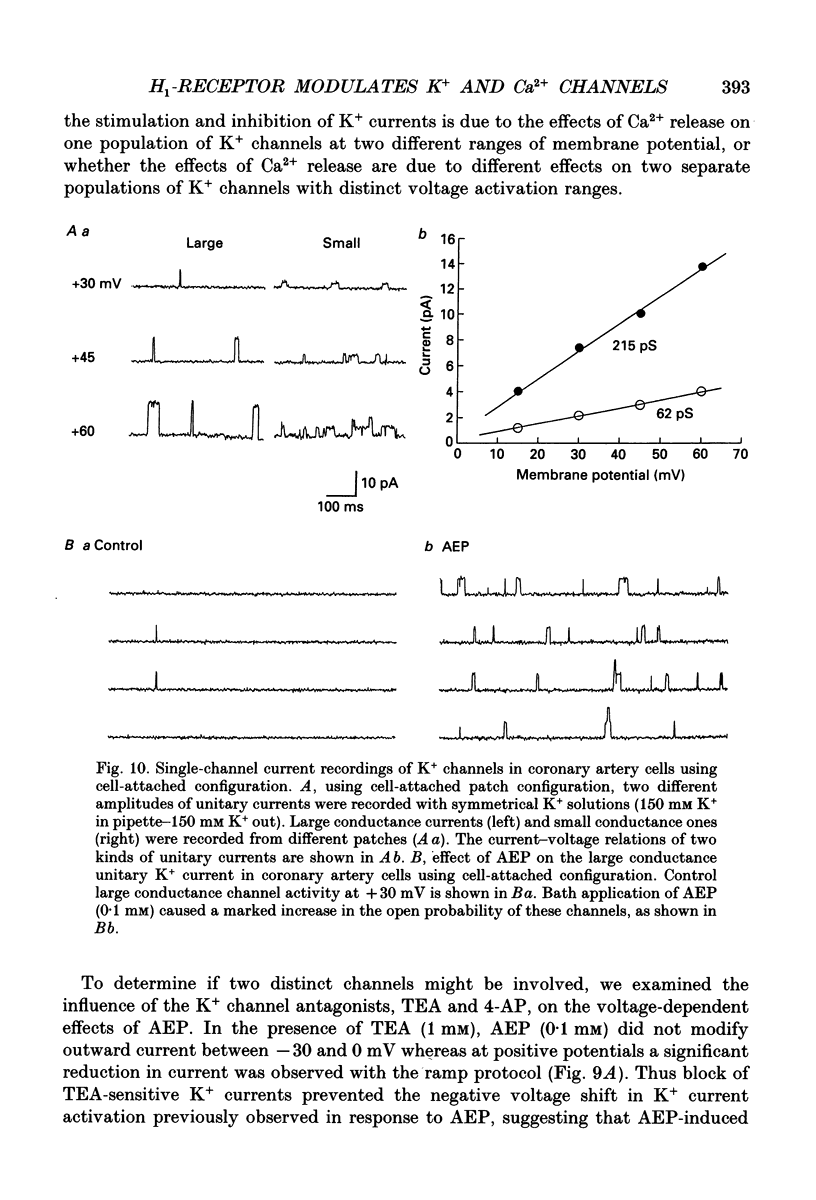

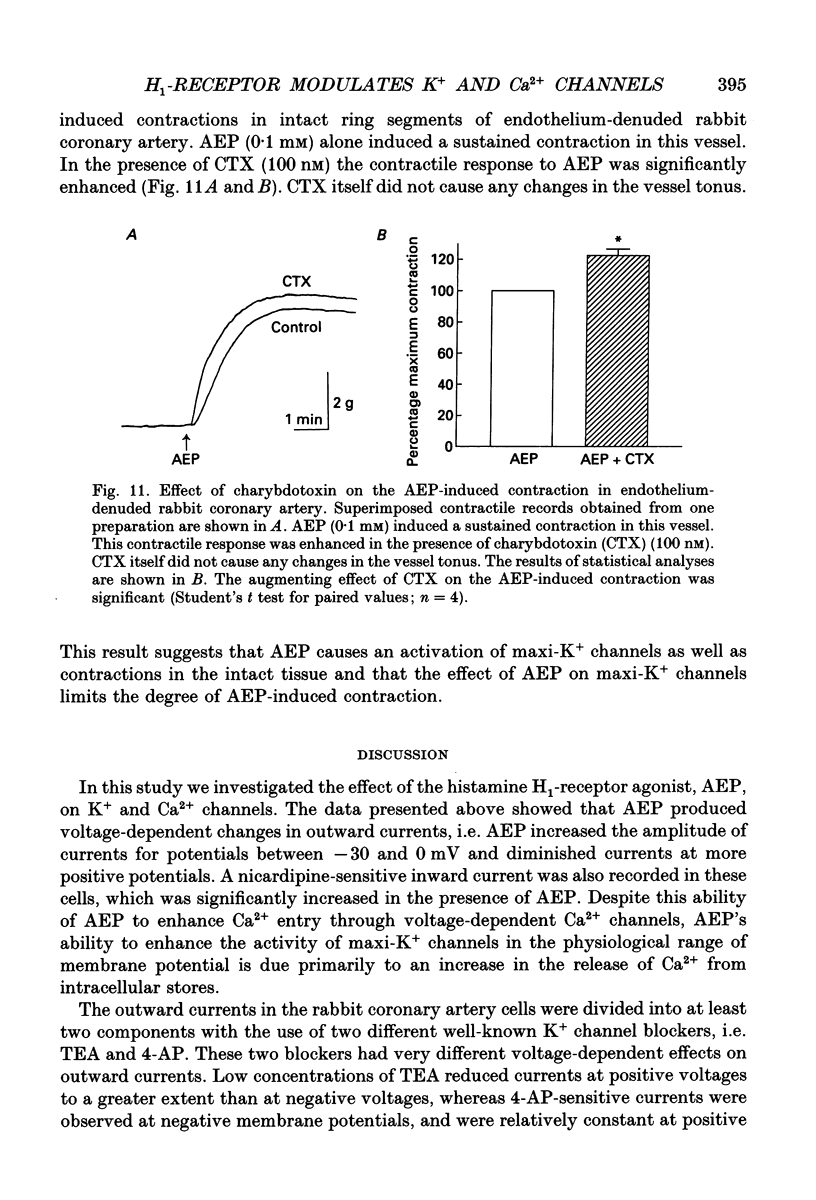





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke C. W., Wier W. G. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991 Mar;68(3):897–902. doi: 10.1161/01.res.68.3.897. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988 Oct;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G., Peyrow M., Sculptoreanu A., Jacques D., Chahine M., Regoli D., Sperelakis N. Angiotensin II increases Isi and blocks IK in single aortic cell of rabbit. Pflugers Arch. 1988 Sep;412(4):448–450. doi: 10.1007/BF01907567. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985 Jun;85(2):499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel P. E., Knickle L. Cytochalasin-B and phloretin depress contraction and relaxation of aortic smooth muscle. Eur J Pharmacol. 1987 Dec 1;144(2):153–157. doi: 10.1016/0014-2999(87)90514-0. [DOI] [PubMed] [Google Scholar]
- Durant G. J., Ganellin C. R., Parsons M. E. Chemical differentiation of histamine H1- and H2-receptor agonists. J Med Chem. 1975 Sep;18(9):905–909. doi: 10.1021/jm00243a009. [DOI] [PubMed] [Google Scholar]
- Désilets M., Driska S. P., Baumgarten C. M. Current fluctuations and oscillations in smooth muscle cells from hog carotid artery. Role of the sarcoplasmic reticulum. Circ Res. 1989 Sep;65(3):708–722. doi: 10.1161/01.res.65.3.708. [DOI] [PubMed] [Google Scholar]
- Ganitkevich V., Isenberg G. Isolated guinea pig coronary smooth muscle cells. Acetylcholine induces hyperpolarization due to sarcoplasmic reticulum calcium release activating potassium channels. Circ Res. 1990 Aug;67(2):525–528. doi: 10.1161/01.res.67.2.525. [DOI] [PubMed] [Google Scholar]
- Gelband C. H., Hume J. R. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res. 1992 Oct;71(4):745–758. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano K., Kanaide H., Abe S., Nakamura M. Temporal changes in the calcium-force relation during histamine-induced contractions of strips of the coronary artery of the pig. Br J Pharmacol. 1991 Jan;102(1):27–34. doi: 10.1111/j.1476-5381.1991.tb12127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Takakura S., Sato K., Sutko J. L. Ryanodine inhibits the release of calcium from intracellular stores in guinea pig aortic smooth muscle. Circ Res. 1986 May;58(5):730–734. doi: 10.1161/01.res.58.5.730. [DOI] [PubMed] [Google Scholar]
- Kajioka S., Nakashima M., Kitamura K., Kuriyama H. Mechanisms of vasodilatation induced by potassium-channel activators. Clin Sci (Lond) 1991 Aug;81(2):129–139. doi: 10.1042/cs0810129. [DOI] [PubMed] [Google Scholar]
- Keef K. D., Ross G. Relaxation induced by KCl, NaCl and sucrose in rabbit coronary arteries. Pflugers Arch. 1987 Jul;409(3):308–313. doi: 10.1007/BF00583481. [DOI] [PubMed] [Google Scholar]
- Khoyi M. A., Bowen S. M., Keef K. D. Effect of membrane potential on H1-receptor-mediated 45Ca influx in coronary artery. Am J Physiol. 1991 Aug;261(2 Pt 2):H554–H560. doi: 10.1152/ajpheart.1991.261.2.H554. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Nelson M. T., Huang Y., Standen N. B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991 Mar;260(3 Pt 2):H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Matsuda J. J., Volk K. A., Shibata E. F. Calcium currents in isolated rabbit coronary arterial smooth muscle myocytes. J Physiol. 1990 Aug;427:657–680. doi: 10.1113/jphysiol.1990.sp018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca modulates outward current through IK1 channels. J Membr Biol. 1990 Jun;116(1):41–45. doi: 10.1007/BF01871670. [DOI] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Worley J. F. Dihydropyridine inhibition of single calcium channels and contraction in rabbit mesenteric artery depends on voltage. J Physiol. 1989 May;412:65–91. doi: 10.1113/jphysiol.1989.sp017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M., Kitamura K., Kuriyama H. Histamine H3-receptor activation augments voltage-dependent Ca2+ current via GTP hydrolysis in rabbit saphenous artery. J Physiol. 1992 Mar;448:133–152. doi: 10.1113/jphysiol.1992.sp019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sakai T., Terada K., Kitamura K., Kuriyama H. Ryanodine inhibits the Ca-dependent K current after depletion of Ca stored in smooth muscle cells of the rabbit ileal longitudinal muscle. Br J Pharmacol. 1988 Dec;95(4):1089–1100. doi: 10.1111/j.1476-5381.1988.tb11743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Toda N. Isolated human coronary arteries in response to vasoconstrictor substances. Am J Physiol. 1983 Dec;245(6):H937–H941. doi: 10.1152/ajpheart.1983.245.6.H937. [DOI] [PubMed] [Google Scholar]
- Trieschmann U., Isenberg G. Ca2+-activated K+ channels contribute to the resting potential of vascular myocytes. Ca2+-sensitivity is increased by intracellular Mg2+-ions. Pflugers Arch. 1989;414 (Suppl 1):S183–S184. doi: 10.1007/BF00582296. [DOI] [PubMed] [Google Scholar]
- Volk K. A., Matsuda J. J., Shibata E. F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J Physiol. 1991 Aug;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Mann C., Hu Q., Sturek M. Multiple effects of ryanodine on intracellular free Ca2+ in smooth muscle cells from bovine and porcine coronary artery: modulation of sarcoplasmic reticulum function. Br J Pharmacol. 1992 Apr;105(4):903–911. doi: 10.1111/j.1476-5381.1992.tb09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


