Abstract
Although vectors derived from adeno-associated virus type 2 (AAV2) promote gene transfer and expression in many somatic tissues, studies with animal models and cultured cells show that the apical surface of airway epithelia is resistant to transduction by AAV2 vectors. Approaches to increase transduction rates include increasing the amount of vector and perturbing the integrity of the epithelia. In this study, we explored the use of vectors based on AAV6 to increase transduction rates in airways. AAV vectors were made using combinations of rep, cap, and packaged genomes from AAV2 or AAV6. The packaged genomes encoded human placental alkaline phosphatase and contained terminal repeat sequences from AAV2 or AAV6. We found that transduction efficiency was primarily dependent on the source of Cap protein, defined here as the vector pseudotype. The AAV6 and AAV2 pseudotype vectors exhibited different tropisms in tissue-cultured cells, and cell transduction by AAV6 vectors was not inhibited by heparin, nor did they compete for entry in a transduction assay, indicating that AAV6 and AAV2 capsid bind different receptors. In vivo analysis of vectors showed that AAV2 pseudotype vectors gave high transduction rates in alveolar cells but much lower rates in the airway epithelium. In contrast, the AAV6 pseudotype vectors exhibited much more efficient transduction of epithelial cells in large and small airways, showing up to 80% transduction in some airways. These results, combined with our previous results showing lower immunogenicity of AAV6 than of AAV2 vectors, indicate that AAV6 vectors may provide significant advantages over AAV2 for gene therapy of lung diseases like cystic fibrosis.
Transfer of therapeutic genes to the lung may provide a cure for diseases such as cystic fibrosis (CF), which affects 1 in 3,000 Caucasian births. Inactivating mutations in the CF transmembrane regulator (CFTR), a chloride ion channel, result in gradual lung destruction, which is the major cause of morbidity. Although the normal CFTR protein has been localized to both the apical surface of the airway epithelium (38) and the submucosal glands beneath the epithelium (10), the airway is the site of microbial obstruction associated with mortality. This clinical manifestation provides the rationale for targeting the airway epithelium for CF gene therapy.
Among the many gene transfer systems being investigated are viral vectors such as those based on adeno-associated virus (AAV), a single-stranded DNA parvovirus. AAV can integrate and promote persistent gene expression in cultured cells and in dividing and nondividing cells in multiple somatic tissues of animals (19, 20, 22–24, 26, and 32). The ability to transduce nondividing cells is an important feature of AAV vectors for gene transfer to the airway epithelium, which has a low rate of proliferation (3, 21). Additionally, the fact that wild-type (wt) AAV has been isolated from human airways (4, 5, 27) is consistent with the idea that AAV has tropism for the lung epithelium. However, although AAV type 2 (AAV2) vectors can transduce multiple cell types in the lung, animal data thus far have shown low to modest rates of transduction by AAV2 vectors in the lung (11, 16, 17). Indeed, even introduction of a high dose of an AAV2 vector (1012 genome-containing particles) resulted in an overall transduction efficiency of 2% in the mouse airway epithelium (2).
Animal studies show that the efficiency of AAV transduction is affected by several factors and can be enhanced by various treatments. AAV transduction in the developing neonatal rabbit lung was more efficient than that in adult lung and was observed in a variety of airway and alveolar cell types (40). AAV vector-transduced cells in adult mouse lungs were rare, but their numbers could be increased by addition of adenovirus to provide helper functions (11), DNA-damaging reagents (1, 23, 30), or tissue injury (13, 16). Additionally, administration of much higher doses of AAV vector could also increase transduction in the lung epithelium (2). Tissue culture models have shown that proliferation rates (29) and polarity of epithelia influence the efficiency of AAV vectors (9). Indeed, reagents that help AAV vectors to bypass the natural resistance of the apical surface to infection can augment AAV transduction 10- to 100-fold (9, 35). These results show that dosage, proliferation rates, and target cell access are factors involved in efficient transduction by AAV vectors in lung epithelia.
Although AAV vector expression can persist for months to years, there may still be a need for readministration of vector to increase or replenish the population of modified cells. In readministration studies, few to no new transduction events have been detected in the rabbit or mouse lung or in skeletal muscle (12, 16, 17, 37), and these results were associated with the detection of neutralizing antibodies (16, 17). Several approaches have been used to achieve effective readministration. These include immune suppression in the lung (17) and muscle (25) and the use of other AAV types in the muscle and liver (36) and the lung (18). We showed that AAV vectors utilizing an AAV6 capsid (AAV6 pseudotype vector) have properties that could be useful for gene therapy, including low immunogenicity and the lack of cross-reactive antibodies generated against AAV2 (18).
Quantitation of the number of vector-expressing cells in our previous study indicated that the AAV6 pseudotype vector was as efficient as, if not more efficient than, AAV2 vectors in the mouse lung and that the transduction rates in various cell types were different between AAV2 and AAV6 (18). In this study, we evaluated the transduction of lung cells by vectors based on the AAV6 serotype more thoroughly to determine the combination of vector components that mediated the most efficient transduction of airway epithelia. To achieve this goal, we made separate expression plasmids for the three components of the AAV6 vector: rep, cap, and the packaged genome containing terminal repeats (TR) from AAV6 and expressing human placental alkaline phosphatase (AP). In conjunction with similar constructs for AAV2 (2), complementation of AAV2 and AAV6 components to generate infectious virions was assessed and then transduction was evaluated in a mouse model of lung gene transfer.
Our data show that AAV2 and AAV6 pseudotype vectors could be generated from all combinations of rep, cap, and genome from the two viruses and that transduction efficiencies of these vectors in tissue-cultured cells were primarily determined by the vector pseudotype, defined as the source of the capsid. While both AAV2 and AAV6 pseudotype vectors bind heparin, only AAV2 is inhibited by heparin in cell transduction assays, indicating that AAV2 and AAV6 interact with different receptors. In mouse lung delivery, the AAV2 vector gave high transduction rates in alveolar cells and much lower rates in airway epithelia, similar to the results obtained in previous studies. In contrast, an AAV6 pseudotype vector showed preferential transduction of epithelial cells in large and small airways at rates up to 80%. Transduction of the mouse airway epithelium by AAV6 pseudotype vectors was 15 to 74 times more efficient than transduction by an AAV2 vector. These results, combined with our previous results showing lower immunogenicity of AAV6 than of AAV2, indicate that AAV6 vectors may provide significant advantages for gene therapy for CF.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney 293 cells (14) and human HT-1080 fibrosarcoma cells (ATCC CCL 121) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin.
Human airway epithelial cells were isolated from nasal polyps of CF patients by digestion with dispase (4 μg/ml; Boehringer Mannheim) as described previously (15) and were maintained in keratinocyte growth medium (Clonetics) at 37°C in 5% CO2–air. Cells were frozen at passage 1 or 2. CF16 cells were derived from CF human airway cells by immortalization with the E6 and E7 genes of human papillomavirus type 16 (15).
Cells grown on porous membranes were prepared according to published procedures (39) but with minor modifications. Human airway cells (106 cells) at passage 1 or 2 after isolation from tissue were seeded on 30-mm collagen-coated, 0.4-μm-pore-size Millicell cellulose filters (Millipore Corp., Bedford, Mass.). Twenty-four hours after seeding, the medium on the surface of the cells (apical side) was removed and the cells were grown at the air-liquid interface by feeding from the basal side. The culture medium consisted of a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 medium, 2% Ultroser G (Biosepra SA, Cergy-Saint-Christophe, France), 100 U of penicillin-streptomycin per ml, and 0.12 U of insulin per ml. Airway epithelia were grown until they were confluent and developed a transepithelial electrical resistance of >1,000 Ω × cm2. Then, 700 μl of keratinocyte growth medium containing a fixed number of vector genome-containing particles was added to the apical side of the transmembrane. After a 4-h exposure, the transmembrane cultures were washed twice with phosphate-buffered saline (PBS) and cultured for an additional 5 days. Transmembrane cultures were fixed in 2% paraformaldehyde in PBS for 20 min and then rinsed three times in PBS and stained in X-Gal solution [25 mM K4Fe(CN)6 · 3H2O, 25 mM K3Fe(CN)6, 25 mM MgCl2, and 1 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Boehringer Mannheim)/ml] at 37°C overnight.
AAV vectors.
The AAV2-based vector ARAP4-2 (previously called ARAP4) contains the AP cDNA expressed from a Rous sarcoma virus promoter and enhancer sequences and the simian virus 40 polyadenylation sequences. The expression cassette was flanked by AAV2 TRs. The ARAP4-2, pCMVE4orfs, and AAV2 packaging plasmids MTrep2 and CMVcap2 have been previously described (2).
The AAV6-based vector ARAP4-6 contains the same expression cassette flanked by AAV6 TRs. It was constructed by isolating the expression cassette containing the Rous sarcoma virus promoter-enhancer, human placental AP cDNA, and the simian virus 40 polyadenylation sequences from pARAP4-2 (2) by digestion with SnaBI. The DNA fragment was ligated to pA6LAPSN (31) previously digested with BglII and NheI to remove a similar expression cassette but containing the Moloney murine leukemia virus promoter-enhancer sequences. CMVcap6 was constructed by isolating the AAV6 cap sequences from pRepCap6 (31) by digestion with Ecl136 and ligating AAV6 cap sequences to CMVcap (2) previously digested with NheI and BglII to remove AAV2 cap sequences. All DNA fragments were end filled prior to ligation. MTrep6 was constructed by PCR amplification of AAV6 rep sequences from pRepCap6 (31). The 5′ oligonucleotide contained a BglII linker and DNA sequences from positions 5101 to 5121 (5′-GAAGATCTGCCATGCCGGGGTTTTACGAG-3′). The 3′ oligonucleotide contained an NheI linker and DNA sequences from positions 3224 to 3244 of pRepCap6 (5′-CACTAGCTAGCCAGCCATACCTGGTTTAAGTC-3′). The PCR product was digested with BglII and NheI and ligated to pMTrep2 (2) previously digested with the same enzymes to remove AAV2 rep sequences.
CWCZn has previously been described (18). It contains an immediate-early promoter and enhancer from cytomegalovirus linked to a nucleus-localizing bacterial β-galactosidase (β-Gal) cDNA and the AAV2 polyadenylation sequences and flanked by AAV2 TRs. Recombinant AAV plasmids ARAP4-2, ARAP4-6, and CWCZn were propagated in the bacterial strain Sure (Stratagene), XL1-Blue (Stratagene), or JC8111 (6). The packaging and helper plasmids were propagated in the DH5α strain of Escherichia coli.
AAV vector production and characterization.
AAV2 and AAV6 pseudotype vectors were generated by cotransfection of vector plasmid (pARAP4-2, pARAP4-6, or pCWCZn), AAV packaging plasmids (pMTrep and pCMVcap from types 2 and/or 6), and pCMVE4orf6 into 293 cells (4 × 106 cells per 10-cm-diameter dish). Concentration and purification of AAV vectors were done as previously described (2). Briefly, clarified crude cell lysates obtained 3 days after transfection were concentrated by centrifugation through a sucrose cushion, followed by density banding in cesium chloride, and dialyzed in Ringer's saline solution (RSS). Titers of ARAP4 vector stocks were determined using HT-1080 cells as targets for transduction. Briefly, 10-fold serial dilutions were made from 10 μl of vector from gradient fractions and applied to cells plated the previous day at 5 × 104 cells per well in six-well dishes (Corning Inc., Corning, N.Y.). Cells were fixed and stained for AP expression 3 days later as previously described (16). Southern analysis was done to determine the number of genome-containing particles in the vector preparations, and the preparations were found to contain approximately 1011 to 1012 genome-containing particles per ml. The CWCZn and ARAP4 vector preparations did not have detectable replication-competent AAV (<50 IU/ml) as determined by replication center assay. Procedures for Southern analysis, transduction titer determination, and replication assay have previously been described (16).
Purification of AAV2 and AAV6 pseudotype vectors on heparin columns.
Clarified crude cell lysates were concentrated by centrifugation through a sucrose cushion. Vector-containing pellets were resuspended in RSS. Vector samples were applied to a heparin column (HiTrap heparin; 1 ml; Amersham catalogue no. 17-0406-01). Then, three 1-ml volumes each of RSS and RSS containing 200, 300, or 500 mM NaCl were applied sequentially, followed by 1-ml volumes of RSS containing 600, 700, or 800 mM NaCl. One-milliliter eluted fractions were collected from the column after each application of saline solution and dialyzed in RSS, and the vector titer in each fraction was determined using HT-1080 cells.
Heparin competition.
Genome-containing particles (108) of AAV2 (Rep2Cap2Genome2) or AAV6 (Rep2Cap6Genome2) vectors were incubated in 2 ml of medium supplemented with 20 μg of heparin (Sigma H3149) per ml or 20 μg of chondroitin sulfate A (Sigma C9819) per ml for 1 h at 37°C. After incubation, 800, 100, and 10 μl of vector (with or without heparin or chondroitin sulfate A) were added to CF16 and HT-1080 cells plated at 105 cells per well of a six-well dish. Competition medium (with or without heparin or chondroitin sulfate A) was added to each well to bring the total volume to 1 ml. The cells were incubated with virus mixtures for 1 h, after which the virus was removed and the cells were rinsed three times with PBS. Two milliliters of medium similar to the previous competition medium but not containing vector was added, and the cells were grown for 3 days for HT-1080 and 5 days for CF16, after which the cells were fixed and stained for AP expression as previously described (16).
Competiton assay for AAV2 and AAV6 vectors.
CF16 cells were plated at 105 cells/well in 12-well dishes 2 to 3 days prior to transduction. The cells were transduced with 5 × 107 genome-containing particles of AAV2-CWCZn (Rep2Cap2Genome2) or 1 × 107 particles of AAV6-CWCZn (Rep2Cap6Genome2) in 500 μl of medium containing increasing amounts of AAV2-ARAP4 (Rep2Cap2Genome2) and AAV6-ARAP4 (Rep2Cap6Genome2). After a 1-h incubation with vectors, the cells were washed three times with 1 ml of PBS and incubated in 1 ml of culture medium for 3 days. The cells were fixed for 7 min in 3.7% formaldehyde in PBS, followed by three rinses in PBS, and stained for β-Gal expression by an overnight incubation at 37°C in β-Gal reaction buffer [25 mM K4Fe(CN)6 · 3H2O, 25 mM K3Fe(CN)6, 25 mM MgCl2, and 1 mg of X-Gal (Boehringer Mannheim)/ml].
AAV vector delivery to mouse airways.
Animal studies were performed in accordance with the guidelines set forth by the Institutional Review Office of the Fred Hutchinson Cancer Research Center. C57BL/6 (B6) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Animals received vector by nasal aspiration according to a previously published protocol (13). Mice were given the AAV vector on day 1 and were euthanatized 4 weeks later, and their lungs were processed for AP staining as previously described (17).
Quantitation of transduction efficiency.
Stained tissue slices from the entire lung were cut into 3-mm blocks and paraffin embedded. Four sections were taken from each lung sample. Three of these were counterstained with nuclear fast red, and the fourth was counterstained with hematoxylin and eosin. Quantitation of transduction efficiency was done by counting the number of AP-positive cells per section on the slides stained with nuclear fast red. Positive alveolar cell counts were estimated from 10 random 1-mm2 areas. For all other cell types, positive counts were obtained by analysis of the entire tissue section. Two sections, approximately 5 μm thick, were scored per lung. The total area was quantitated by scanning stained slides (Adobe Photoshop) and found to be approximately 0.5 to 1 cm2 per slide. Values were expressed as AP-positive cells per square centimeter.
RESULTS
All combinations of Cap, Rep, and vector genomes derived from AAV2 and AAV6 generate infectious virions.
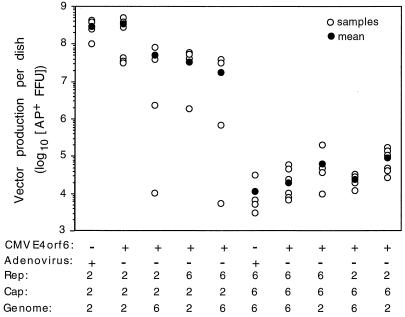
We evaluated the ability of different combinations of AAV2 and AAV6 Rep proteins, Cap proteins, and vector genomes to complement and produce infectious vectors. Vector production was assessed by measuring transduction of HT-1080 human fibroblasts. Cells exposed to vector preparations were stained for expression of vector-encoded AP (Fig. 1). Vector production from transfected 293 cells was significantly higher for vector stocks produced using the AAV2 capsid than for stocks produced using the AAV6 capsid (107 to 108 compared to 104 to 105 AP-positive focus-forming units [FFU] per 10-cm dish of transfected 293 cells). Vector production from separate MTrep and CMVcap constructs for the AAV2 and AAV6 vectors was similar whether the cells were infected with wt adenovirus or were cotransfected with CMVE4orf6 (Fig. 1, compare Rep2Cap2Genome2 and Rep6Cap6Genome6, with and without infectious adenovirus). All combinations of Rep proteins, Cap proteins, and vector genomes derived from AAV2 and AAV6 generated infectious virions.
FIG. 1.
Yields of vectors made with components from AAV2 and AAV6. The vector yield per 10-cm dish of 293 cells (4 × 106) transfected with plasmids encoding Rep and Cap proteins and vector genome and cotransfected with pCMVE4orf6 or infected with wt adenovirus is shown. The serotype from which the encoded Rep, Cap, and ITR are derived is indicated (2 for AAV2 or 6 for AAV6), and n = 4 to 6. Vector yield is shown as total AP-positive FFU per 10-cm dish. Vector yield was obtained by determining the titer of vector stocks (clarified crude cell lysates from 293 cells) on HT-1080 target cells according to a procedure outlined in Materials and Methods.
The yield of vector as measured by transduction of HT-1080 cells with clarified crude cell lysates from transfected 293 cells (AP-positive FFU per plate of 293 cells) showed a 100- to 1,000-fold difference between AAV2 and AAV6 pseudotype vectors. To determine if the lower yield of vector for the AAV6 pseudotype vector was due to differences in packaging functions, Southern analysis was done on vector preparations to quantitate the number of genome-containing particles produced. The particle-to-infectivity ratio for the AAV2 pseudotype vectors ranged from 74 to 125 particles per AP-positive FFU, whereas AAV6 pseudotype vectors ranged from 1 × 104 to 5 × 104 genome-containing particles per AP-positive FFU (Table 1). Approximately 100- to 700-fold-more genome-containing particles were required per transduction event for the AAV6 pseudotype vectors than for the AAV2 vectors. The increase in particle-to-infectivity ratio accounted for the majority of the drop in vector yields as measured by transduction (100- to 1,000-fold decrease).
TABLE 1.
Particle-to-infectivity ratios of vectors
| Vector | No. of vector genomes per AP-positive FFUa | n |
|---|---|---|
| Rep2Cap2Genome2 | 74 ± 37 | 7 |
| Rep2Cap2Genome6 | 125 | 1 |
| Rep6Cap2Genome2 | 75 | 1 |
| Rep6Cap2Genome6 | 80 | 1 |
| Rep6Cap6Genome6 | 3.4 × 104 | 2 |
| Rep6Cap6Genome2 | 5.3 × 104 | 2 |
| Rep2Cap6Genome6 | 1.2 × 104 | 1 |
| Rep2Cap6Genome2 | (2.7 ± 1.1) × 104 | 4 |
Southern analysis was performed on DNase-resistant genomes extracted from virions produced with the indicated components from AAV2 or AAV6 serotypes to determine the concentration of genome-containing particles. The titer was determined by infecting HT-1080 cells with dilutions of vector and counting AP-positive FFU. n denotes the number of vector samples. When n is 2, the mean value is shown. When n is >2, the mean and standard deviation are shown.
Rep and vector genome components also affected vector yields as measured by transduction (Fig. 1). Within the AAV6 pseudotype vectors, there was a trend for the Rep6Cap6 Genome6 vector to have lower vector yields than combinations having vector genome or Rep from AAV2. The combination with both Rep and vector genome from AAV2 had the highest vector yields of the AAV6 pseudotype vectors. Within the AAV2 pseudotype vectors, the Rep2Cap2Genome2 combination gave the best yield of vector. Replacement of either or both Rep2 and AAV2 vector genomes with their AAV6 counterparts resulted in a 5- to 10-fold drop in vector yield. The particle-to-infectivity ratios were not significantly different for all AAV2 pseudotype vectors (Table 1), suggesting that substituting AAV6 Rep or vector genome decreased replication, packaging, or virion stability.
Comparison of transduction in human fibroblasts and airway cells by AAV2 and AAV6 pseudotype vectors.
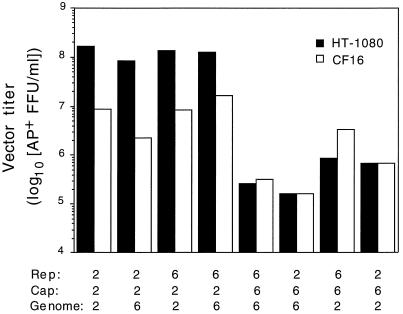
The particle-to-infectivity ratio was much higher for all AAV6 pseudotype vectors than for the analogous AAV2 vectors in human HT-1080 fibroblasts (Table 1), showing that AAV2 more efficiently transduced these cells. We extended this analysis to include CF16, a cell line derived from a nasal polyp of a CF individual (Fig. 2). CF16 is an immortalized but nontransformed cell line. Previously, we found that the titers of AAV2 vectors on primary airway cells derived from the nasal polyps of CF patients were similar to their titers on CF16 cells (data not shown). Therefore, CF16 was used as a tissue culture model of target cells for CF gene therapy. The high titers of AAV2 pseudotype vectors observed in HT-1080 cells dropped dramatically when tested on CF16 cells. In contrast, the low titers observed for the AAV6 pseudotype vectors in HT-1080 did not change significantly in CF16 cells. In fact, there was a tendency for titers to increase. The titers of the AAV2 and AAV6 pseudotype vectors in human airway cells and fibroblasts show that transduction efficiency segregated with the vector capsid and suggest that AAV2 and AAV6 may utilize different receptors for cell entry.
FIG. 2.
Titers of vector with mixed components from AAV2 and AAV6 were assayed using human fibroblasts (HT-1080) and airway epithelial cells (CF16). Vector preparations were adjusted to contain 1010 genome-containing particles per ml, and then the titer was determined by exposure of cells to limiting dilutions of vector. Values are averages of two experiments with duplicate determinations per dilution.
AAV6 capsid interacts with heparin, but transduction of cultured cells is not inhibited by soluble heparin sulfate.
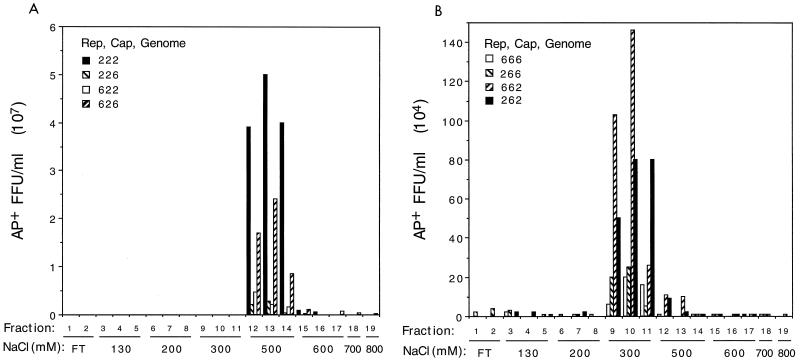
Membrane-associated heparan sulfate proteoglycan can serve as a primary attachment receptor for AAV2 (34), with the human fibroblast growth factor receptor 1 (28) and α5β5 integrin (33) acting as coreceptors. Consistent with this is the observation that heparin-based affinity chromatography works well for AAV2 vector purification (8, 41). We tested whether AAV6 capsid could also interact with heparin by determining if AAV6 pseudotype vectors could also bind to a heparin column. The binding of all AAV2 pseudotype vectors to the heparin column could be disrupted by 500 mM NaCl (Fig. 3A). The elution profile showed that more than 90% of recovered vectors eluted from the heparin column at this salt molarity, with vector yields ranging from 50 to greater than 90% (n = 11). The AAV6 pseudotype vectors could also bind to heparin, but the vectors eluted from the column using 300 mM NaCl (Fig. 3B). Again, greater than 90% of recovered vector eluted at this salt molarity, and the final yield ranged from 50 to 90% (n = 6). These results show that the AAV6 capsid interacted with heparin but that the affinity of the interaction was weaker than that of the AAV2 capsid with heparin.
FIG. 3.
Binding of AAV2 and AAV6 pseudotype vectors to heparin columns. The vector titers in fractions eluted after binding of vectors to heparin columns are shown. Vectors are indicated with the serotypes of vector components listed in the order Rep, Cap, and vector genome. The fraction number is indicated with the corresponding salt concentration. The figure shows elution profiles for AAV2 (A) and AAV6 (B) pseudotype vectors. FT, flowthrough fractions having a salt concentration of 130 mM.
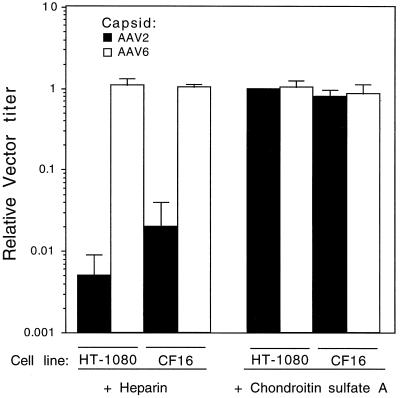
The ability to purify AAV6 vectors by heparin column binding suggested that AAV6, like AAV2, may use membrane-bound heparan sulfate proteoglycan as a receptor for cell attachment and entry. To test this, a competition experiment using heparin, a soluble receptor analog, was done. AAV6 and AAV2 pseudotype vectors that differed only in the capsid serotype were preincubated with soluble heparin before addition to target cells. Chondroitin sulfate A was also used as a control for the specificity of the inhibition by heparin. Soluble heparin inhibited transduction of HT-1080 or CF16 cells by an AAV2 capsid-containing vector, whereas it did not inhibit transduction of these cells by an analogous vector packaged in the AAV6 capsid (Fig. 4). Chrondroitin sulfate A did not inhibit transduction by either vector pseudotype (Fig. 4).
FIG. 4.
Effect of heparin and chondroitin sulfate A on transduction of human cells by AAV2 and AAV6 pseudotype vectors. Vectors (Rep2Cap2Genome2 and Rep2Cap6Genome2) were incubated with heparin or chondroitin sulfate A prior to placement on cells as described in Materials and Methods. Relative transduction efficiency was obtained by dividing the number of AP-positive FFU in samples incubated with heparin or chondroitin sulfate A by the number in samples incubated without heparin or chondroitin sulfate A. Means and standard deviations obtained from three experiments are shown.
AAV6 and AAV6 use different cellular receptors.
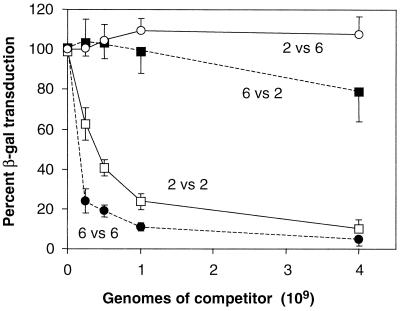
The resistance of AAV6 pseudotype vectors to competition by soluble heparin suggested that AAV6 capsid recognized a receptor distinct from that used by AAV2. This hypothesis was tested by competition experiments with AAV6 and AAV2 vectors (Fig. 5). CF16 cells were transduced with a constant amount of AAV6 vector expressing β-Gal in the presence of increasing amounts of either an AAV2 vector or an AAV6 vector expressing AP. At a 100-fold excess of competitor over target, a 90% inhibition of AAV6 vector transduction was observed with the AAV6 competitor, but no significant inhibition was observed with the AAV2 competitor. Similarly, when cells were exposed to a constant amount of an AAV2 vector in the presence of increasing amounts of AAV2 or AAV6 vectors, significant inhibition of AAV2 vector transduction was observed only with the AAV2 vector competitor. This result and the result of the heparin competition experiment are both consistent with the utilization of distinct cellular receptors by AAV2 and AAV6.
FIG. 5.
Transduction by β-Gal-expressing AAV2 or AAV6 pseudotype vectors in the presence of competing AP-expressing AAV2 or AAV6 pseudotype vectors. CF16 cells were transduced with a constant amount of AAV2-CWCZn or AAV6-CWCZn vectors in the presence of increasing amounts of competing AAV2 or AAV6 encapsidated AP-expressing vector. The number of β-Gal-positive cells detected in the cotransduced wells was divided by the number of positive cells in the control wells (β-Gal vector only) and expressed as a percentage of the control (percent β-Gal transduction). The percentage is plotted versus the number of genome-containing particles of the competing AP-expressing vector. The lines are labeled with the target β-Gal vector pseudotype versus the competitor AP vector pseudotype (for example, 2 versus 2 means AAV2 β-Gal vector versus an AAV2 AP vector). The means and standard deviations of three experiments are given for the AAV6 β-Gal transduction experiments, and the means and standard deviations of two experiments done with quadruplicate wells are given for the AAV2 β-Gal transduction experiments.
AAV6 vectors transduce airway epithelia more efficiently than do AAV2 vectors.
We next tested both vectors on polarized airway cells because it is known that, while human airway cells in monolayer cultures are relatively permissive for AAV2 infection, cells in a polarized epithelium are resistant to infection, particularly from the apical side. We compared the transduction rates of AAV2 (Rep2Cap2Genome2) and AAV6 (Rep2Cap6Genome2) pseudotype vectors that expressed β-Gal in transmembrane cultures of primary human airway cells. The epithelia were exposed to the vectors from the apical side for 4 h at low particle numbers (50 to 500 per cell). AAV6 was more efficient than was AAV2 in transmembrane cultures of human airway epithelial cells, giving β-Gal focus numbers that were 37- to >200-fold higher (Table 2).
TABLE 2.
Transduction of polarized human airway epithelia by AAV2 and AAV6 vectorsa
| Vector pseudotype | Age of epithelia (days) | No. of vector particles/cell | β-Gal FFU |
|---|---|---|---|
| AAV2 | 4 | 50 | 0 |
| 4 | 500 | 17 | |
| 11 | 50 | 0, 0 | |
| 11 | 500 | 3, 3 | |
| AAV6 | 4 | 50 | 212 |
| 4 | 500 | 640 | |
| 11 | 50 | 108, 388 | |
| 11 | 500 | 485, 420 |
AAV2 and AAV6 pseudotype vectors, Rep2Cap2Genome2 and Rep2Cap6Genome2, encoding nucleus-localizing β-Gal were used to infect airway epithelia (106 cells seeded per transmembrane culture) by apical exposure for 4 h. Epithelia were fixed and stained for β-Gal expression 5 days later. Primary bronchial airway cells used to generate epithelia were derived from two CF individuals.
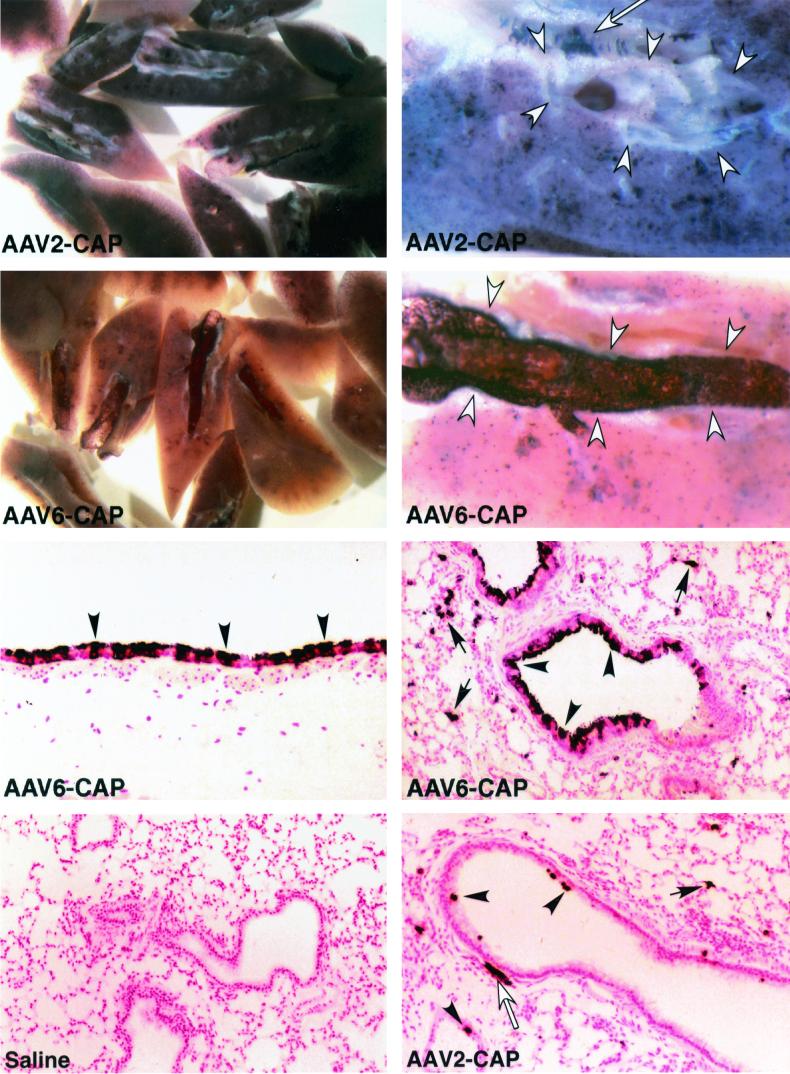
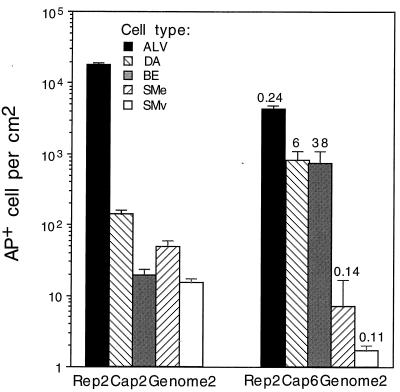
A mouse model of lung gene transfer was used to compare the performances of an AAV2 vector and several AAV6 pseudotype vectors in vivo. AAV2 and AAV6 pseudotype vectors were delivered to mouse airways by nasal aspiration, and the mice's lungs were examined for AP marker gene expression 1 month later. In one experiment, an AAV2 vector (Rep2Cap2Genome2) was compared to an AAV6 pseudotype vector (Rep2Cap6Genome2). Both were given at 7 × 1011 genome-containing particles per mouse. Mouse lungs treated with the AAV2 vector exhibited abundant AP staining in the lung parenchyma and much lower staining in the airway epithelium (Fig. 6, top panels). Robust AP expression in smooth muscle cells underlying the epithelium or in vasculature was also observed (Fig. 6, top right panel). Mouse lungs treated with the AAV6 pseudotype vector exhibited a different pattern of staining. There were abundant AP-positive-staining cells in the airway and much less staining in the lung parenchyma (Fig. 6, second row) than that seen with the AAV2 vector. Histologic analysis of mouse lungs showed that the cells in airways exhibiting abundant staining were cells of the airway epithelium and that transduction efficiency in some airways was high, up to 80% of the cells in the airway (Fig. 6, third row). AAV2 vector-treated lungs exhibited few AP-positive airway epithelial cells (Fig. 6, fourth row, right panel), while saline-treated lungs did not exhibit any AP-positive cells (Fig. 6, fourth row, left panel). Quantitation of the number of AP-positive cells shows that the AAV2 vector transduced alveolar cells at 100- to 1,000-fold-higher rates than it transduced cells of the distal airway and bronchial epithelium, respectively (Fig. 7). Transduction of smooth muscle cells was also lower than transduction of alveolar cells. The Rep2Cap6Genome2 pseudotype showed a decrease in transduction of alveolar cells to 24% of the value obtained for the AAV2 vector. In contrast, transduction of epithelial cells in distal airways and larger bronchial airways increased 6- and 38-fold, respectively. Interestingly, transduction of smooth muscle cells decreased to approximately 11 and 14% of that observed for the AAV2 vector.
FIG. 6.
Histochemical detection of AP expression in mouse lungs 1 month after vector exposure. The left panels of the top two rows display mouse lungs cut along the main bronchial airway in each lobe, and all the lung portions from one mouse are shown in each panel (magnification, ×8). The right panels of the top two rows show bisected lung portions with the main bronchial airway outlined by open arrowheads (magnification, ×32). The bottom four panels show histologic sections of AP-stained mouse lungs. Mice were given saline or an AAV2 or an AAV6 pseudotype vector expression AP as indicated by saline, AAV2-CAP, or AAV6-CAP (7 × 1011 genome-containing particles each). Saline-treated lungs did not exhibit AP-positive cells, while AAV vector-treated lungs exhibited AP-positive alveolar cells (arrows), airway epithelial cells (arrowheads), and smooth muscle cells (open arrows). Magnifications of photographs of histologic sections are ×400 for the left panel second from the bottom and ×200 for all other panels showing histologic sections.
FIG. 7.
Quantitation of transduction rates in mouse lungs 1 month after vector exposure to 7 × 1011 genome-containing particles. Vector pseudotypes are indicated at the bottom; ratios of the transduction values for the AAV6 pseudotype divided by the values for the AAV2 vector are shown above the histogram bars for each cell type. Abbreviations: ALV, alveolar cells; DA, distal airway epithelial cells; BE, bronchial airway epithelial cells (having underlying cartilage); SMe, smooth muscle cells underlying epithelium; SMv, smooth muscle in vascular walls. Means and standard errors of the means are given. n = 5 for Rep2Cap2Genome2, and n = 3 for Rep2Cap6Genome2.
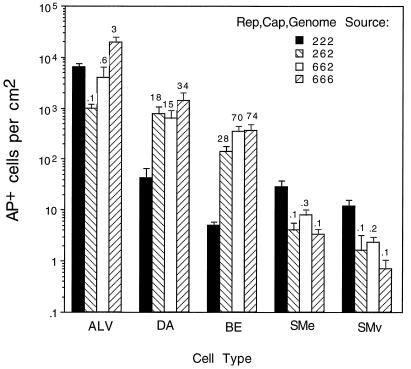
In a parallel experiment, we tested the effects of various components of AAV6 in addition to the AAV6 capsid proteins on transduction in mouse lung. Vector doses of 1011 genome-containing particles of the AAV2 vector (Rep2Cap2Genome2) and three AAV6 pseudotype vectors (Rep2Cap6Genome2, Rep6Cap6Genome2, and Rep6Cap6Genome6) were given. Quantitation of AP-positive cells in the lung showed that all AAV6 pseudotype vectors exhibited higher transduction rates in distal airway epithelium (15- to 34-fold increase) and bronchial airway epithelium (28- to 74-fold increase) (Fig. 8). In contrast, transduction of smooth muscle cells decreased significantly (3- to 10-fold decrease). The transduction rates in alveolar cells varied with the vector components. In comparison to the AAV2 vector, Rep2Cap6Genome2 was 10-fold lower in the number of AP-positive alveolar cells, Rep6Cap6Genome2 was approximately 2-fold lower, and Rep6Cap6Genome6 vector was actually 3-fold higher. The experiments with mouse lungs show that AAV6 pseudotype vectors were more efficient at mediating transduction of airway epithelial cells.
FIG. 8.
Quantitation of transduction rates in mouse lungs 1 month after vector exposure (1011 genome-containing particles). Vector components are indicated at the right. The ratios of the transduction values for the AAV6 pseudotype divided by the values for the AAV2 vector are shown above the histogram bars for each cell type. Cell designations are the same as for Fig. 7. Means and standard errors of the means are given. n = 5 for the Rep2Cap2Genome2 group, and n = 3 for all other groups.
DISCUSSION
In this study, we evaluated vectors based on AAV6 for lung gene therapy. AAV2 and AAV6 pseudotype vectors were generated from combinations of vector components from both viruses. Vectors were characterized for particle yields, transduction efficiencies, and biological properties in tissue culture and then tested in a mouse model of lung gene transfer to determine which vector mediated the most efficient transduction of airway epithelia. We found that AAV6 vectors have properties distinct from those of AAV2 vectors and that they transduce airway epithelia more efficiently than do AAV2 vectors.
The AAV6 vectors used in our previous study (18) were produced by using plasmids encoding both Rep and Cap linked to their AAV promoters and polyadenylation elements; consequently, the expression of Rep and Cap proteins was regulated in a manner analogous to that for the wt virus. Production of high-titer vectors occurred when wt adenovirus was used to provide helper functions but not when only one adenovirus gene, E4orf6, was substituted (data not shown). In this study, placement of AAV6 replication and packaging functions on separate plasmids and under heterologous promoters enabled the production of vectors using the adenovirus E4orf6 gene, minimizing contaminating amounts of adenovirus proteins that could be highly immunogenic. Indeed, AAV6 vector production using MTrep6 and CMVcap6 constructs gave slightly higher yields when CMVE4orf6 was cotransfected than when wt adenovirus was used. Additionally, placement of the Rep and Cap functions on separate plasmids also decreases the probability of generating replication-competent recombinant AAV, and none was detected in the vector preparations (<50 IU/1012 genome-containing particles of vector). The data show that the technology used for production of high titers of AAV2 with minimal adenovirus genes and free of replication-competent AAV (2) can be utilized for AAV6.
The effects of Rep on vector behavior could be separated from those of Cap when they exist on separate expression cassettes. There are many similarities between AAV2 and AAV6 Rep proteins and between their binding sites in the inverted TR (ITR) sequences. Rep78 of AAV6 has the exact same ATP binding motif and conserved cysteine and histidine residues of the zinc finger binding motif as does Rep78 of AAV2 (31). However, there are still 71 different amino acids out of 623. Rep binding sites (RBS) in the 5′ ITR of AAV6 are identical to those in AAV2, while there are two changes in each of the two RBS found in the AAV6 3′ TR. Thus, while there are similarities in Rep proteins and RBS between AAV2 and AAV6, there are also some differences that could result in functional changes. Exchange of Rep6 for Rep2 (Rep6Cap2Genome2 versus Rep2Cap2Genome2) lowered the overall production but did not affect the transduction efficiency (particle-to-infectivity ratio). Likewise, exchange of AAV6 ITR for AAV2 ITR (Rep2Cap2Genome6 versus Rep2Cap2Genome2) affected production yields but not transduction efficiency. One possible explanation for this is that the interactions of heterologous Rep and ITR components were not as efficient as the homologous interaction in terms of replication and packaging of AAV2 pseudotype vectors. However, this phenomenon did not occur in the AAV6 group because vector production by components derived completely from AAV6 had the lowest vector yields. We concluded from these results that production yields for vectors with either pseudotype were higher when AAV2 Rep and AAV2 vector genomes were used, indicating an inherently higher efficiency in replication and/or packaging for this combination than for the AAV6 counterparts.
We found that of the three vector components, Rep, Cap, and ITR in the vector genomes, the capsid serotype was the major determinant of transduction efficiency. The differences in infectivity for different cells by AAV2 and AAV6 pseudotype vectors suggested that AAV6 utilized a different cellular receptor from the one proposed for AAV2. Results from competition studies indicate that heparan sulfate is the major receptor for AAV2 (34) with fibroblast growth factor receptor 1 (28) and an integrin acting as a coreceptor (33). Therefore, we tested whether AAV6 could interact with heparin by determining its ability to bind to a heparin column. We found that all AAV6 pseudotype vectors could bind to a heparin column but were eluted at a lower salt concentration than that for AAV2. This result showed that the AAV6 capsid interacted in some manner with heparin sulfate. However, AAV6 transduction of cells by AAV6 was not inhibited by soluble heparin, suggesting that the weak affinity of AAV6 for heparin did not prevent its binding to a functional receptor. Although AAV2 and AAV6 are 83 to 88% identical in their VP1 proteins, allowing for some similar properties, they also contain five highly variable regions that presumably are on the outer surface of the capsid, allowing for differences in receptor usage. Indeed, competition assays showed that AAV2 and AAV6 vectors did not interfere in cell transduction. The results indicate that the AAV6 capsid can utilize a receptor different from heparin sulfate proteoglycans on the cell surface for internalization.
Recently, an AAV vector based on serotype 5 was shown to transduce the apical surface of cultured airway epithelia more efficiently than did AAV2 (39). The sequence of AAV5 indicates that it has many areas of divergence from AAV2 and AAV6, particularly in the capsid variable regions (7). Heparin also did not inhibit binding to cells by AAV5, although it is not known whether AAV5 might interact weakly with heparin. Additionally, it is not known whether AAV5 exhibited lower immunogenicity than did AAV2 in eliciting neutralizing antibodies, as has been shown for AAV6. Certainly, there was a lack of cross-complementation between the AAV2 and AAV5 Rep proteins and ITRs (7), which was not observed between AAV2 and AAV6. In a mouse model of lung transduction, AAV5 performed fivefold better than did AAV2 in airway epithelia. Thus, AAV5 is an example of another serotype exhibiting better airway tropism than that of AAV2.
We show here that, at a vector dose of 1011 genome-containing particles, AAV6 could infect distal airways 15- to 34-fold better than AAV2 and large airways 28- to 74-fold better. The results obtained in vivo were in contrast to the tissue culture data where the infectious units determined in monolayer cultures of cells were higher for AAV2 than for AAV6 vectors and underestimated the performance of AAV6 vectors in the mouse lung. Indeed, transduction efficiency achieved rates as high as 80% in some airways exposed to AAV6 pseudotype vectors. Additionally, complementation of Rep proteins and ITRs between AAV2 and AAV6 enabled us to determine the combination of vector components exhibiting more targeted transduction of airway epithelia. We found that AAV2 vectors transduce alveolar cells 100-fold more frequently than airway epithelial cells, similar to previous results. The present data show that not only did the Rep2Cap6 Genome2 combination have significantly higher transduction in airway epithelia but it also had a significantly lower rate of transduction in alveolar cells than did AAV2 vectors. Our results show that AAV6 vector pseudotypes provide significant advantages over AAV2 vectors for lung gene therapy and support the exploration of other AAV vector pseudotypes to determine the optimal vector pseudotype to target specific tissues.
ACKNOWLEDGMENTS
We thank J. M. Alfano, S. R. Moe, and A. J. Ebbert for excellent technical assistance.
This work was supported by grants DK47754 and HL66947 from the National Institutes of Health and grants from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- 2.Allen J M, Halbert C L, Miller A D. Improved adeno-associated virus vector production with transfection of a single helper adenovirus gene, E4orf6. Mol Ther. 2000;1:88–95. doi: 10.1006/mthe.1999.0010. [DOI] [PubMed] [Google Scholar]
- 3.Ayers M M, Jeffery P K. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- 4.Blacklow N R, Hoggan M D, Kapikian A Z, Austin J B, Rowe W P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:363–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 5.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 6.Boissy R, Astell C R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5′-terminal palindrome of minute virus of mice. Gene. 1985;35:179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- 7.Chiorini J A, Kim F, Yang L, Kotin R M. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark K R, Liu X, McGrath J P, Johnson P R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 9.Duan D, Yue Y, Yan Z, Engelhardt J F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Investig. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt J F, Yankaskas J R, Ernst S A, Yang Y, Marino C R, Boucher R C, Cohn J A, Wilson J M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 11.Fisher K J, Gao G, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K J, Jooss K, Alson J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 13.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P Z, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham F L, Smiley J. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 15.Halbert C L, Alexander I E, Wolgamot G M, Miller A D. Adeno-associated virus vectors transduce primary cells much less efficiently than immortalized cells. J Virol. 1995;69:1473–1479. doi: 10.1128/jvi.69.3.1473-1479.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbert C L, Rutledge E A, Allen J M, Russell D W, Miller A D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzog R W, Yang E Y, Couto L B, Hagstrom J N, Elwell D, Fields P A, Burton M, Bellinger D A, Read M S, Brinkhous K M, Podsakoff G M, Nichols T C, Kurtzman G J, High K A. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 20.Herzog R W, Hagstrom J N, Kung S, Tai S J, Wilson J M, Fisher K J, High K A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauffman S L. Cell proliferation in the mammalian lung. Int Rev Exp Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- 22.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of AAV vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koeberl D D, Bonham L, Halbert C L, Allen J M, Birkebak T, Miller A D. Persistent, therapeutically relevant levels of human granulocyte colony-stimulating factor in mice after systemic delivery of adeno-associated virus vectors. Hum Gene Ther. 1999;10:2133–2140. doi: 10.1089/10430349950017121. [DOI] [PubMed] [Google Scholar]
- 25.Manning W C, Zhou S, Bland M P, Escobedo J A, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 26.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 27.Parks W P, Boucher D W, Melnick J L, Taber L H, Yow M D. Seroepidemiological and ecological studies of the adenovirus-associated satellite viruses. Infect Immun. 1970;2:716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 29.Russell D W, Miller A D, Alexander I E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell D W, Alexander I E, Miller A D. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 33.Summerford C, Bartlett J S, Samulski R J. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 34.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters R W, Dongsheng D, Engelhardt J F, Welsh M J. Incorporation of adeno-associated virus in calcium phosphate coprecipitate improves gene transfer to airway epithelia in vitro and in vivo. J Virol. 2000;74:535–540. doi: 10.1128/jvi.74.1.535-540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao W, Chirmule N, Berta S C, McCullough B, Gao G, Wilson J M. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yankaskas R J, Suchindran H, Sarkodi B, Nettesheim P, Randell S. Cystic fibrosis transmembrane conductance regulator (CFTR) protein is selectively expressed in ciliated airway epithelial cells. Am Rev Respir Dis. 1993;147:A26. [Google Scholar]
- 39.Zabner J, Seiler M, Walters R, Kotin R M, Fulgeras W, Davidson B L, Chiorini J A. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeitlin P L, Chu S, Conrad C, McVeigh U, Ferguson K, Flotte T R, Guggino W B. Alveolar stem cell transduction by an adeno-associated viral vector. Gene Ther. 1995;2:623–631. [PubMed] [Google Scholar]
- 41.Zolotukhin S, Byrne B J, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski R J, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]