Abstract
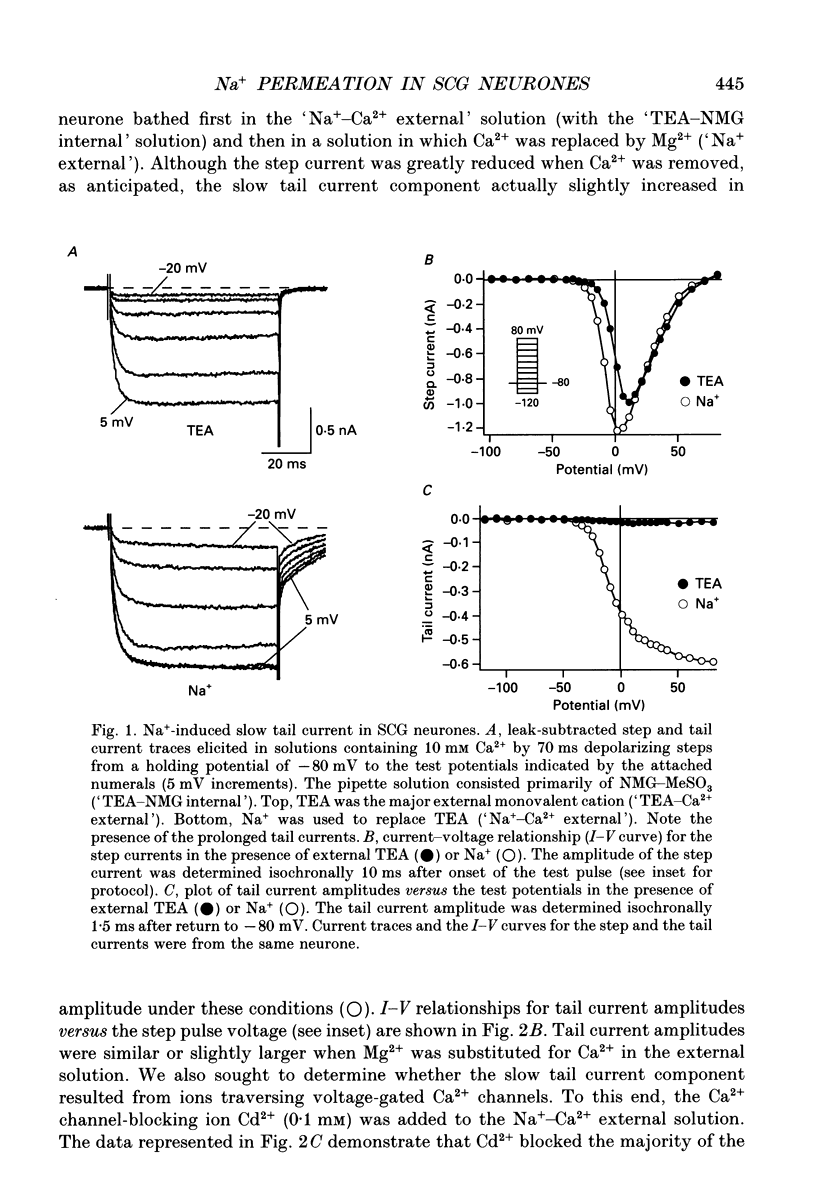
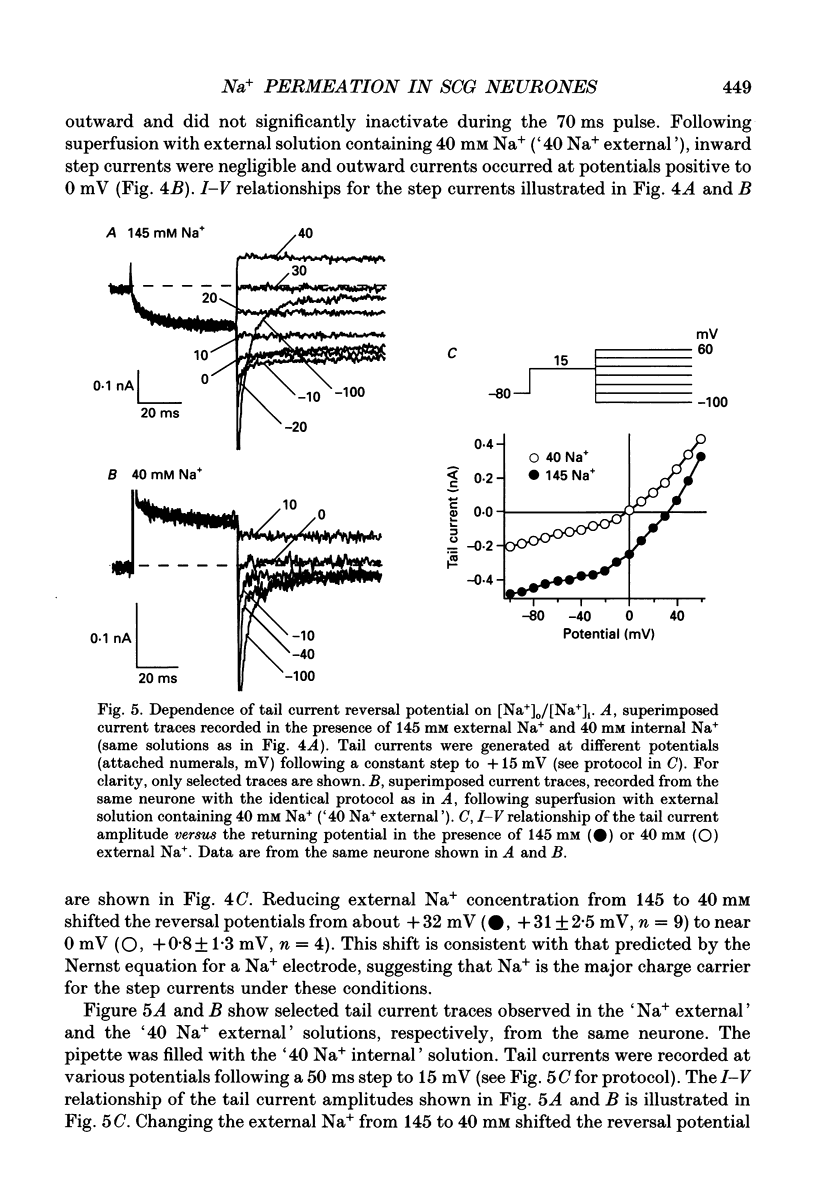
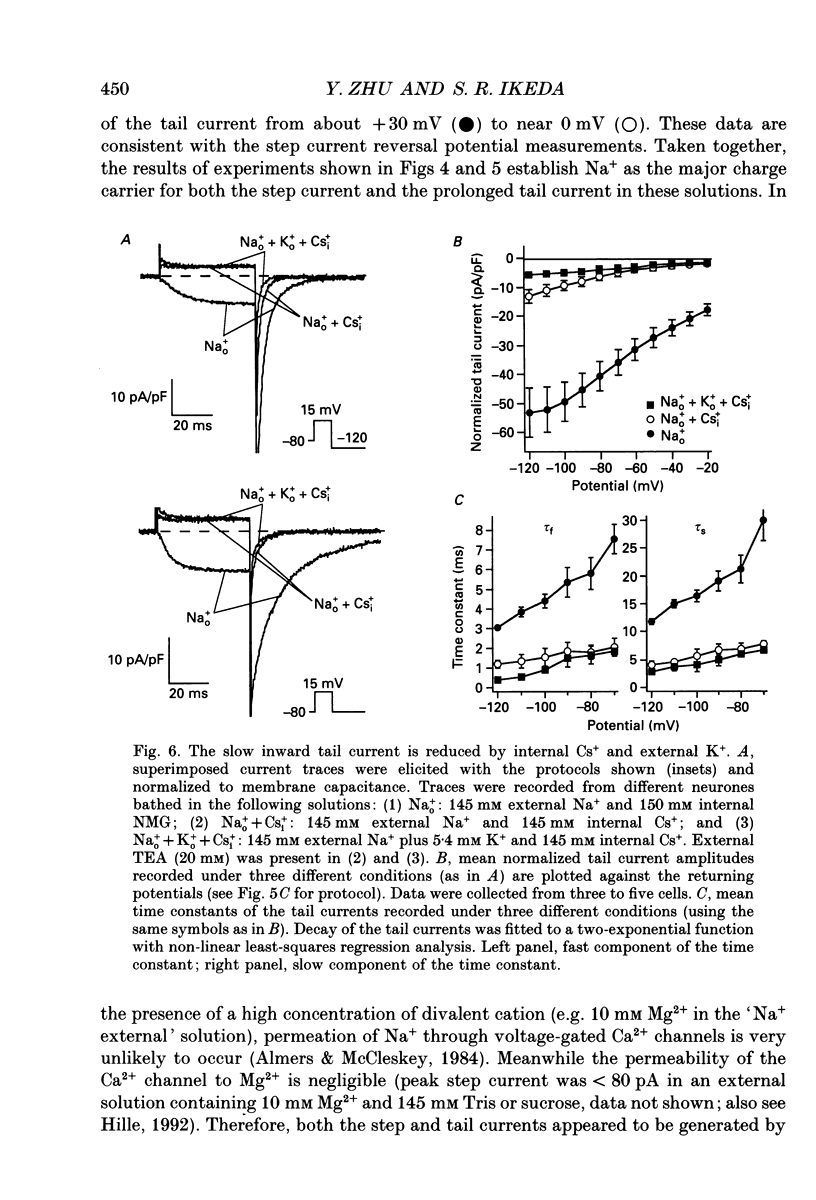
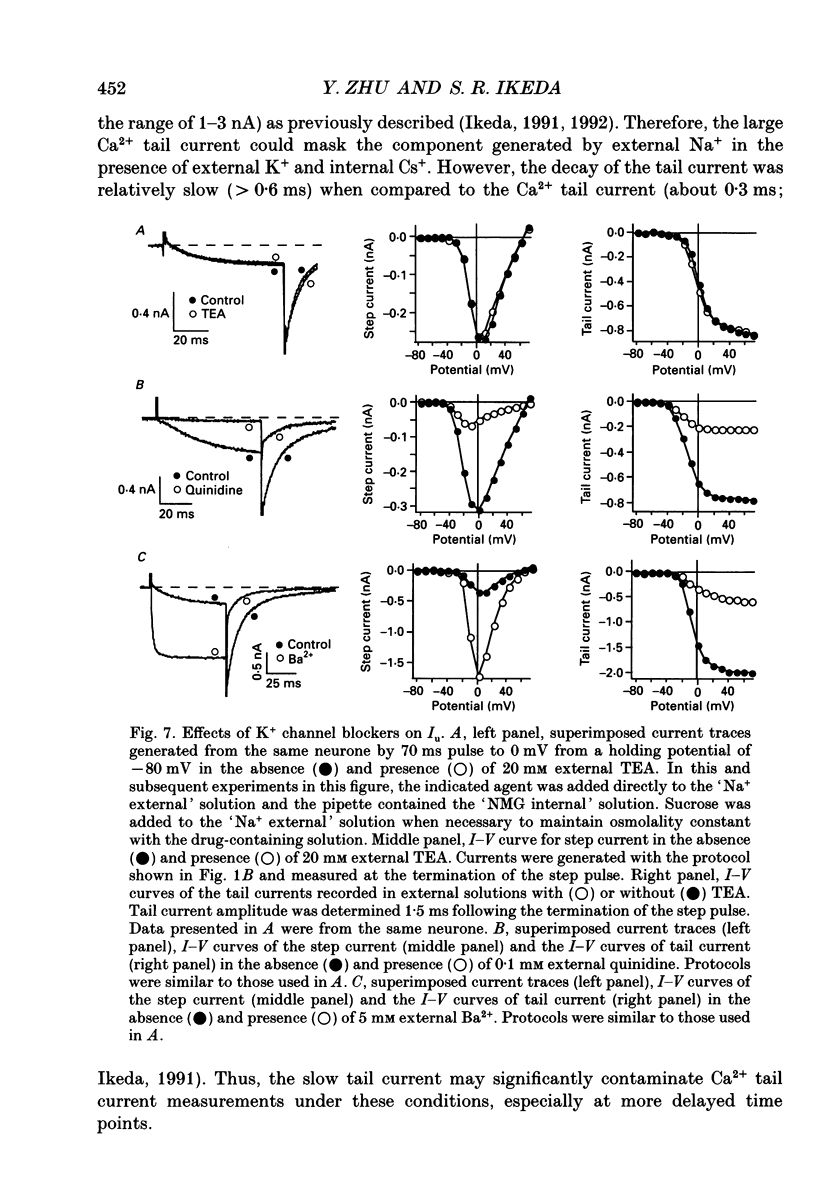
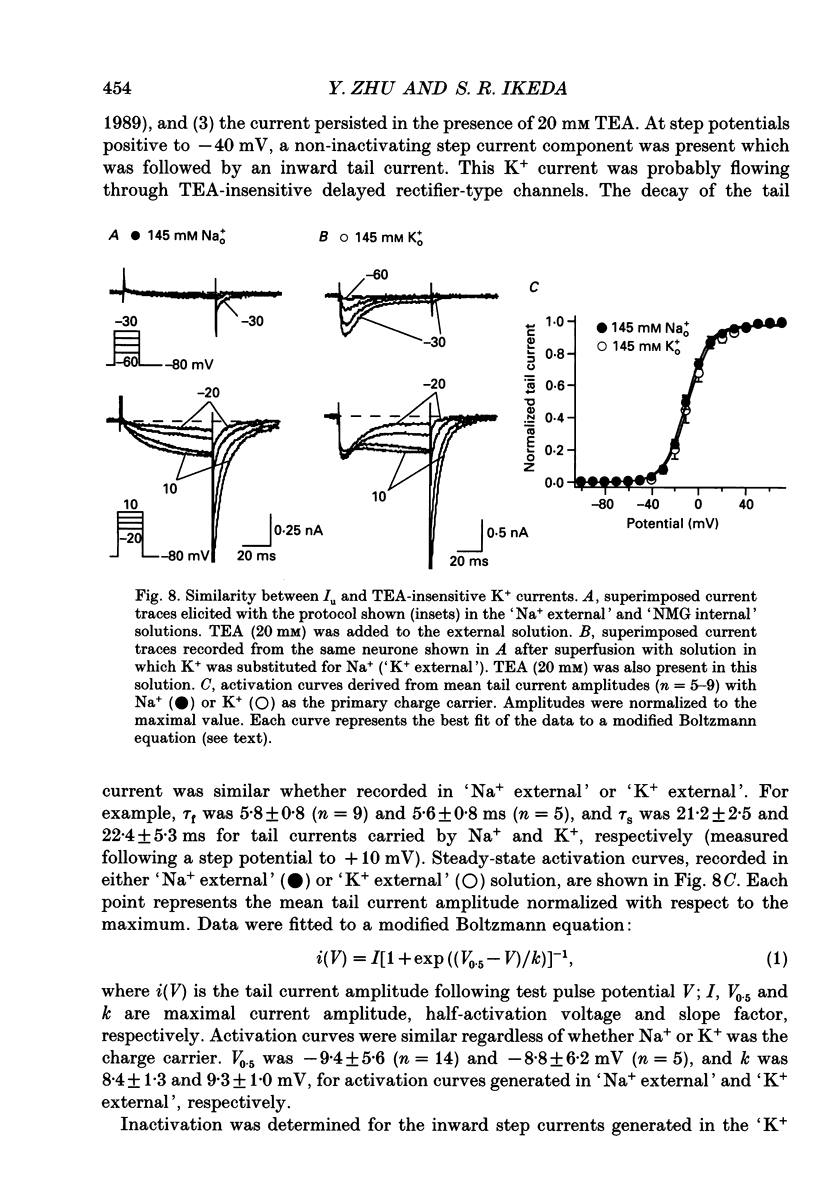
1. An unanticipated inward tail current was recorded from freshly isolated adult rat superior cervical ganglion (SCG) neurones using the whole-cell variant of the patch-clamp technique. The tail current was present when Na+ was substituted for tetraethylammonium (TEA) as the primary monovalent cation in external solutions designed to isolate Ca2+ channel currents (0.5 microM tetrodotoxin present and K+ omitted). 2. The tail current was observed following step potentials positive to -30 mV and reached half-activation near -9.0 mV. The decay of the tail current was voltage dependent and could be described with two time constants. Between potentials of -120 and -70 mV, tau f, the fast component, varied from 3 to 8 ms and tau s, the slow component, changed from 12 to 30 ms, respectively. 3. The tail current was not carried by Ca2+, and did not appear to flow through a voltage-gated Ca2+ channel or a Ca(2+)-dependent channel as it persisted in the absence of external Ca2+ or in the presence of the Ca2+ channel blocker, Cd2+ (0.1 mM). 4. Varying the external [Cl-] did not alter the reversal potential of the tail current indicating that Cl- was not the charge carrier. 5. The reversal potential of the tail current changed in accordance with the Nernst relationship when [Na+]i/[Na]o was altered. Our results suggested that this 'unusual or unanticipated current' (Iu) was carried primarily by Na+. 6. Iu was inhibited by the K+ channel-blocking agents quinidine (0.1 mM), external Ba2+ (5 mM) and internal Cs+ (145 mM). TEA (20 mM either internally or externally) and dendrotoxin (10 microM) were not effective inhibitors of Iu. 7. The decay time constants of the tail current and parameters of activation and inactivation of Iu were similar to those of TEA-insensitive delayed rectifier-type K+ channel currents observed in the presence of 145 mM external K+. 8. Iu was reduced in the presence of either external or internal K+. The interaction of external K+ with Na+ on the Iu tail amplitude was reminiscent of anomalous mole-fraction behaviour. 9. Ion permeability studies revealed that the channel producing Iu had a permeability sequence to monovalent cations of 3.5:2.5:2:1:0.5 for Rb+, K+, Cs+, Na+ and Li+, respectively. 10. These data suggest that in the absence of external K+, the ion selectivity of a TEA-insensitive K+ channel in sympathetic neurones is profoundly diminished. Under these conditions, Na+ traversing a K+ channel can generate an unanticipated inward current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
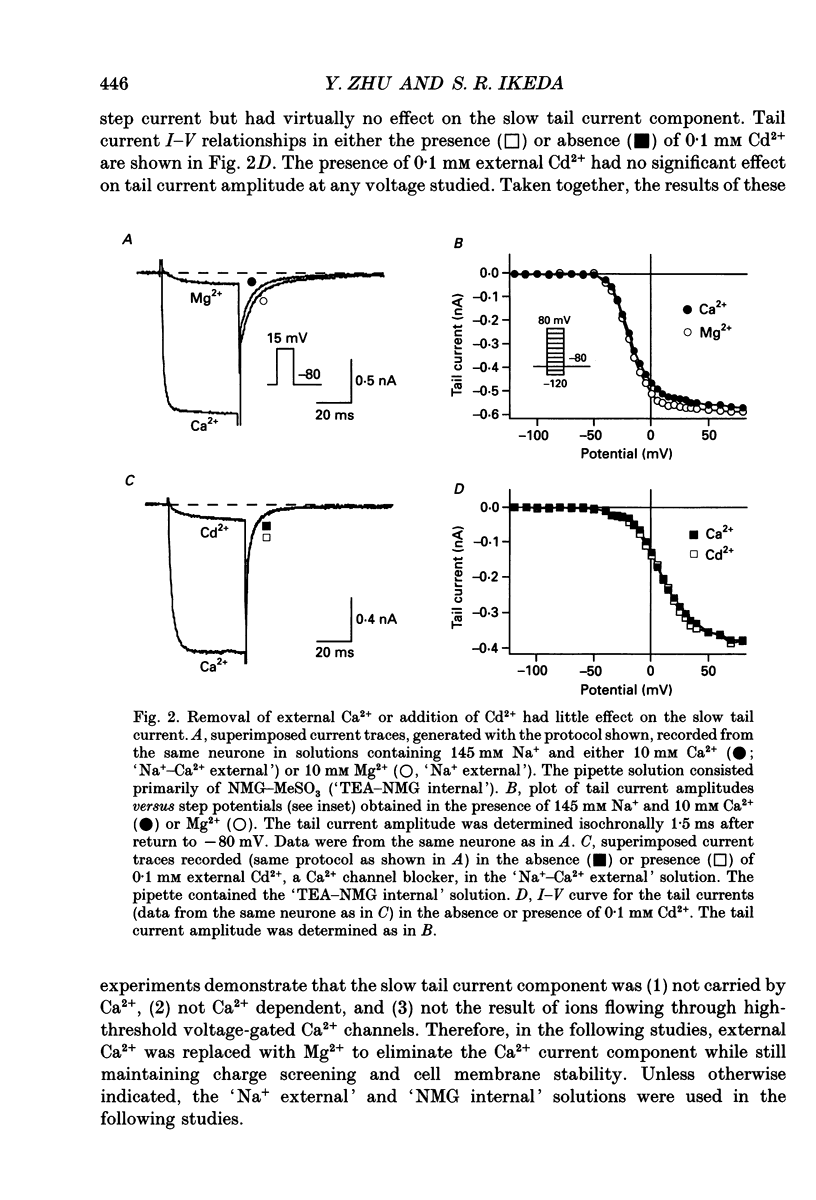
PDF



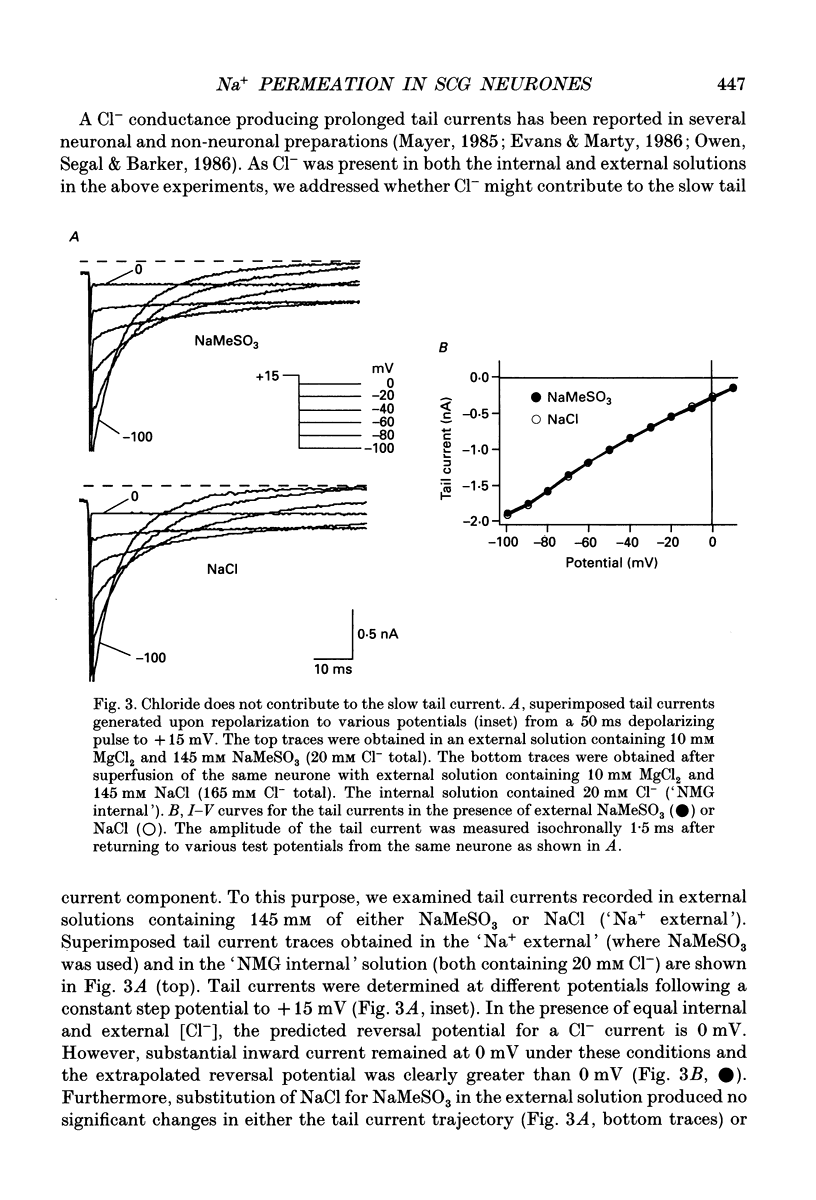







Selected References
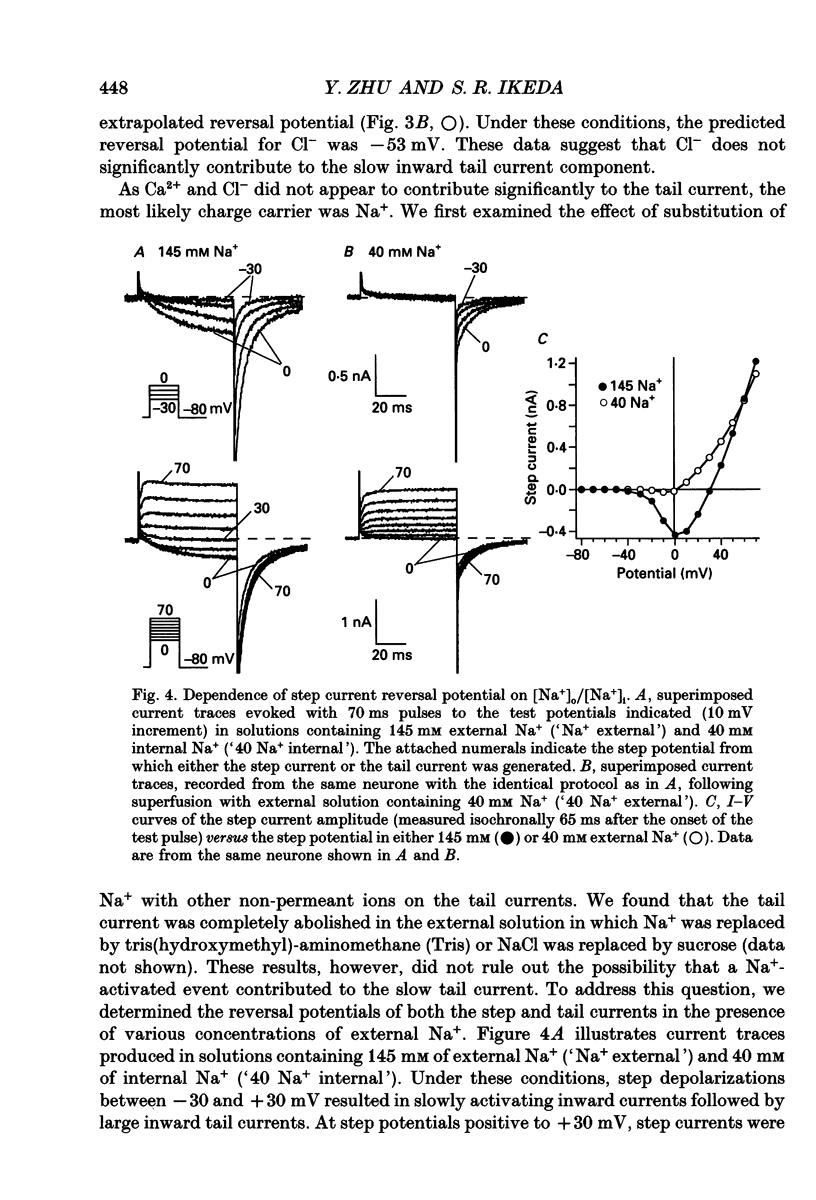
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Miller C. Do voltage-dependent K+ channels require Ca2+? A critical test employing a heterologous expression system. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7579–7582. doi: 10.1073/pnas.87.19.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
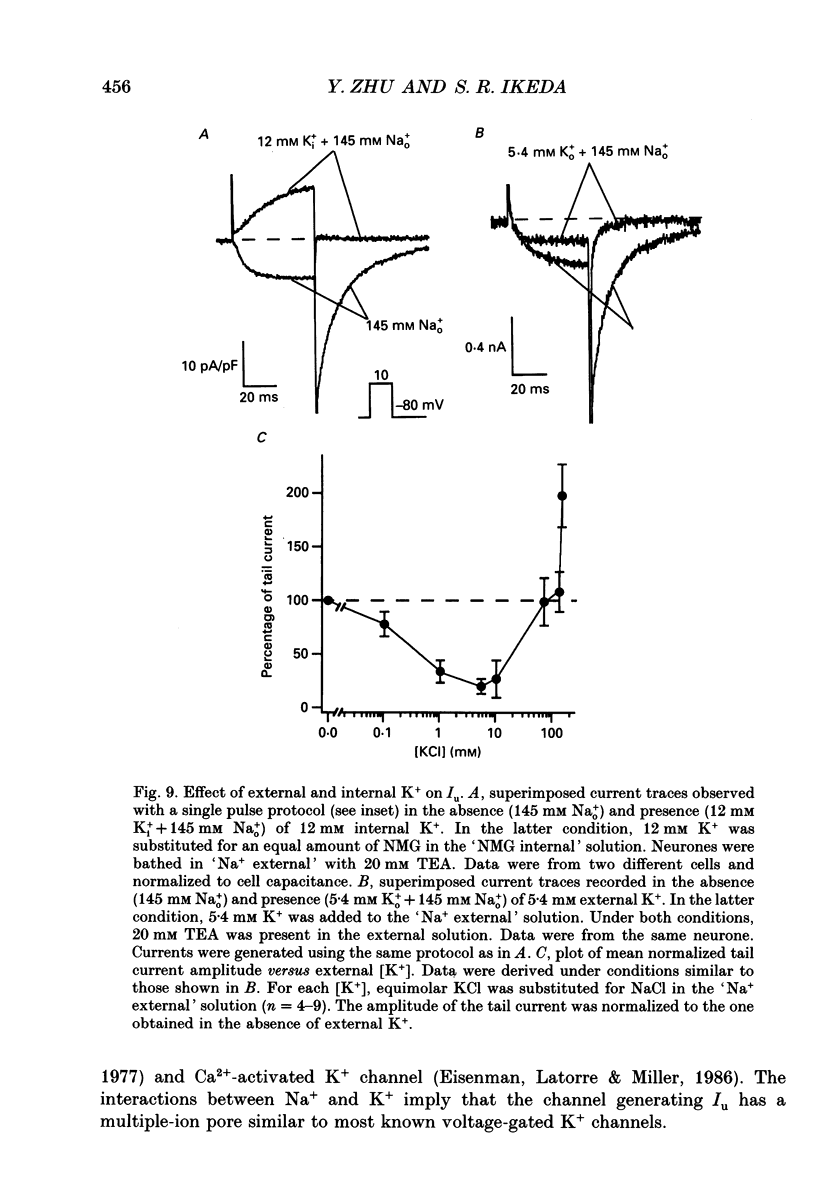
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
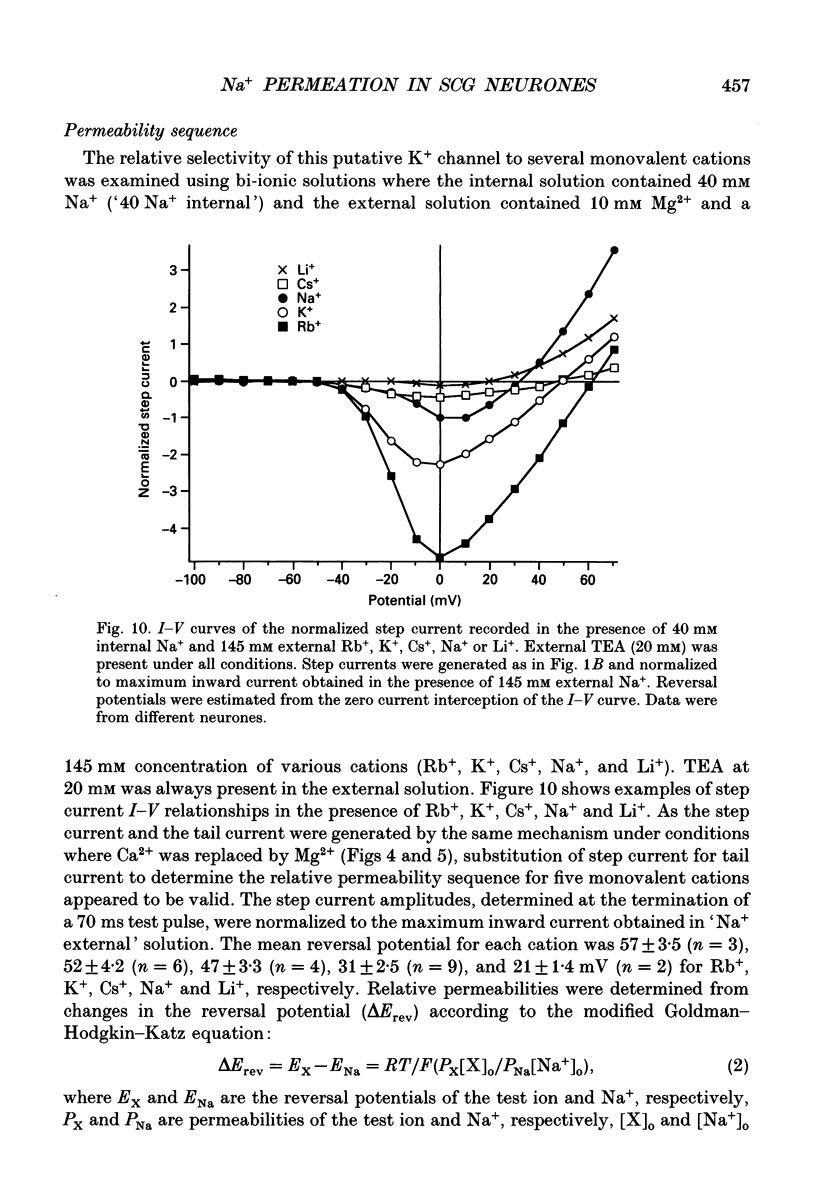
- Beech D. J., Bernheim L., Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992 Jan;8(1):97–106. doi: 10.1016/0896-6273(92)90111-p. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Dupont J. L., Feltz A. IA current compared to low threshold calcium current in cranial sensory neurons. Neurosci Lett. 1985 Dec 4;62(2):249–254. doi: 10.1016/0304-3940(85)90363-5. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P. Muscarinic receptor-mediated inhibition of voltage-activated Ca current in neuroblastoma x glioma hybrid (NG 108-15) cells--reduction of muscarinic agonist and antagonist potency by tetraethylammonium (TEA). Neurosci Lett. 1991 Jun 24;127(2):165–168. doi: 10.1016/0304-3940(91)90785-r. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Latorre R., Miller C. Multi-ion conduction and selectivity in the high-conductance Ca++-activated K+ channel from skeletal muscle. Biophys J. 1986 Dec;50(6):1025–1034. doi: 10.1016/S0006-3495(86)83546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Krasne S., Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+-Tl+ mixtures. J Gen Physiol. 1977 Sep;70(3):269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Action of quinidine on ionic currents of molluscan pacemaker neurons. J Gen Physiol. 1984 Jun;83(6):919–940. doi: 10.1085/jgp.83.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992 Dec;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Tetrodotoxin-resistant sodium current of rat nodose neurones: monovalent cation selectivity and divalent cation block. J Physiol. 1987 Aug;389:255–270. doi: 10.1113/jphysiol.1987.sp016656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Jacobs L. S. Dihydropyridine actions on calcium currents of frog sympathetic neurons. J Neurosci. 1990 Jul;10(7):2261–2267. doi: 10.1523/JNEUROSCI.10-07-02261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. Voltage-clamp analysis of a Ca2+- and voltage-dependent chloride conductance in cultured mouse spinal neurons. J Neurophysiol. 1986 Jun;55(6):1115–1135. doi: 10.1152/jn.1986.55.6.1115. [DOI] [PubMed] [Google Scholar]
- Pardo L. A., Heinemann S. H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Potassium currents of acutely isolated adult rat superior cervical ganglion neurons. Brain Res. 1989 Apr 24;485(2):205–214. doi: 10.1016/0006-8993(89)90563-5. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D., Armstrong C. M. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988 Aug;92(2):197–218. doi: 10.1085/jgp.92.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner P. K., Oxford G. S. Cation permeation through the voltage-dependent potassium channel in the squid axon. Characteristics and mechanisms. J Gen Physiol. 1987 Aug;90(2):261–290. doi: 10.1085/jgp.90.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]


