Abstract
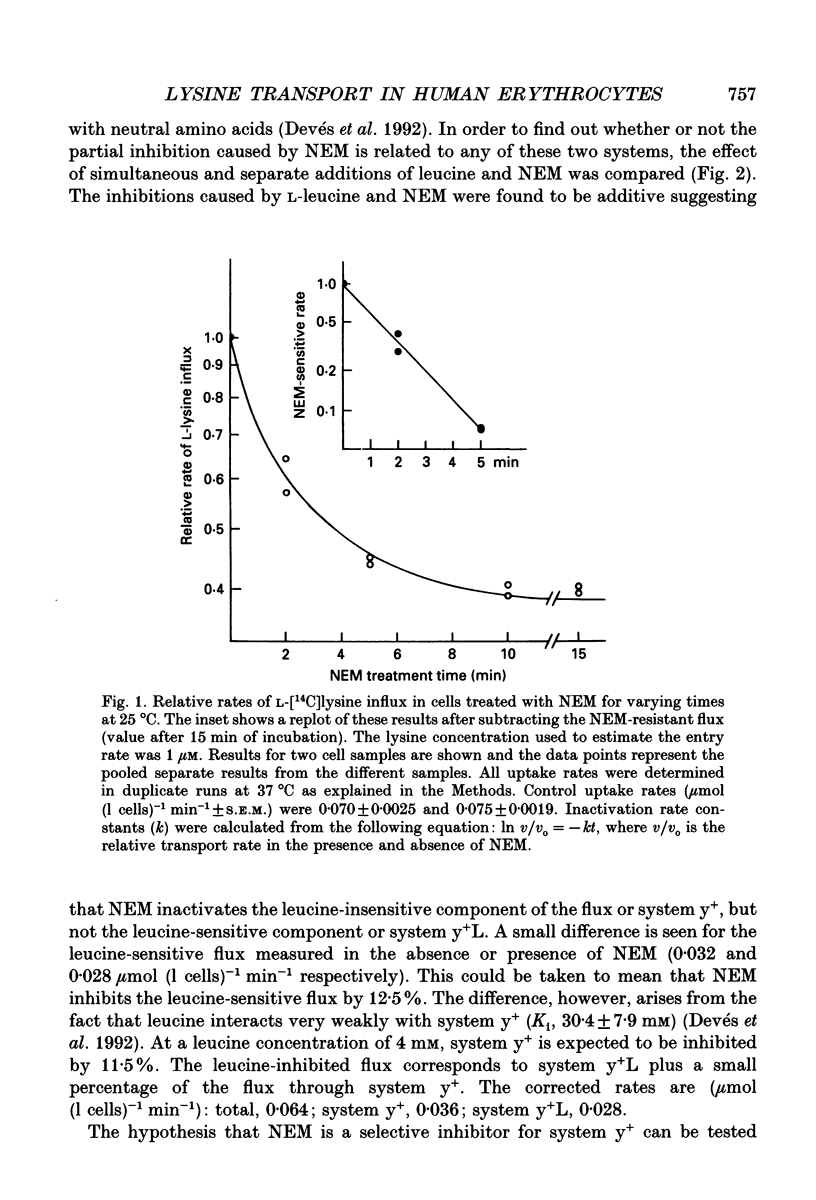
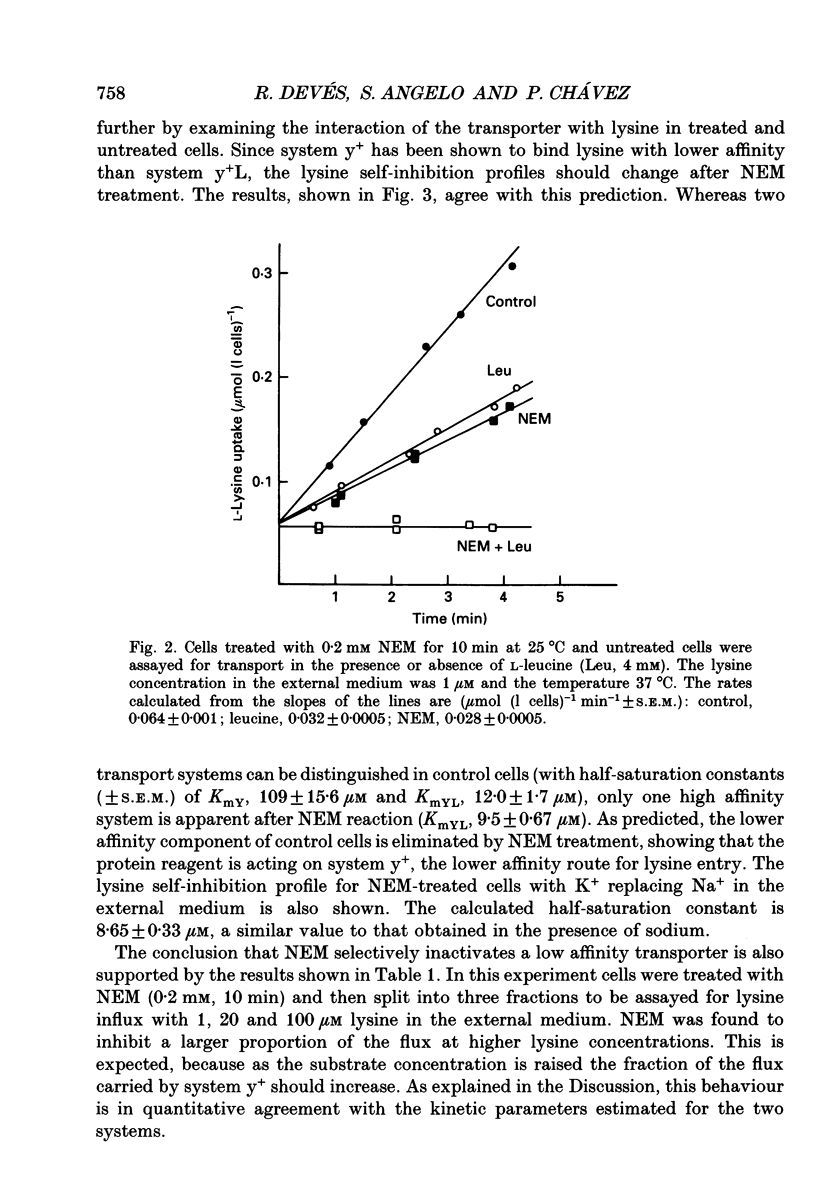
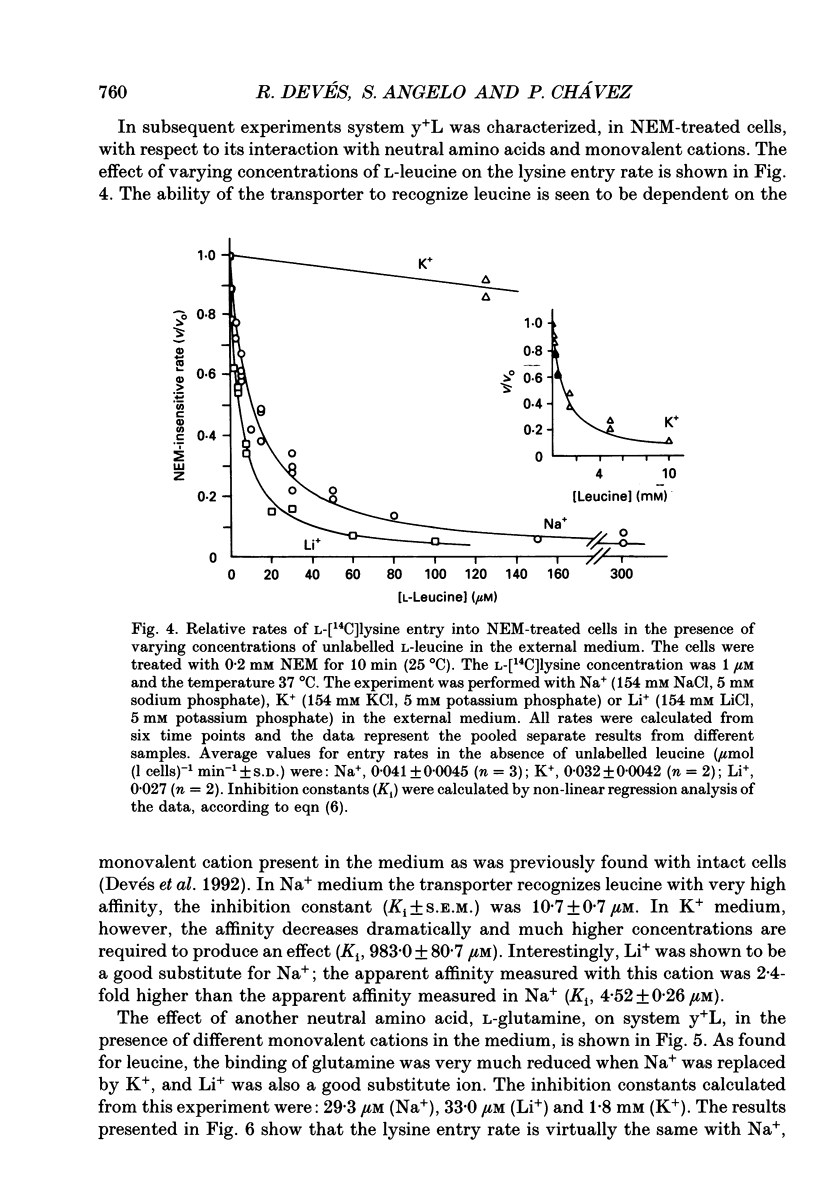
1. The sulfhydryl reagent N-ethylmaleimide (NEM) was shown to inactivate the low affinity lysine transporter in human erythrocytes (system y+) without affecting the high affinity transporter (system y+L). 2. Pre-treatment of the cells with NEM reduced the rate of entry of L-[14C]lysine (1 microM) by approximately 50% (maximum effect). 3. NEM (0.2 mM) inhibited the NEM-sensitive component of the flux with mono-exponential kinetics. The inactivation rate constant (k, +/- S.E.M.) was 0.53 +/- 0.027 min-1 (25 degrees C). The substrate did not protect against inactivation. 4. Lysine self-inhibition experiments revealed two transport systems in untreated cells (half-saturation constants Km; +/- S.E.M.), 12.0 +/- 1.7 microM and 109 +/- 15.6 microM) and only one high affinity system in NEM-treated cells (Km 9.5 +/- 0.67 microM), indicating that NEM inactivates system y+. 5. The NEM-insensitive L-[14C]lysine influx (system y+L) was inhibited with high affinity by unlabelled neutral amino acids. The inhibition constant for L-leucine in sodium medium (Ki +/- S.E.M.) was 10.7 +/- 0.72 microM (37 degrees C). The system was also strongly inhibited by L-methionine, L-glutamine and with less affinity by L-phenylalanine and L-serine. N-methyl-L-leucine, L-proline and 2-amino-2-norbornane-carboxylic acid, a bicyclic analogue of leucine, did not exert a significant effect. 6. Lysine transport through system y+L occurred at the same rate in Na+, K+ or Li+ medium and the binding of lysine to the transporter was unaffected by Na+ replacement. 7. The interaction of system y+L with neutral amino acids was dependent on the cation present in the medium. The inhibition constant for leucine and glutamine increased approximately 90- and 60-fold respectively when Na+ was replaced by K+. Li+ was shown to be a very good substitute for Na+.
Full text
PDF



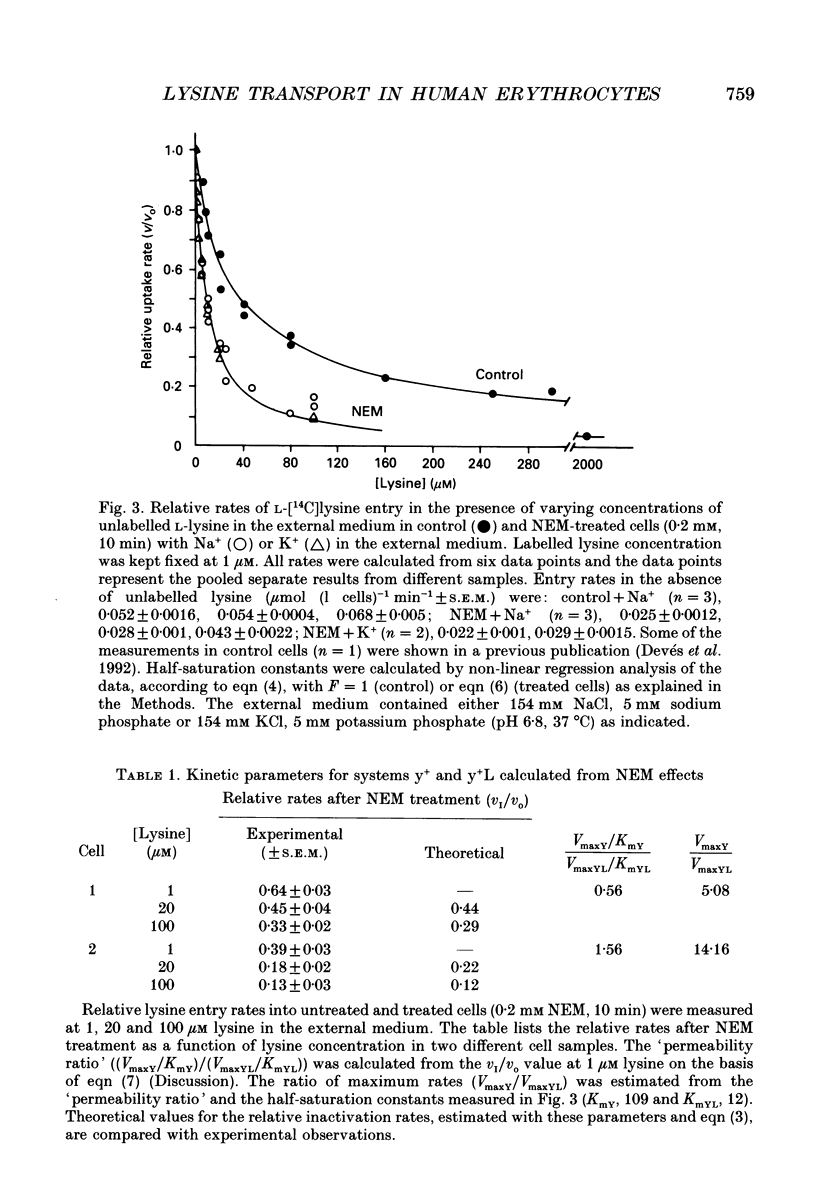
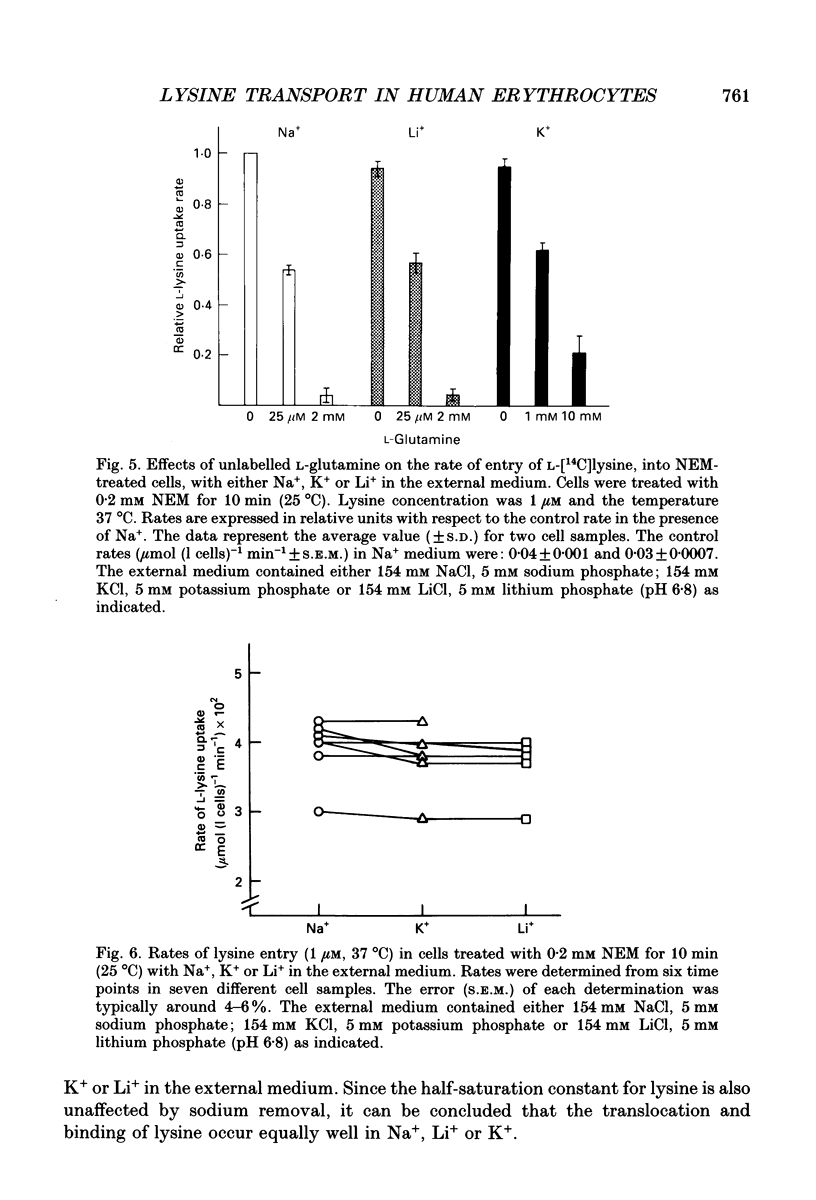
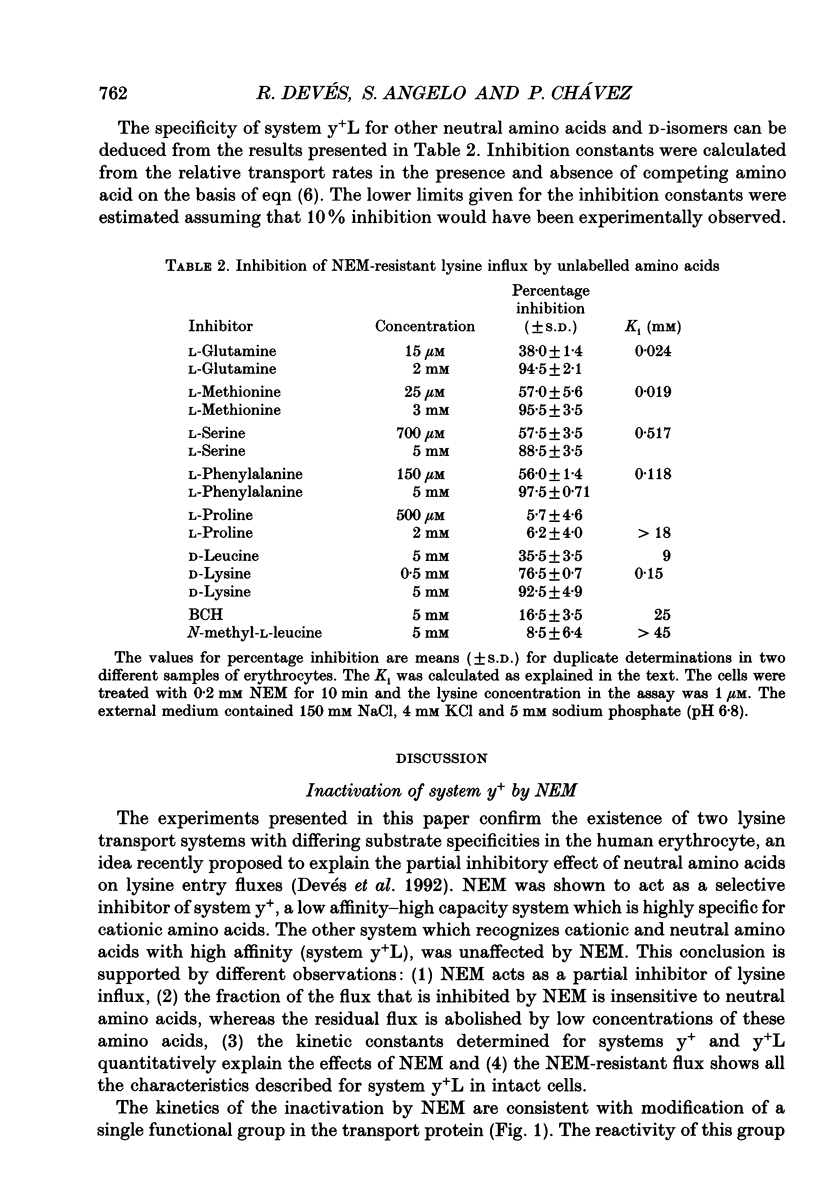




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Saleh E. A., Wheeler K. P. Transport of neutral amino acids by human erythrocytes. Biochim Biophys Acta. 1982 Jan 22;684(2):157–171. doi: 10.1016/0005-2736(82)90001-3. [DOI] [PubMed] [Google Scholar]
- Bertran J., Werner A., Moore M. L., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5601–5605. doi: 10.1073/pnas.89.12.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N., Antonioli J. A. Cationic amino acid transport in the rabbit reticulocyte. Na+-dependent inhibition of Na+-independent transport. J Biol Chem. 1969 Mar 25;244(6):1497–1504. [PubMed] [Google Scholar]
- Devés R., Chavez P., Boyd C. A. Identification of a new transport system (y+L) in human erythrocytes that recognizes lysine and leucine with high affinity. J Physiol. 1992 Aug;454:491–501. doi: 10.1113/jphysiol.1992.sp019275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Evidence for a two-state mobile carrier mechanism in erythrocyte choline transport: effects of substrate analogs on inactivation of the carrier by N-ethylmaleimide. J Membr Biol. 1981;61(1):21–30. doi: 10.1007/BF01870749. [DOI] [PubMed] [Google Scholar]
- Furesz T. C., Moe A. J., Smith C. H. Two cationic amino acid transport systems in human placental basal plasma membranes. Am J Physiol. 1991 Aug;261(2 Pt 1):C246–C252. doi: 10.1152/ajpcell.1991.261.2.C246. [DOI] [PubMed] [Google Scholar]
- Harvey C. M., Ellory J. C. Identification of amino acid transporters in the red blood cell. Methods Enzymol. 1989;173:122–160. doi: 10.1016/s0076-6879(89)73010-x. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Krupka R. M., Devés R. Looking for probes of gated channels: studies of the inhibition of glucose and choline transport in erythrocytes. Biochem Cell Biol. 1986 Nov;64(11):1099–1107. doi: 10.1139/o86-145. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Shao T. C., Christensen H. N. Structural selectivity in interaction of neutral amino acids and alkali metal ions with a cationic amino acid transport system. J Biol Chem. 1971 Mar 25;246(6):1677–1681. [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem. 1988 Mar 5;263(7):3150–3163. [PubMed] [Google Scholar]
- Van Winkle L. J., Christensen H. N., Campione A. L. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem. 1985 Oct 5;260(22):12118–12123. [PubMed] [Google Scholar]
- Wells R. G., Hediger M. A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5596–5600. doi: 10.1073/pnas.89.12.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Gazzola G. C., Christensen H. N. Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J Biol Chem. 1982 Apr 25;257(8):4443–4449. [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jones S. E., Ellory J. C. Amino acid transport in human and in sheep erythrocytes. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):355–375. doi: 10.1098/rspb.1980.0100. [DOI] [PubMed] [Google Scholar]
- Young J. D., Mason D. K., Fincham D. A. Topographical similarities between harmaline inhibition sites on Na+-dependent amino acid transport system ASC in human erythrocytes and Na+-independent system asc in horse erythrocytes. J Biol Chem. 1988 Jan 5;263(1):140–143. [PubMed] [Google Scholar]


