Abstract
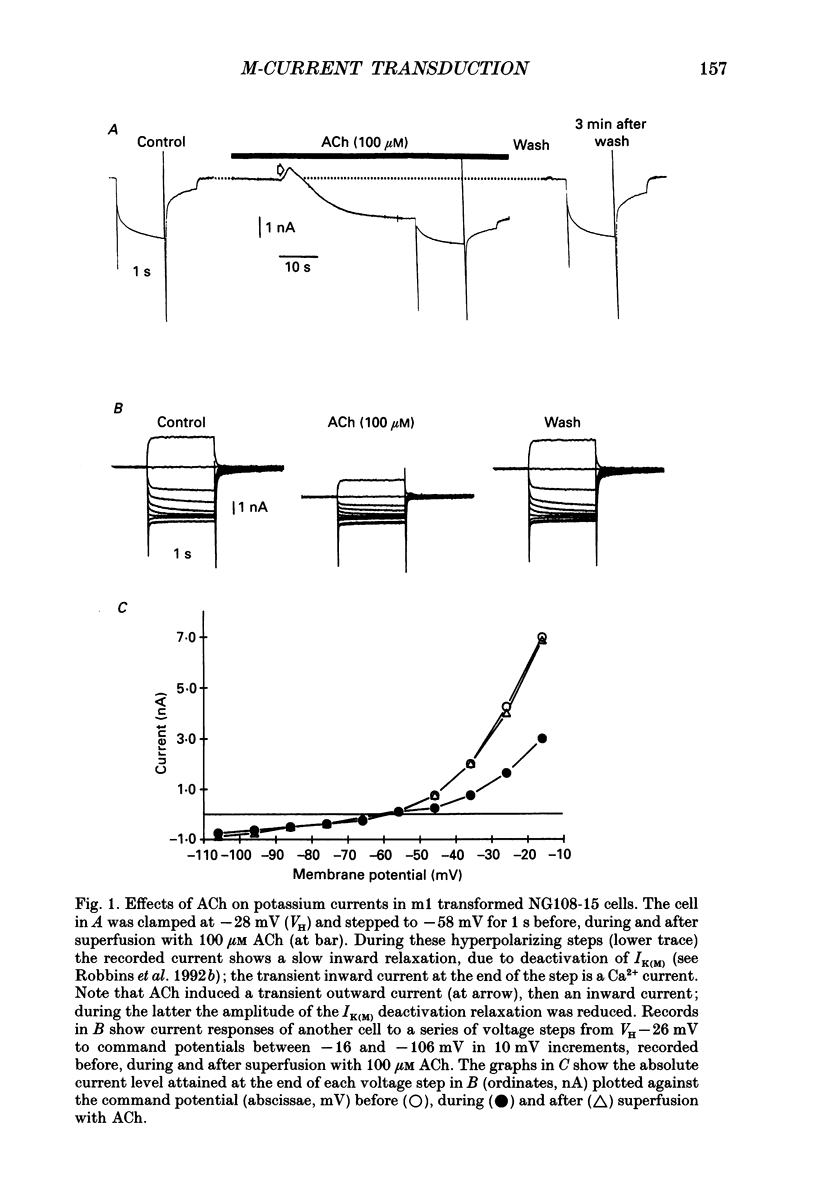
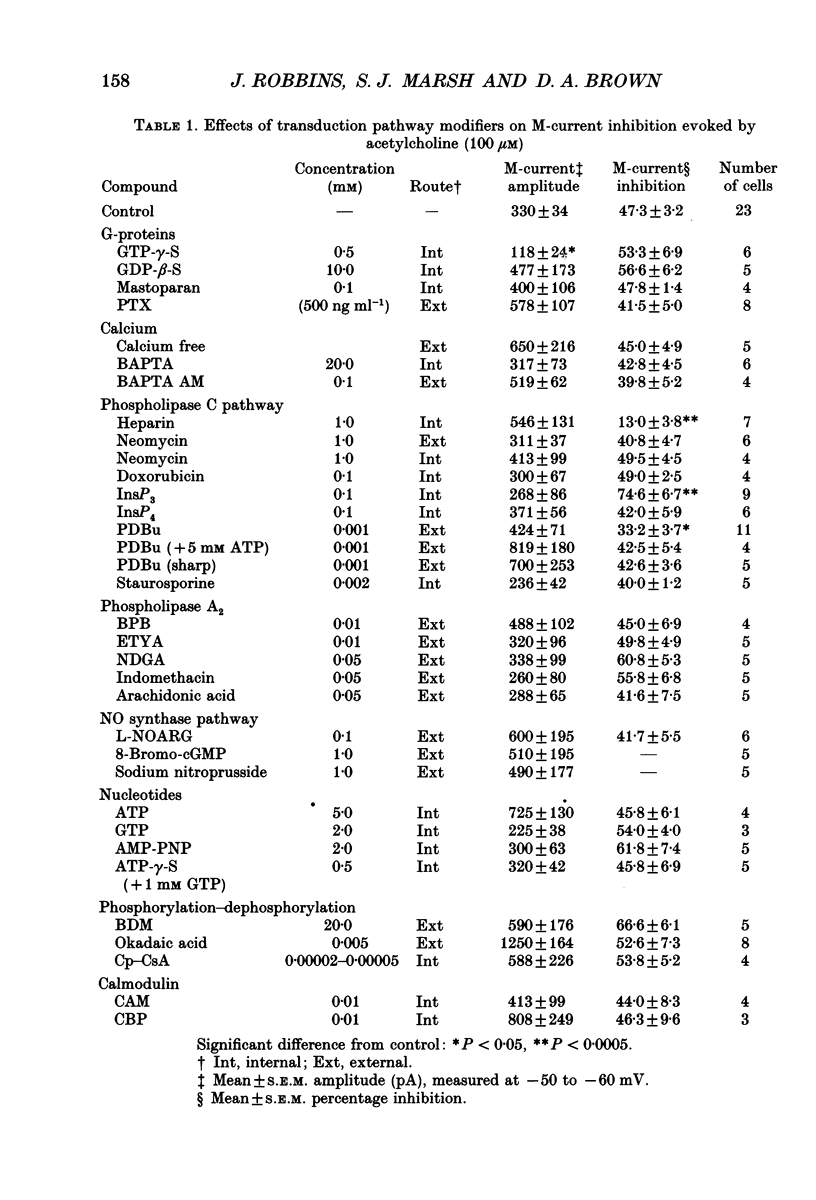
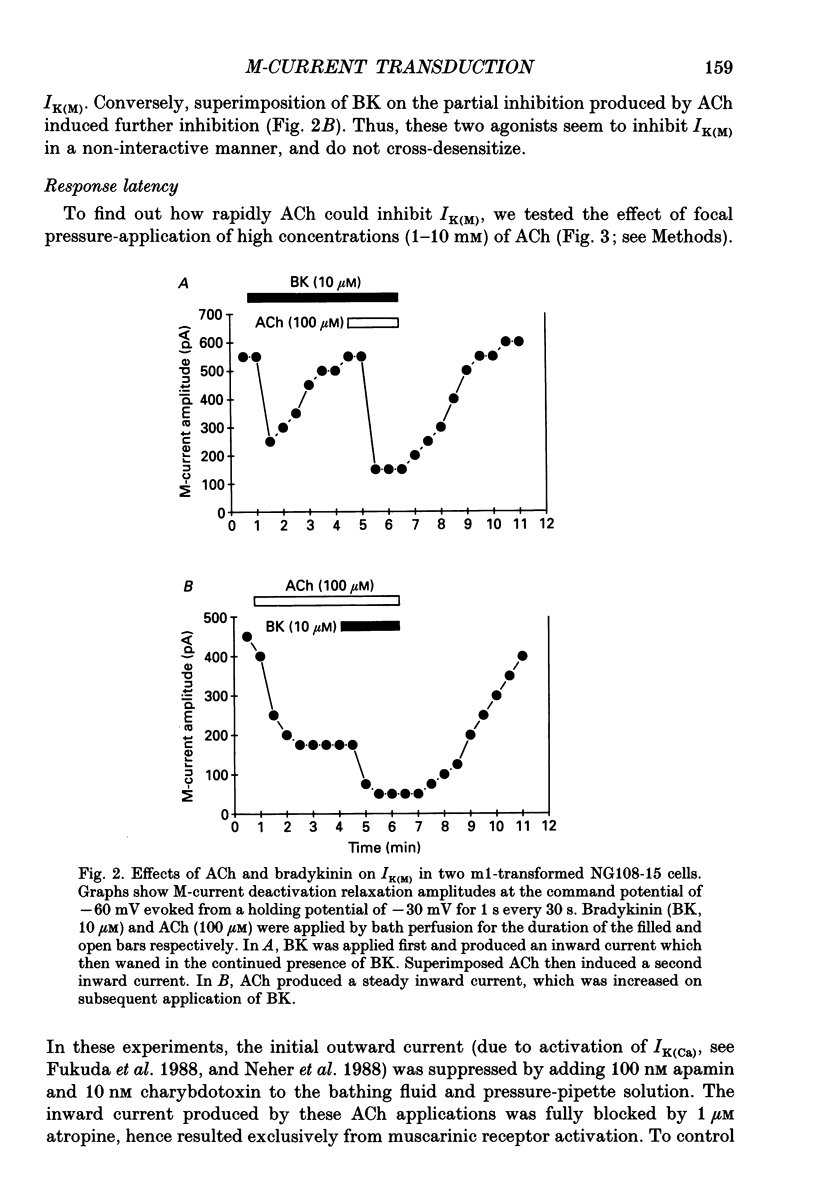
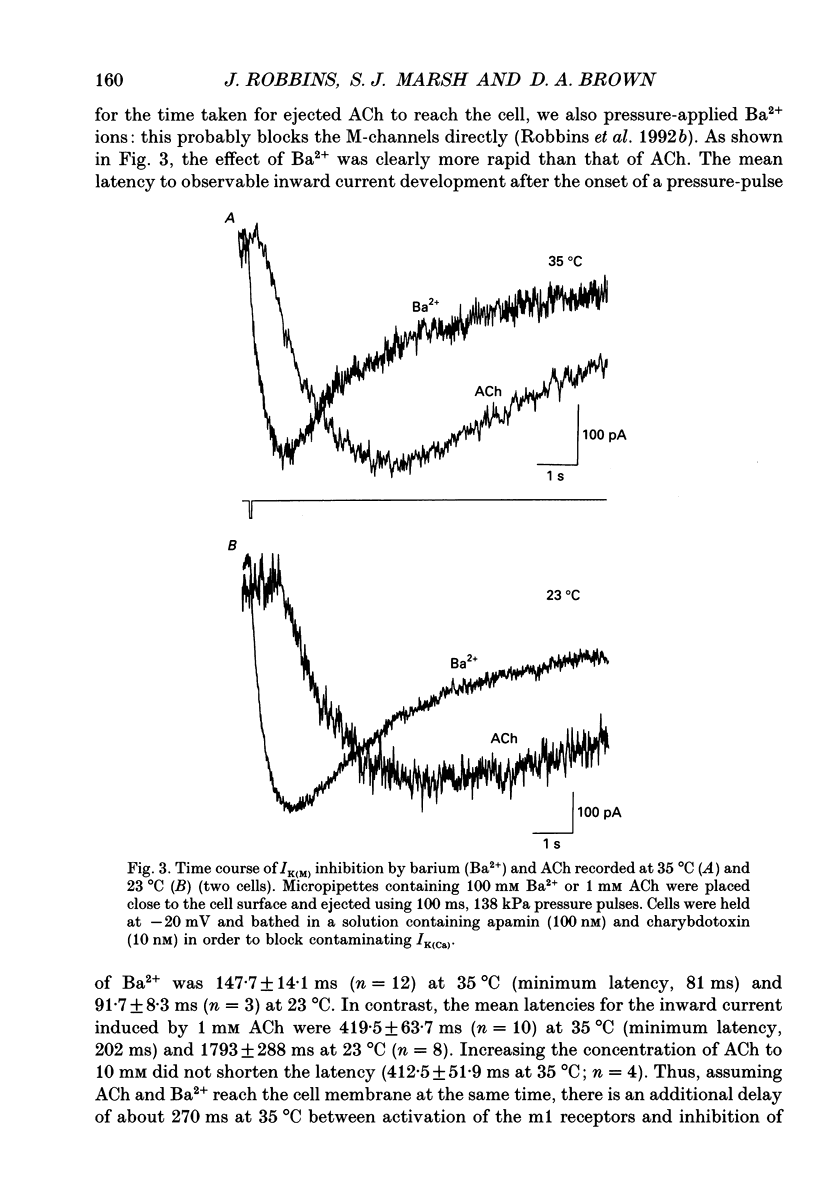
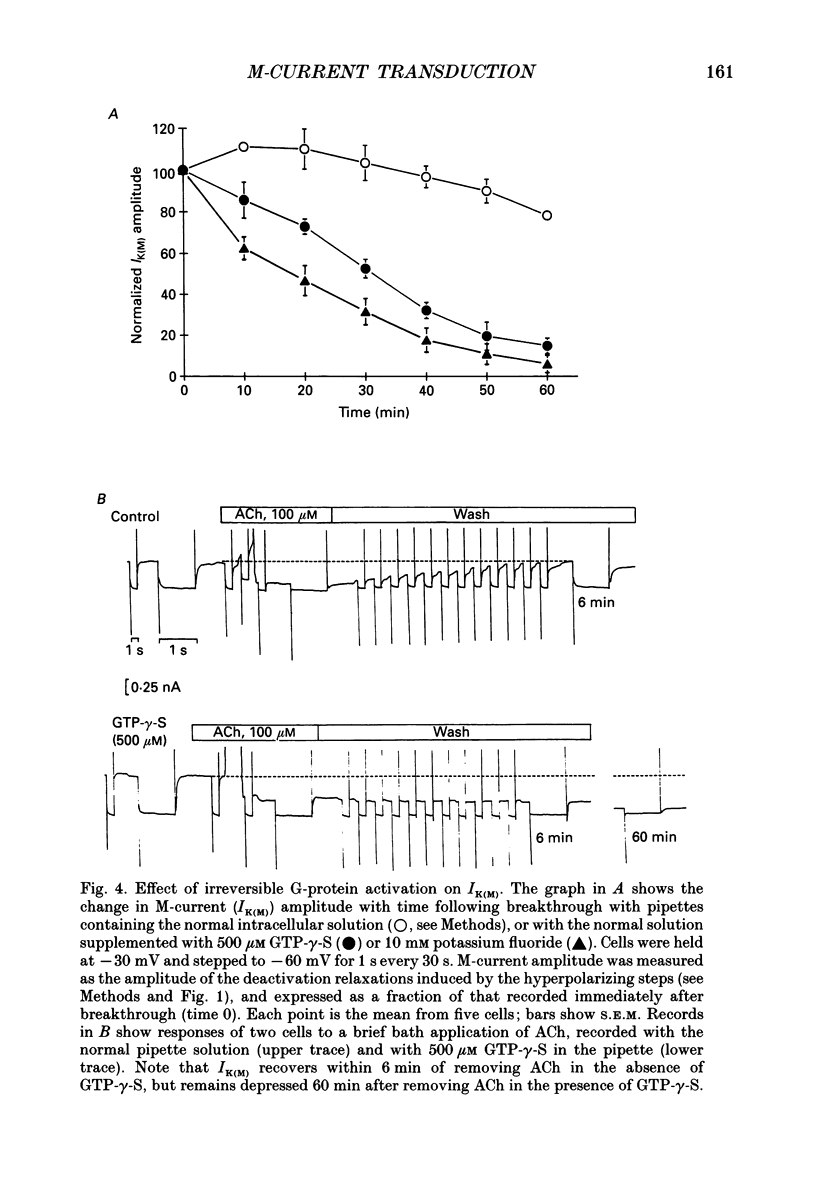
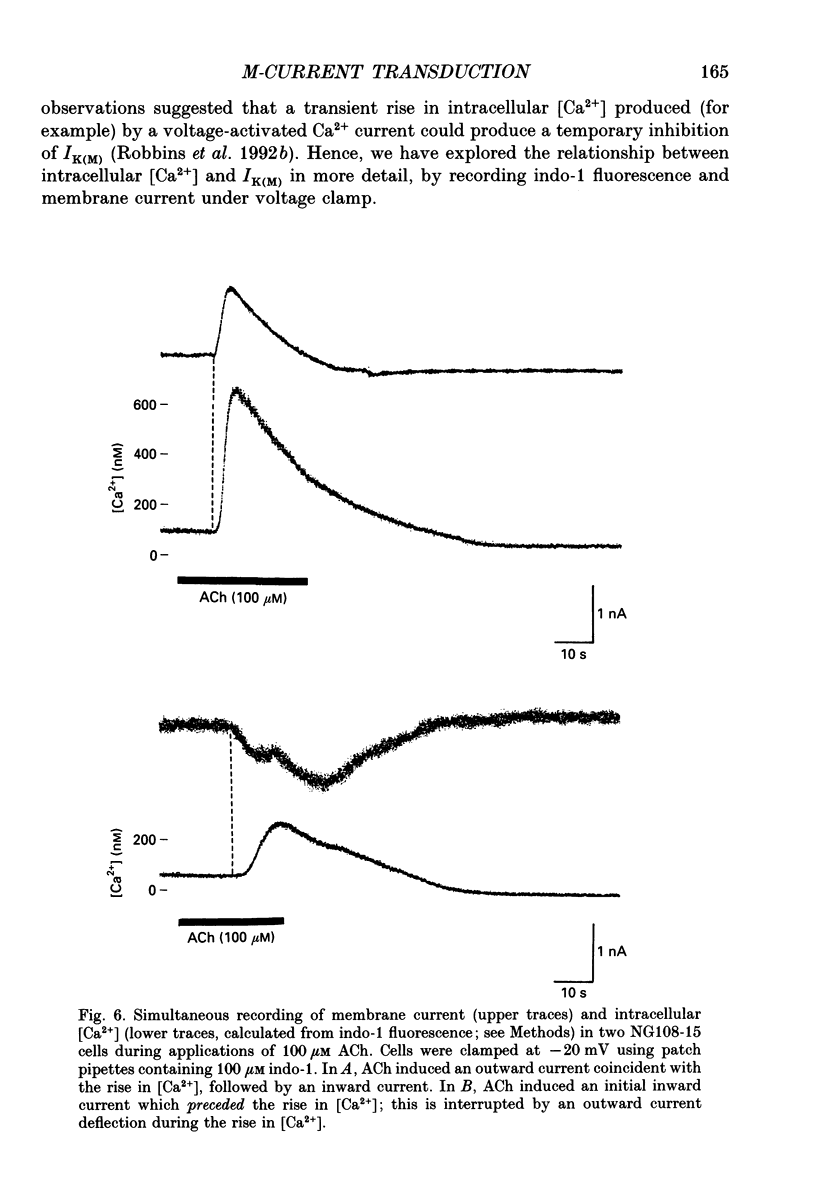
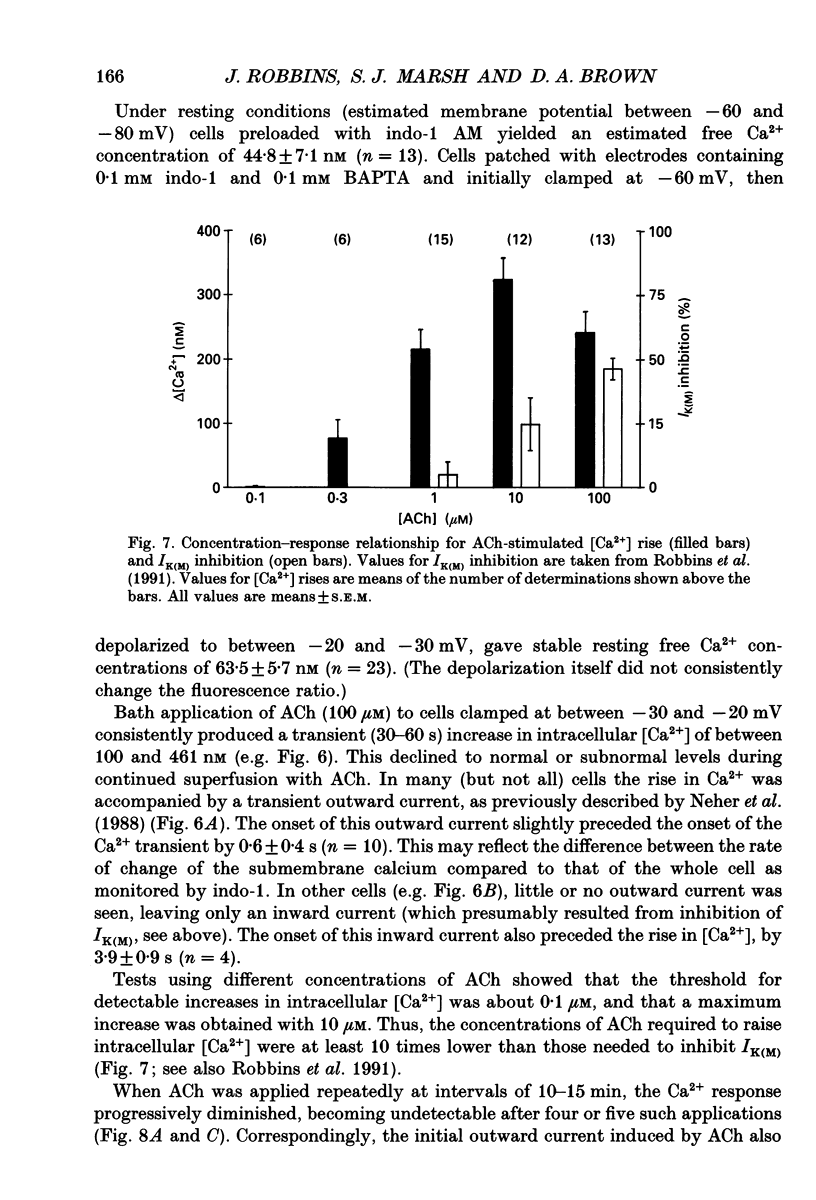
1. Acetylcholine (ACh) produces two membrane current changes when applied to NG108-15 mouse neuroblastoma x rat glioma hybrid cells transformed (by DNA transfection) to express m1 muscarinic receptors: it activates a Ca(2+)-dependent K+ conductance, producing an outward current, and it inhibits a voltage-dependent K+ conductance (the M conductance), thus diminishing the M-type voltage-dependent K+ current (IK(M)) and producing an inward current. The present experiments were undertaken to find out how far inhibition of IK(M) might be secondary to stimulation of phospholipase C, by recording membrane currents and intracellular Ca2+ changes with indo-1 using whole-cell patch-clamp methods. 2. Bath application of 100 microM ACh reversibly inhibited IK(M) by 47.3 +/- 3.2% (n = 23). Following pressure-application of 1 mM ACh, the mean latency to inhibition was 420 ms at 35 degrees C and 1.79 s at 23 degrees C. Latencies to inhibition by Ba2+ ions were 148 ms at 35 degrees C and 92 ms at 23 degrees C. 3. The involvement of a G-protein was tested by adding 0.5 mM GTP-gamma-S or 10 mM potassium fluoride to the pipette solution. These slowly reduced IK(M), with half-times of about 30 and 20 min respectively, and rendered the effect of superimposed ACh irreversible. Effects of ACh were not significantly changed after pretreatment for 24 h with 500 ng ml-1 pertussis toxin or on adding up to 10 mM GDP-beta-S to the pipette solution. 4. The role of phospholipase C and its products was tested using neomycin (to inhibit phospholipase C), inositol 1,4,5-trisphosphate (InsP3) and inositol 1,3,4,5-tetrakisphosphate (InsP4), heparin, and phorbol dibutyrate (PDBu) and staurosporin (to activate and inhibit protein kinase C respectively). Both neomycin (1 mM external) and InsP3 (100 microM intrapipette) inhibited the ACh-induced outward current and/or intracellular Ca2+ transient but did not block ACh-induced inhibition of IK(M). Intrapipette heparin (1 mM) blocked activation of IK(Ca) and reduced Ach-induced inhibitions of IK(M), but also reduced inhibition of ICa via endogeneous m4 receptors. PDBu (with or without intrapipette ATP) and staurosporin had no significant effects.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





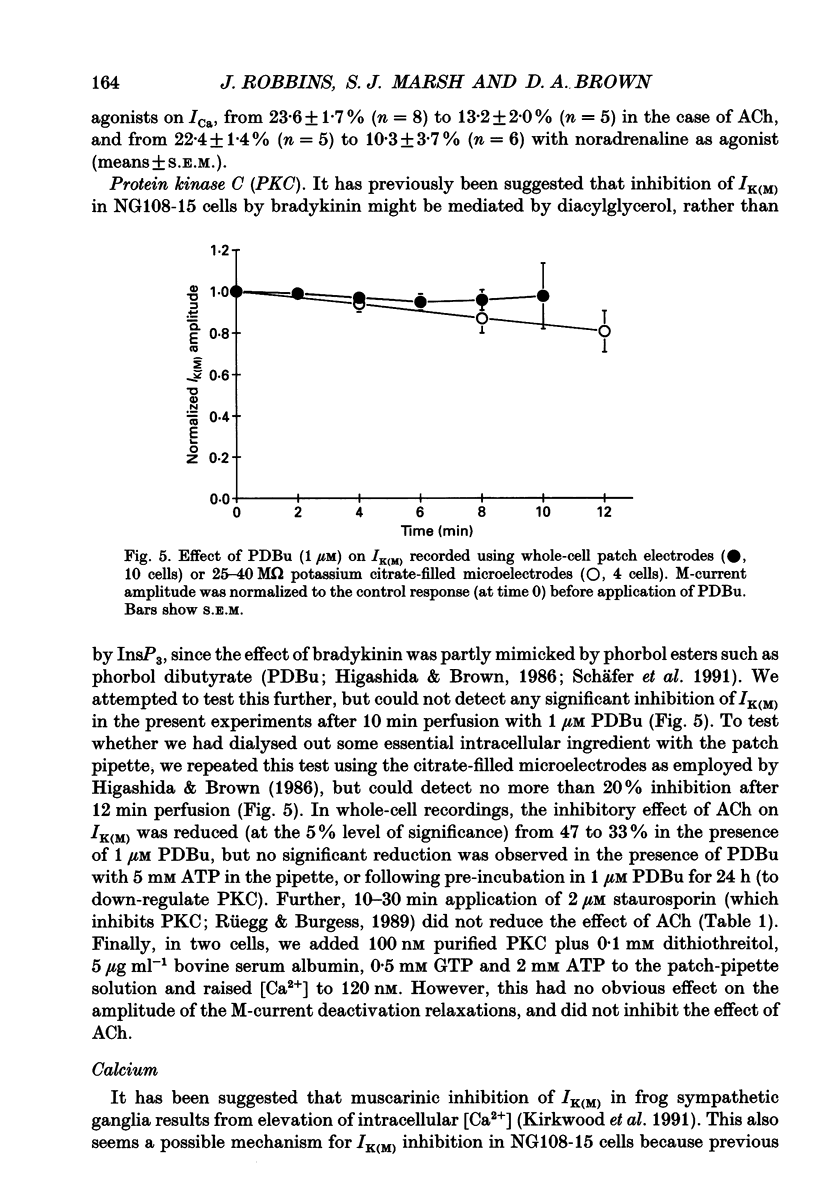
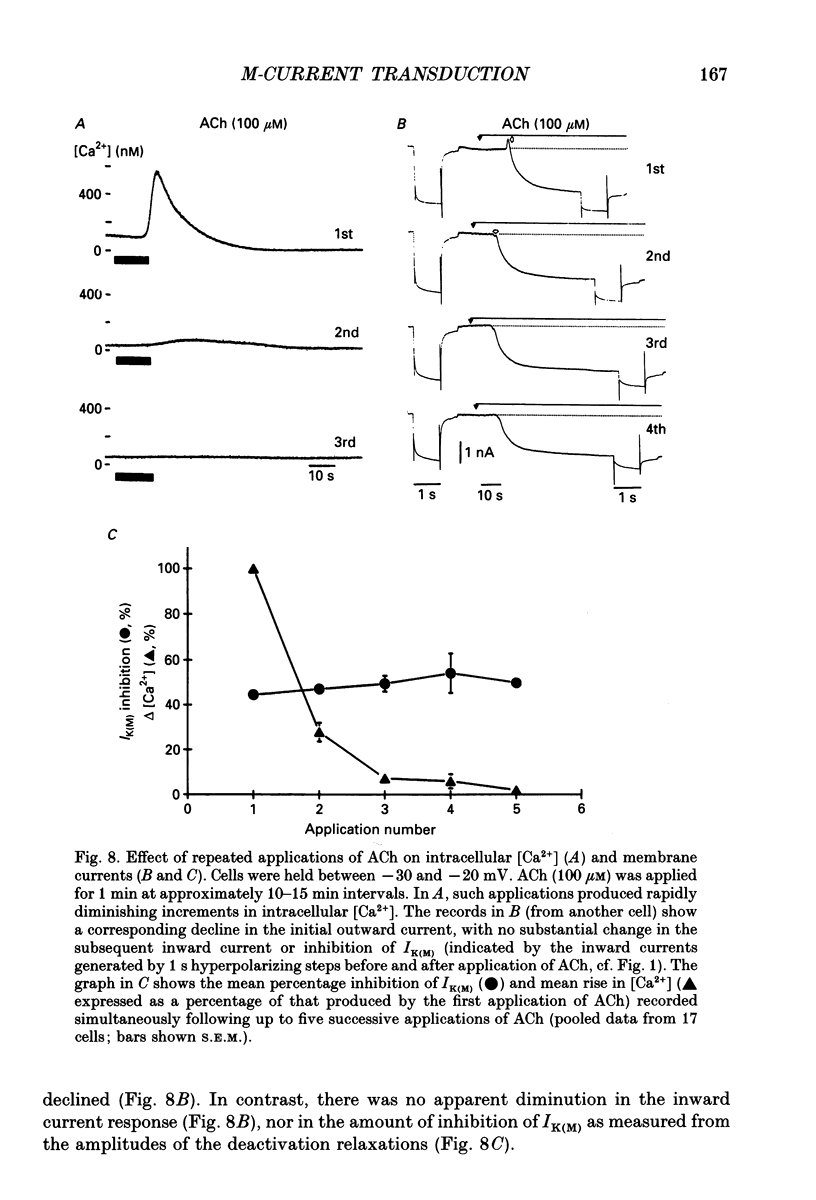
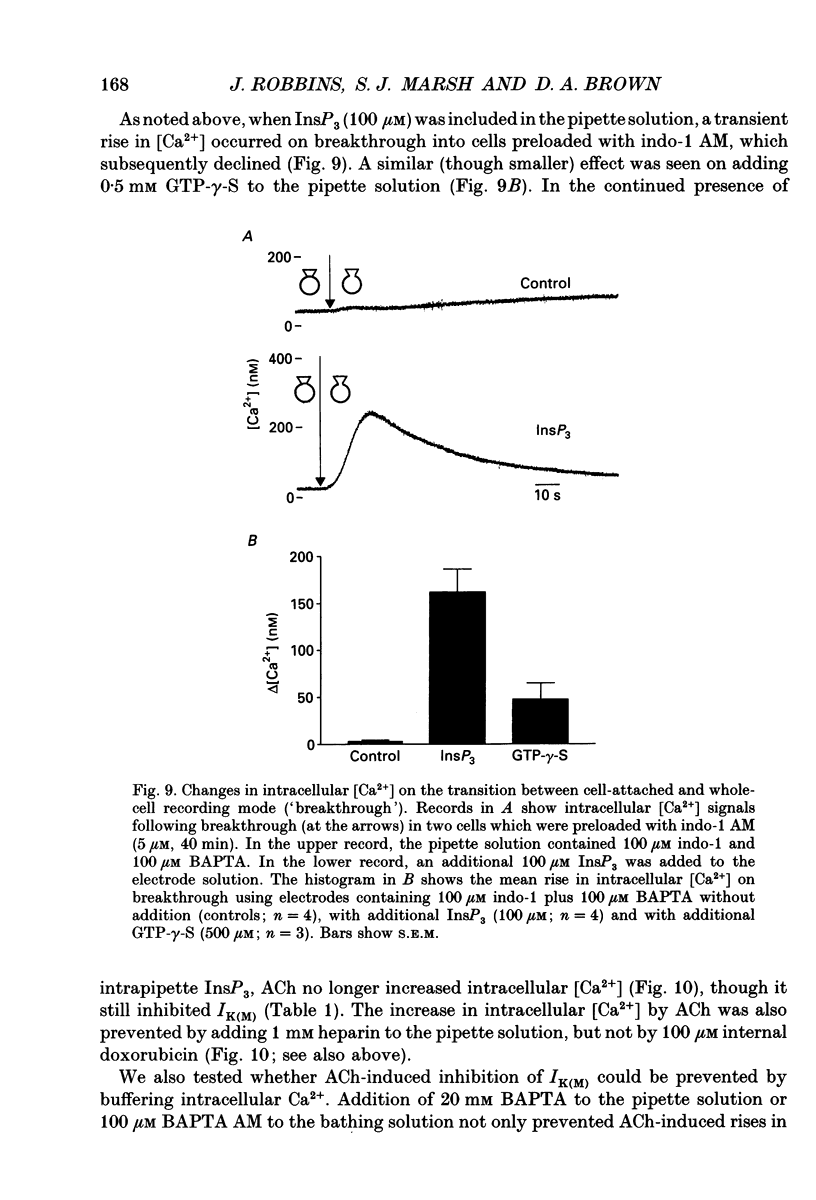
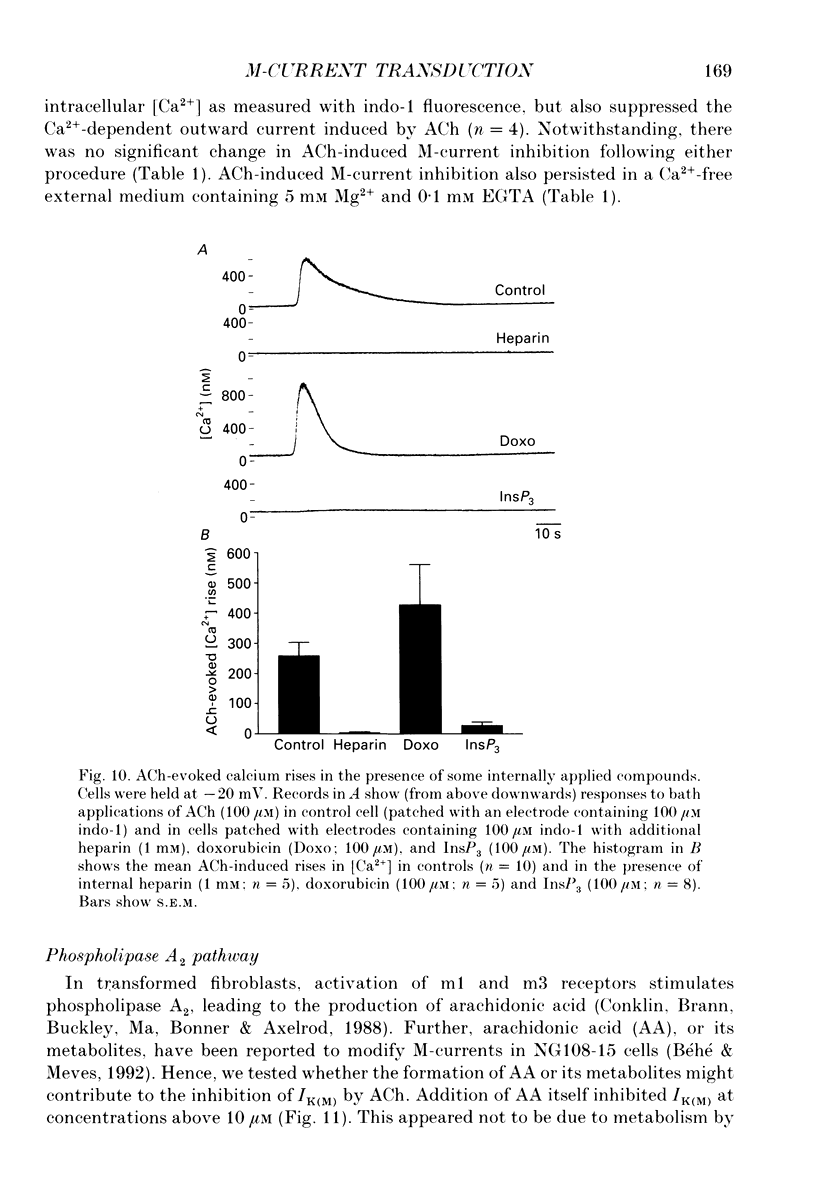
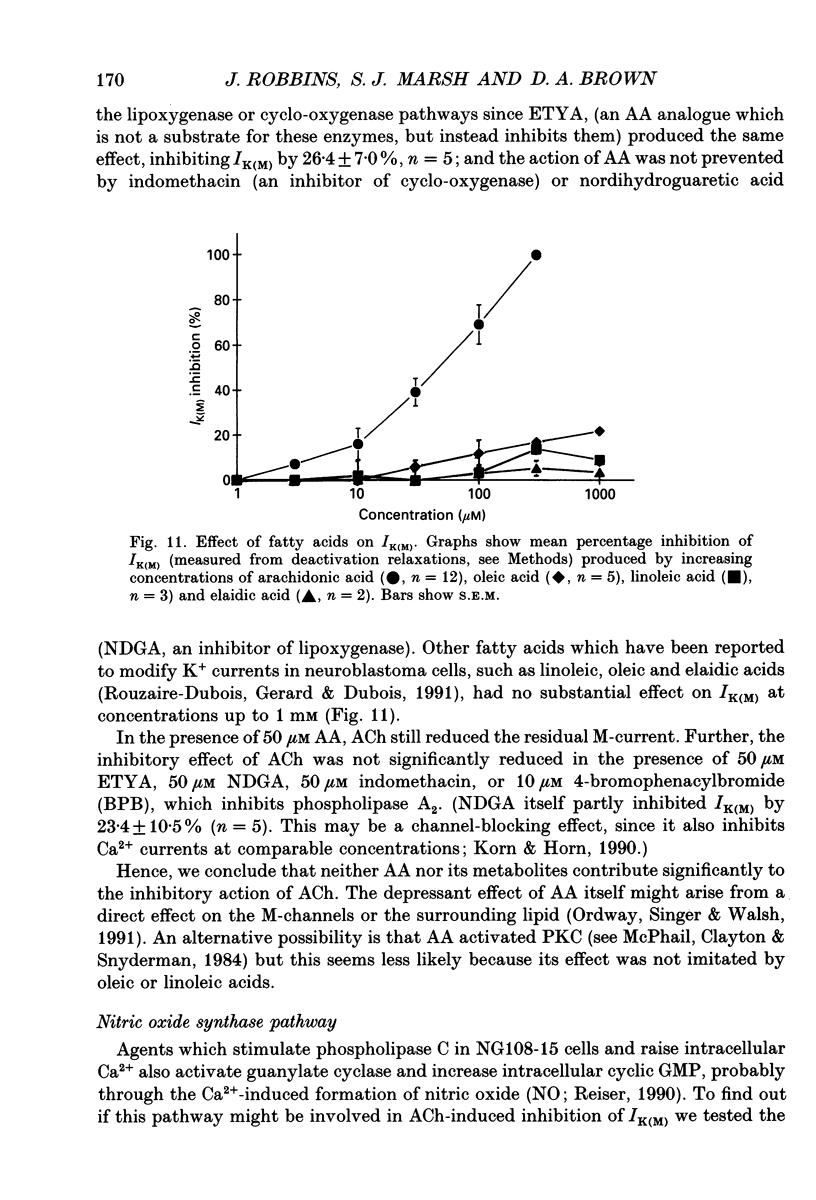








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beech D. J., Bernheim L., Mathie A., Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G. J., Oliver K. G., Horstman D. A., Obie J., Putney J. W., Jr Relationship between the calcium-mobilizing action of inositol 1,4,5-trisphosphate in permeable AR4-2J cells and the estimated levels of inositol 1,4,5-trisphosphate in intact AR4-2J cells. Biochem J. 1991 Feb 1;273(Pt 3):541–546. doi: 10.1042/bj2730541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M. M., Hille B. Protein kinase C is not necessary for peptide-induced suppression of M current or for desensitization of the peptide receptors. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2943–2947. doi: 10.1073/pnas.86.8.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Two components of muscarine-sensitive membrane current in rat sympathetic neurones. J Physiol. 1985 Jan;358:335–363. doi: 10.1113/jphysiol.1985.sp015554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Smith P. A. M-currents in frog sympathetic ganglion cells: manipulation of membrane phosphorylation. Br J Pharmacol. 1992 Feb;105(2):329–334. doi: 10.1111/j.1476-5381.1992.tb14254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Higashida H., Kubo T., Maeda A., Akiba I., Bujo H., Mishina M., Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature. 1988 Sep 22;335(6188):355–358. doi: 10.1038/335355a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Hashii M., Fukuda K., Caulfield M. P., Numa S., Brown D. A. Selective coupling of different muscarinic acetylcholine receptors to neuronal calcium currents in DNA-transfected cells. Proc Biol Sci. 1990 Oct 22;242(1303):68–74. doi: 10.1098/rspb.1990.0105. [DOI] [PubMed] [Google Scholar]
- Higashida H., Yokoyama S., Hoshi N., Myojo Y., Kawamura T., Ito Y., Hashii M., Sagara J., Furuya K. Phosphoinositides and synaptic function in NG108-15 neuroblastoma x glioma hybrid cells. Comp Biochem Physiol C. 1991;98(1):129–137. [PubMed] [Google Scholar]
- Higashijima T., Uzu S., Nakajima T., Ross E. M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem. 1988 May 15;263(14):6491–6494. [PubMed] [Google Scholar]
- Huang G. J., McArdle J. J. Novel suppression of an L-type calcium channel in neurones of murine dorsal root ganglia by 2,3-butanedione monoxime. J Physiol. 1992 Feb;447:257–274. doi: 10.1113/jphysiol.1992.sp019001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Time course of receptor-channel coupling in frog sympathetic neurons. Biophys J. 1991 Aug;60(2):502–507. doi: 10.1016/S0006-3495(91)82077-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Weinberger R. P., Waxham M. N. Active site-directed inhibition of Ca2+/calmodulin-dependent protein kinase type II by a bifunctional calmodulin-binding peptide. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4991–4995. doi: 10.1073/pnas.85.14.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A., Simmons M. A., Mather R. J., Lisman J. Muscarinic suppression of the M-current is mediated by a rise in internal Ca2+ concentration. Neuron. 1991 Jun;6(6):1009–1014. doi: 10.1016/0896-6273(91)90240-z. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Nordihydroguaiaretic acid inhibits voltage-activated Ca2+ currents independently of lipoxygenase inhibition. Mol Pharmacol. 1990 Oct;38(4):524–530. [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Kongsamut S., Tsien R. W. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989 Aug 24;340(6235):639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lopez H. S., Adams P. R. A G Protein Mediates the Inhibition of the Voltage-Dependent Potassium M Current by Muscarine, LHRH, Substance P and UTP in Bullfrog Sympathetic Neurons. Eur J Neurosci. 1989 Sep;1(5):529–542. doi: 10.1111/j.1460-9568.1989.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Lopez H. S. Kinetics of G protein-mediated modulation of the potassium M-current in bullfrog sympathetic neurons. Neuron. 1992 Apr;8(4):725–736. doi: 10.1016/0896-6273(92)90093-s. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Zucker R. S., Marsh S. J., Adams P. R. Modulation of M-current by intracellular Ca2+. Neuron. 1991 Apr;6(4):533–545. doi: 10.1016/0896-6273(91)90056-6. [DOI] [PubMed] [Google Scholar]
- Mathie A., Bernheim L., Hille B. Inhibition of N- and L-type calcium channels by muscarinic receptor activation in rat sympathetic neurons. Neuron. 1992 May;8(5):907–914. doi: 10.1016/0896-6273(92)90205-r. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A., Fukuda K., Kubo T., Numa S. Intracellular calcium release mediated by two muscarinic receptor subtypes. FEBS Lett. 1988 Nov 21;240(1-2):88–94. doi: 10.1016/0014-5793(88)80345-4. [DOI] [PubMed] [Google Scholar]
- Oakes S. G., Schlager J. J., Santone K. S., Abraham R. T., Powis G. Doxorubicin blocks the increase in intracellular Ca++, part of a second messenger system in N1E-115 murine neuroblastoma cells. J Pharmacol Exp Ther. 1990 Mar;252(3):979–983. [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Otero A. D. Transphosphorylation and G protein activation. Biochem Pharmacol. 1990 May 1;39(9):1399–1404. doi: 10.1016/0006-2952(90)90420-p. [DOI] [PubMed] [Google Scholar]
- Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser G. Endothelin and a Ca2+ ionophore raise cyclic GMP levels in a neuronal cell line via formation of nitric oxide. Br J Pharmacol. 1990 Nov;101(3):722–726. doi: 10.1111/j.1476-5381.1990.tb14147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Caulfield M. P., Higashida H., Brown D. A. Genotypic m3-Muscarinic Receptors Preferentially Inhibit M-currents in DNA-transfected NG108-15 Neuroblastoma x Glioma Hybrid Cells. Eur J Neurosci. 1991;3(8):820–824. doi: 10.1111/j.1460-9568.1991.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Robbins J., Cloues R., Brown D. A. Intracellular Mg2+ inhibits the IP3-activated IK(Ca) in NG108-15 cells. [Why intracellular citrate can be useful for recording IK(Ca)]. Pflugers Arch. 1992 Mar;420(3-4):347–353. doi: 10.1007/BF00374469. [DOI] [PubMed] [Google Scholar]
- Robbins J., Trouslard J., Marsh S. J., Brown D. A. Kinetic and pharmacological properties of the M-current in rodent neuroblastoma x glioma hybrid cells. J Physiol. 1992;451:159–185. doi: 10.1113/jphysiol.1992.sp019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Gérard V., Dubois J. M. Modification of K+ channel properties induced by fatty acids in neuroblastoma cells. Pflugers Arch. 1991 Nov;419(5):467–471. doi: 10.1007/BF00370790. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Béhé P., Meves H. Inhibition of the M current in NG 108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1991 Jul;418(6):581–591. doi: 10.1007/BF00370575. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A., Stansfeld C. E., Brown D. A. Closure of potassium M-channels by muscarinic acetylcholine-receptor stimulants requires a diffusible messenger. Proc Biol Sci. 1992 Nov 23;250(1328):119–125. doi: 10.1098/rspb.1992.0139. [DOI] [PubMed] [Google Scholar]
- Sim J. A., Gerber U., Knöpfel T., Brown D. A. Evidence Against a Role for Protein Kinase C in the Inhibition of the Calcium-activated Potassium Current IAHP by Muscarinic Stimulants in Rat Hippocampal Neurons. Eur J Neurosci. 1992;4(9):785–791. doi: 10.1111/j.1460-9568.1992.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Simmons M. A., Mather R. J. Selectivity of the effects of guanosine-5'-O-(2-thiodiphosphate) on agonist inhibition of the M-current in amphibian sympathetic neurons. J Neurosci. 1991 Jul;11(7):2130–2134. doi: 10.1523/JNEUROSCI.11-07-02130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Willuweit B., Aktories K. Heparin uncouples alpha 2-adrenoceptors from the Gi-protein in membranes of human platelets. Biochem J. 1988 Feb 1;249(3):857–863. doi: 10.1042/bj2490857. [DOI] [PMC free article] [PubMed] [Google Scholar]


