Abstract
1. We recorded from 239 neurons located in the magnocellular division of the red nucleus of four alert macaque monkeys. At the same time, we recorded electromyographic (EMG) signals from as many as twenty electrodes chronically implanted on muscles of the shoulder, arm, forearm and hand. We recorded EMG signals for periods ranging from several months to a year. 2. The monkeys were trained to perform three free-form food retrieval tasks, each of which activated all of the recorded muscles and most of the neurons. The 'prehension' task required simply that the monkey grasp a piece of food from a fixed point in space. The 'barrier' task required the monkey to reach around a small barrier to obtain the food, and the 'Kluver' task required that food be removed from small holes. During the prehension task, we found approximately equal numbers of neurons that were strongly active while the hand was being moved toward the target (70% of units), and while the food was being grasped (60%). Relatively few units were active as the hand was returned to the mouth (15%). 3. Data files of 1-2 min duration were collected while the monkey performed a single behavioural task. Whenever possible, we recorded files for all three tasks from each neuron. For each file we calculated long time-span analog cross-correlations (+/- 1.28 s) between instantaneous neuronal firing rate and each of the full-wave rectified, low-pass filtered EMG signals. We used the peak correlation and the time of the peak as two summary measures of the functional relation between modulation of neuronal activity and EMG. 4. The magnitude of the strongest correlations was between 0.4 and 0.5 (normalized to a perfect correlation of +/- 1.0). Distal muscles were the most frequently correlated, and extensors were more frequently correlated than flexors. For all monkeys, the lags for well correlated muscles were distributed broadly about a uni-modal value near 0 ms. Eighty five per cent of the correlations larger than or equal to 0.25 had peaks between -150 and 200 ms. 5. The activity of each neuron was represented in a muscle co-ordinate system by an n-dimensional 'functional linkage vector', each element of which was the peak correlation with one of n muscles. The vector for any given neuron points in a particular direction in muscle space, depending on the similarity between the activity of the neuron and the activity of each muscle.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







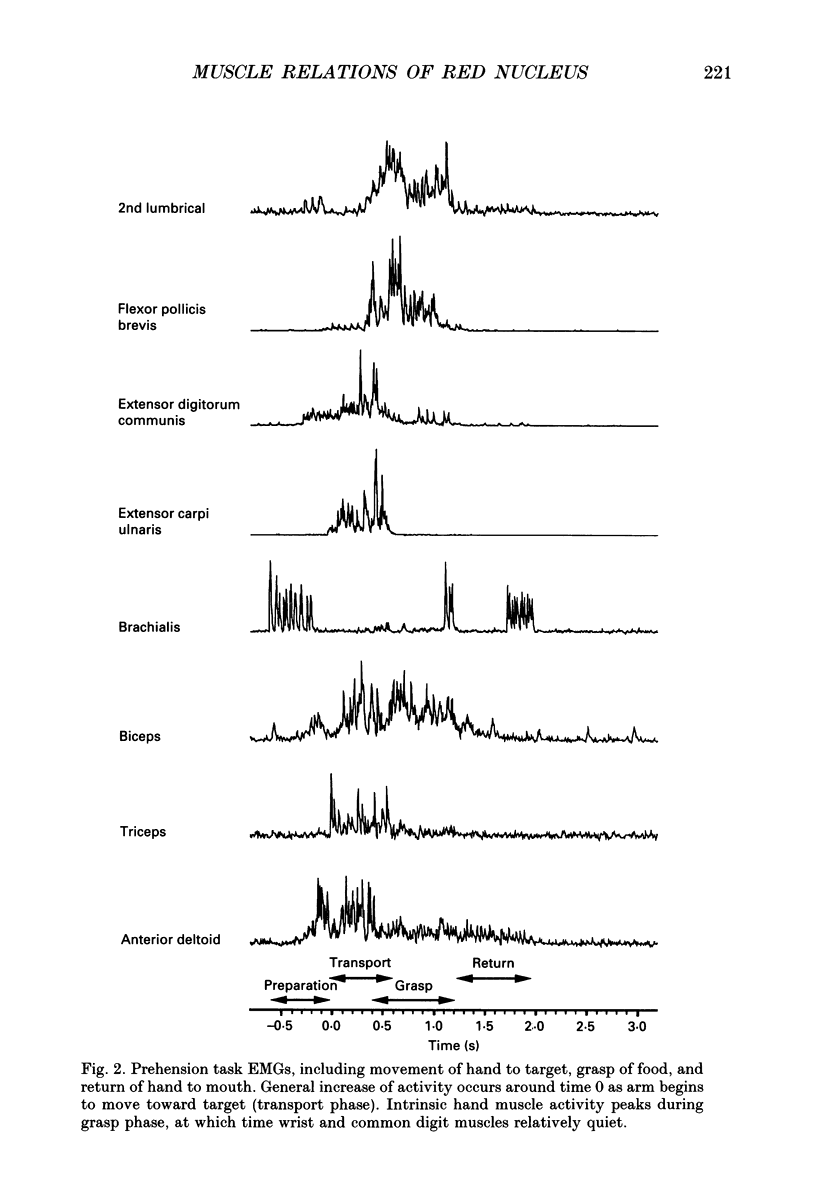
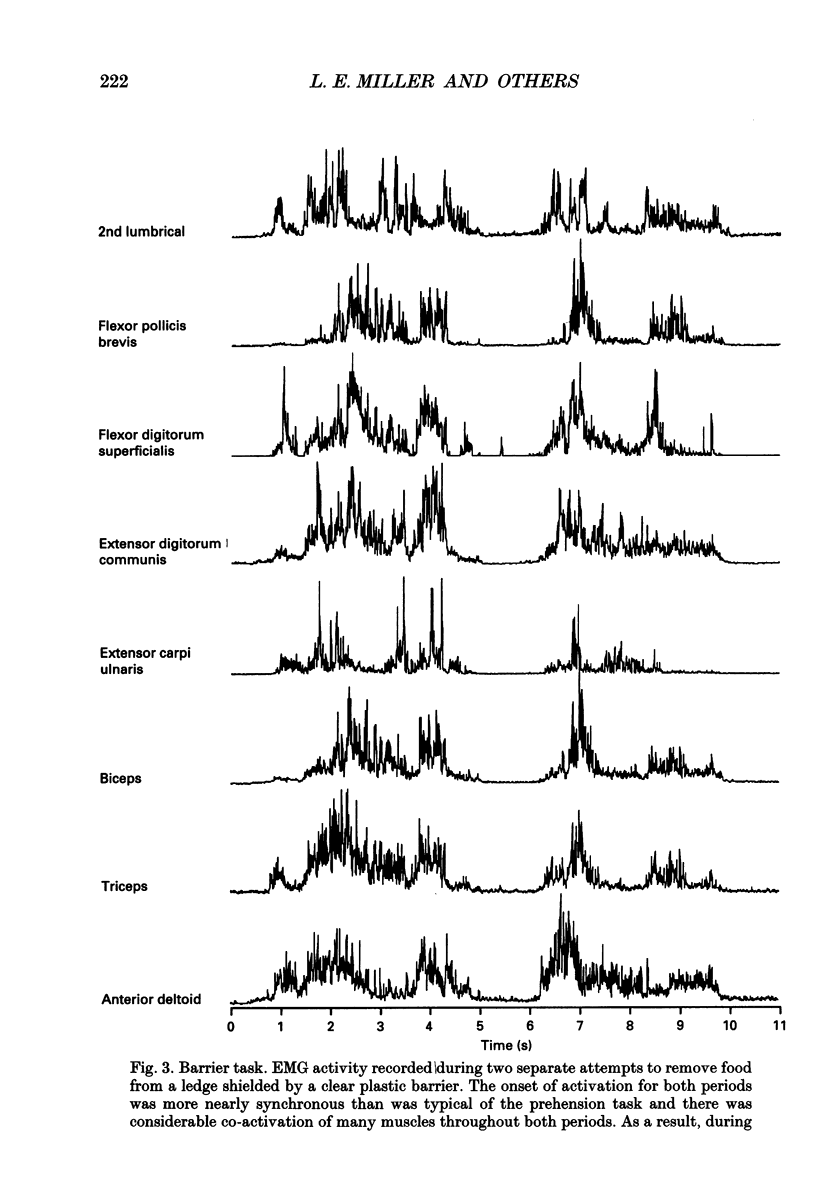


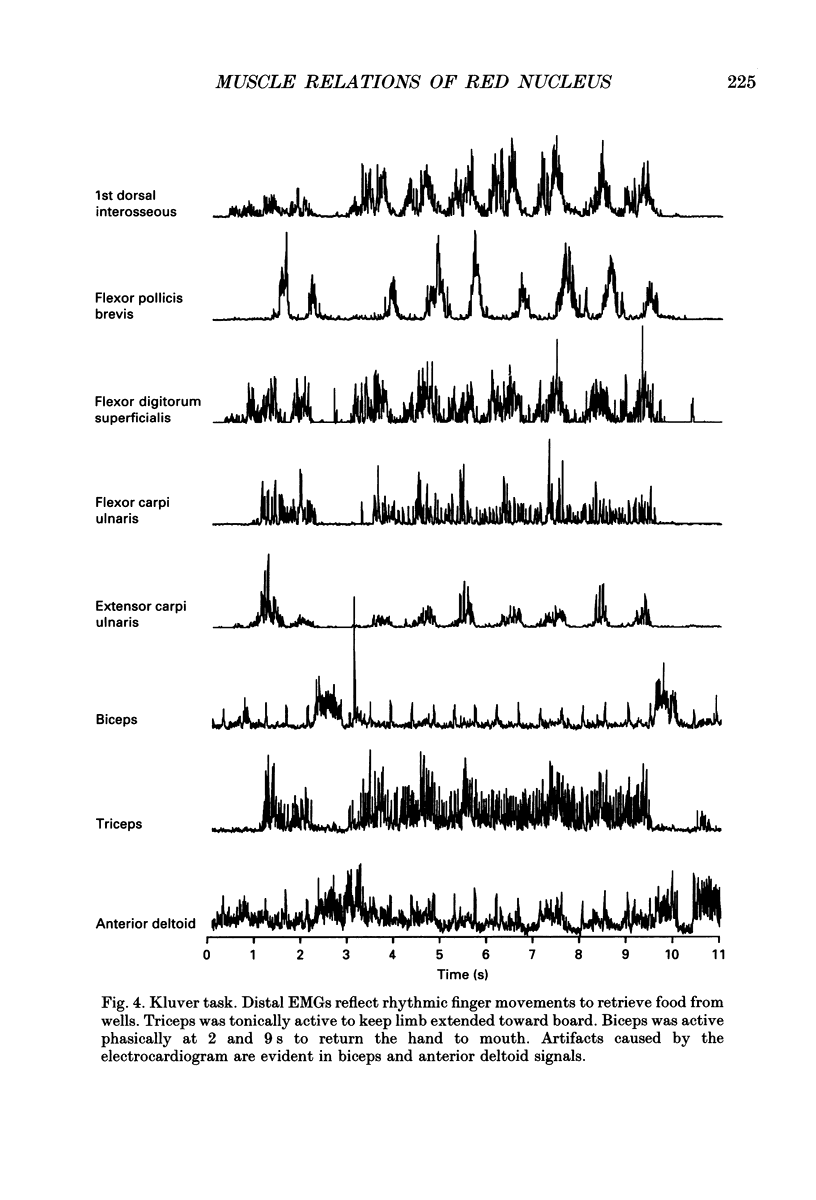
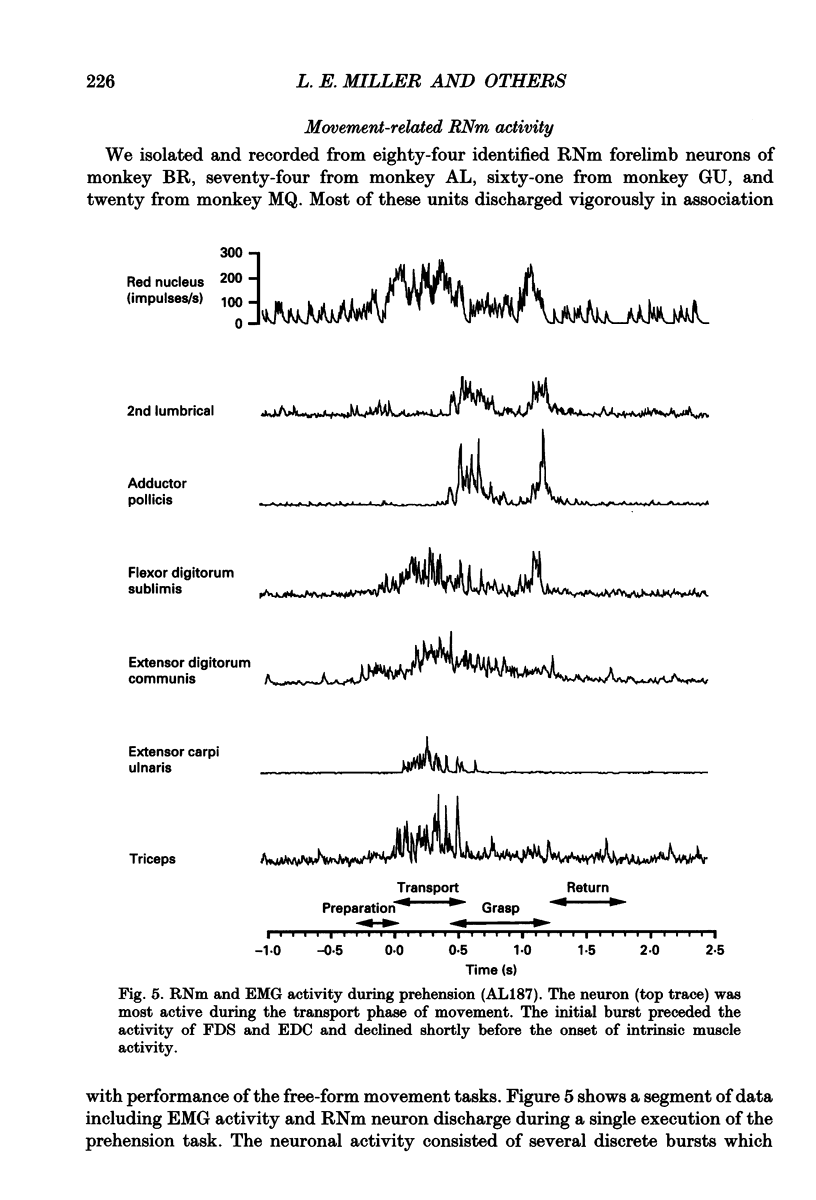

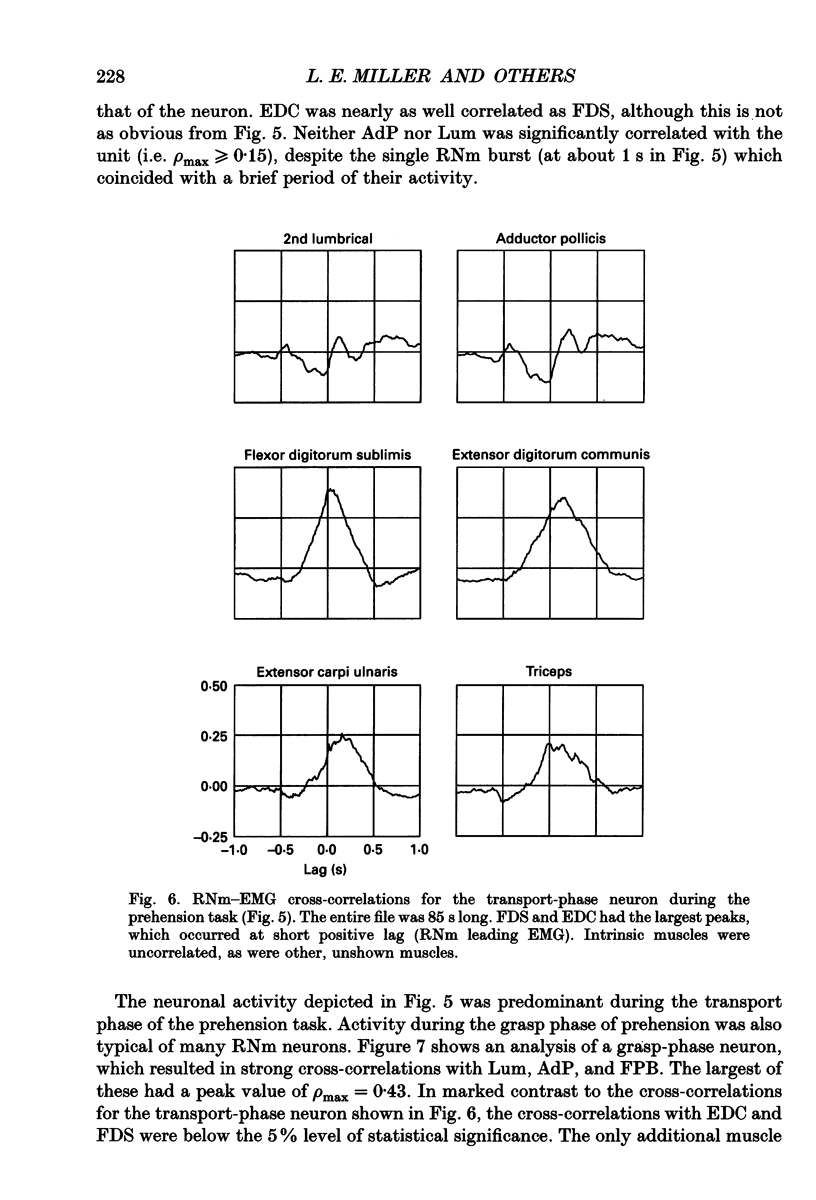
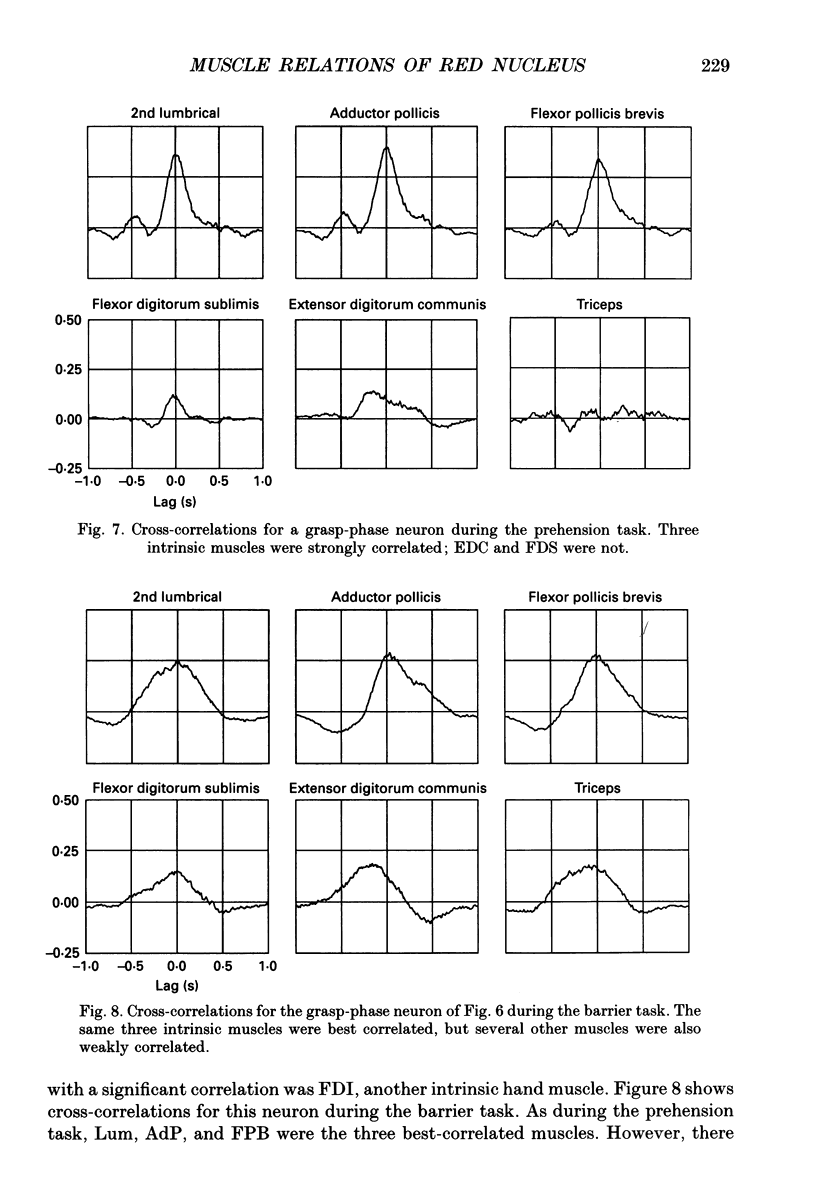
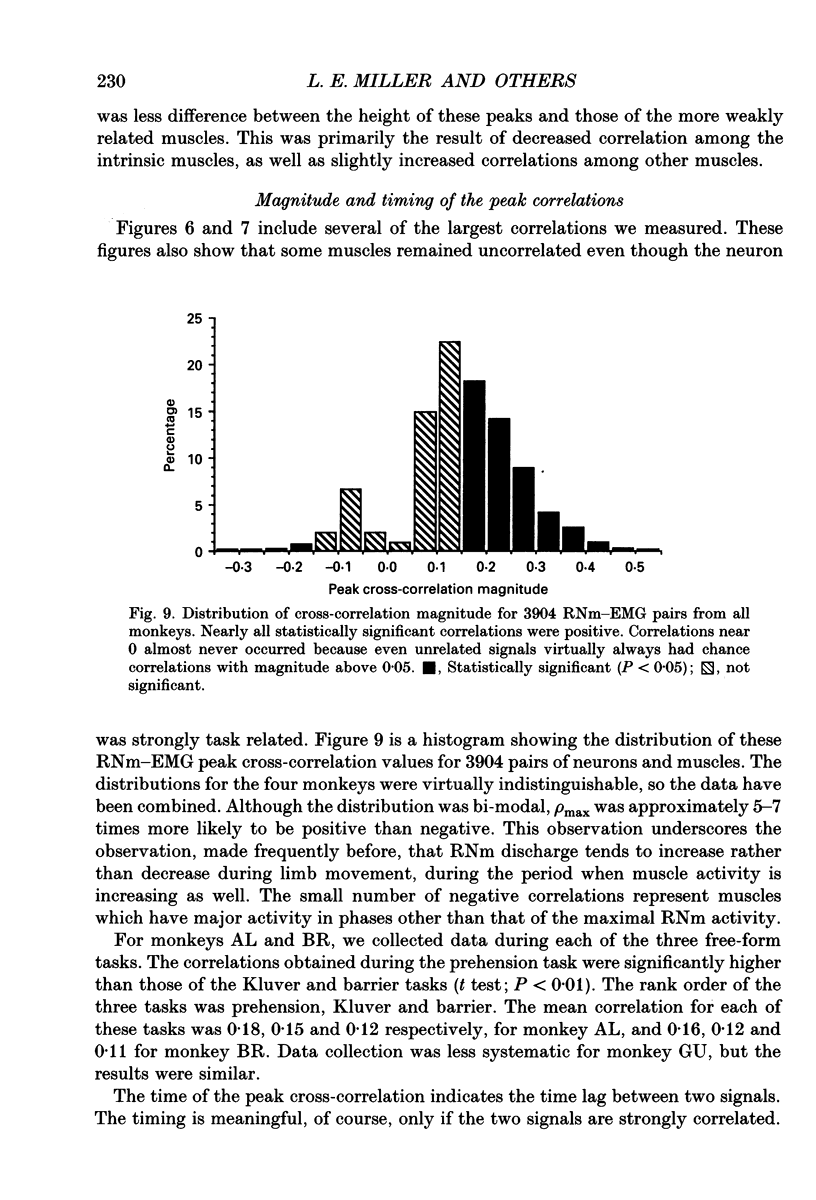
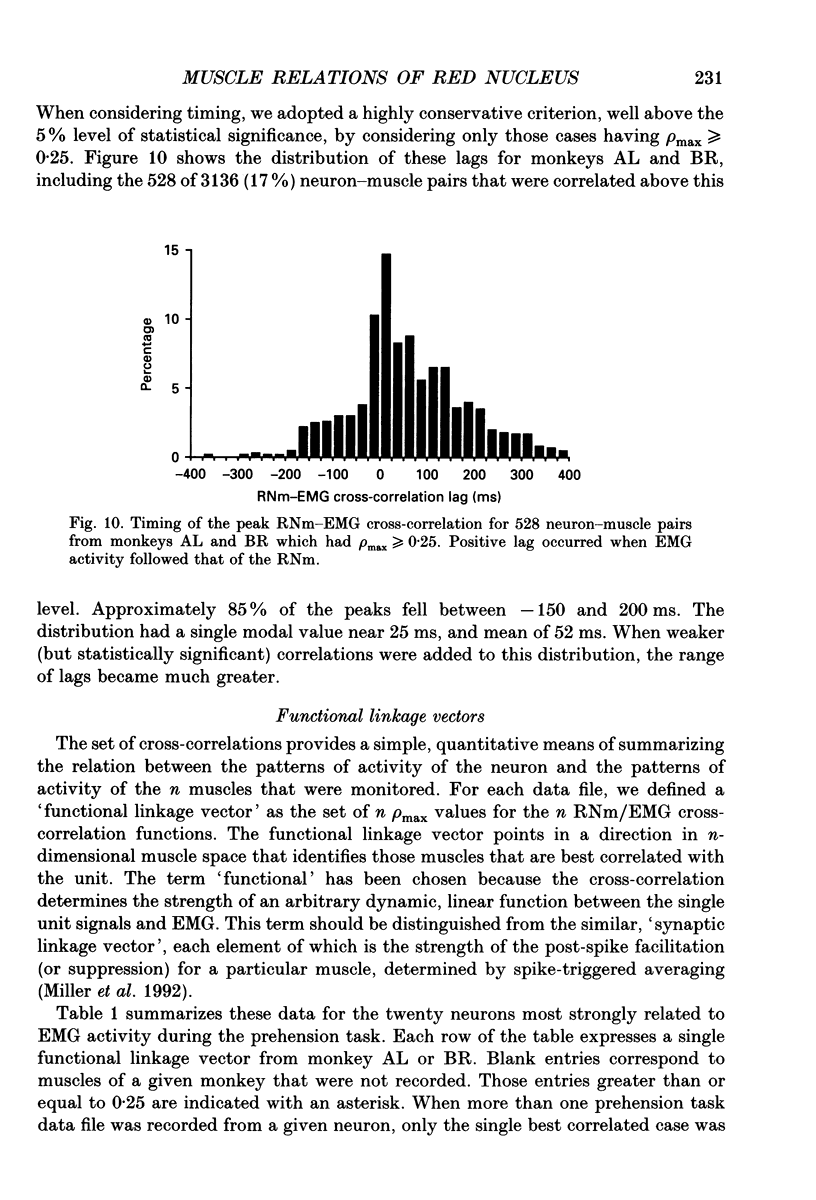
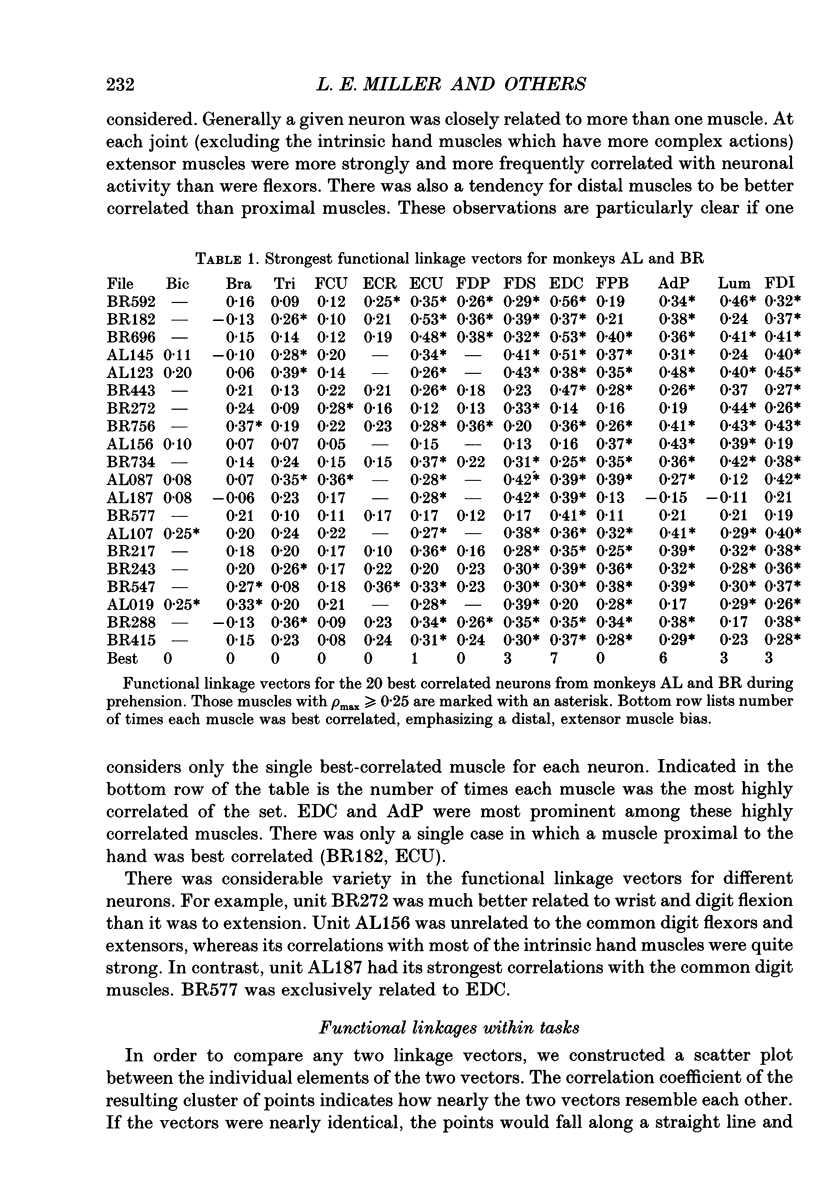

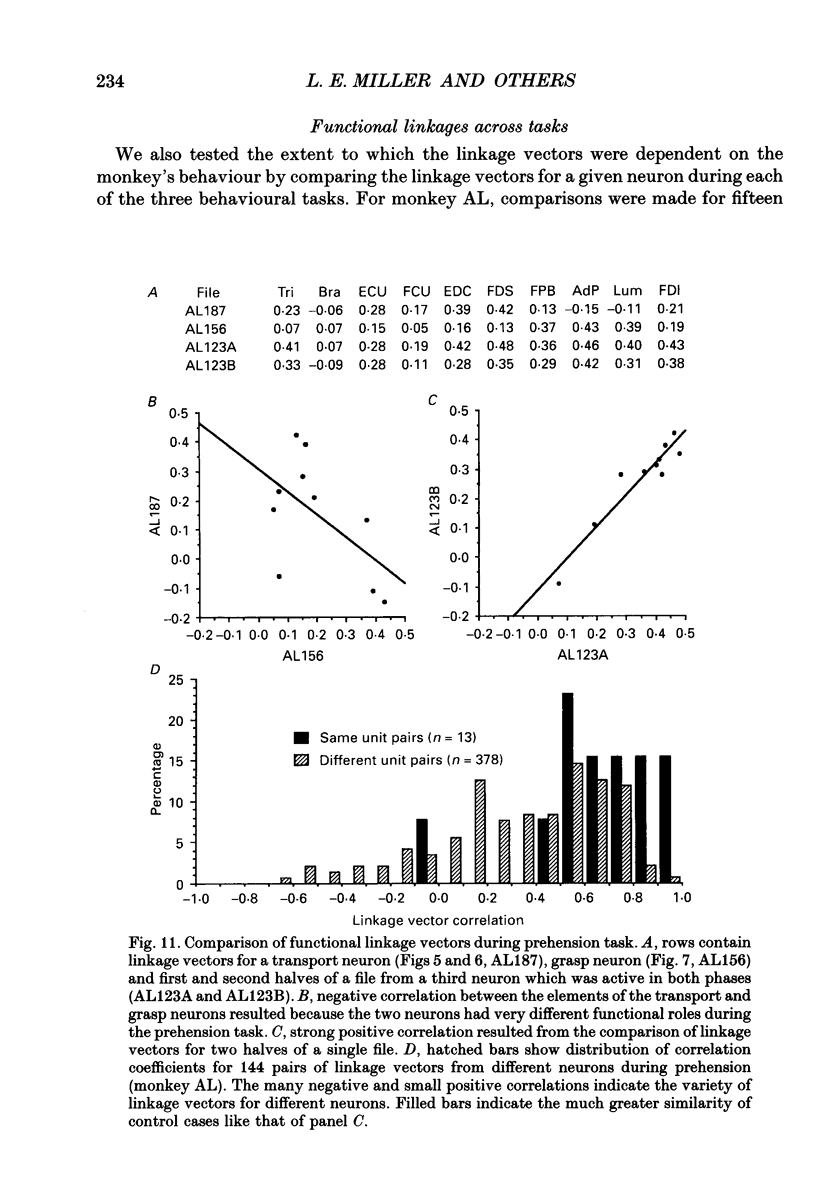
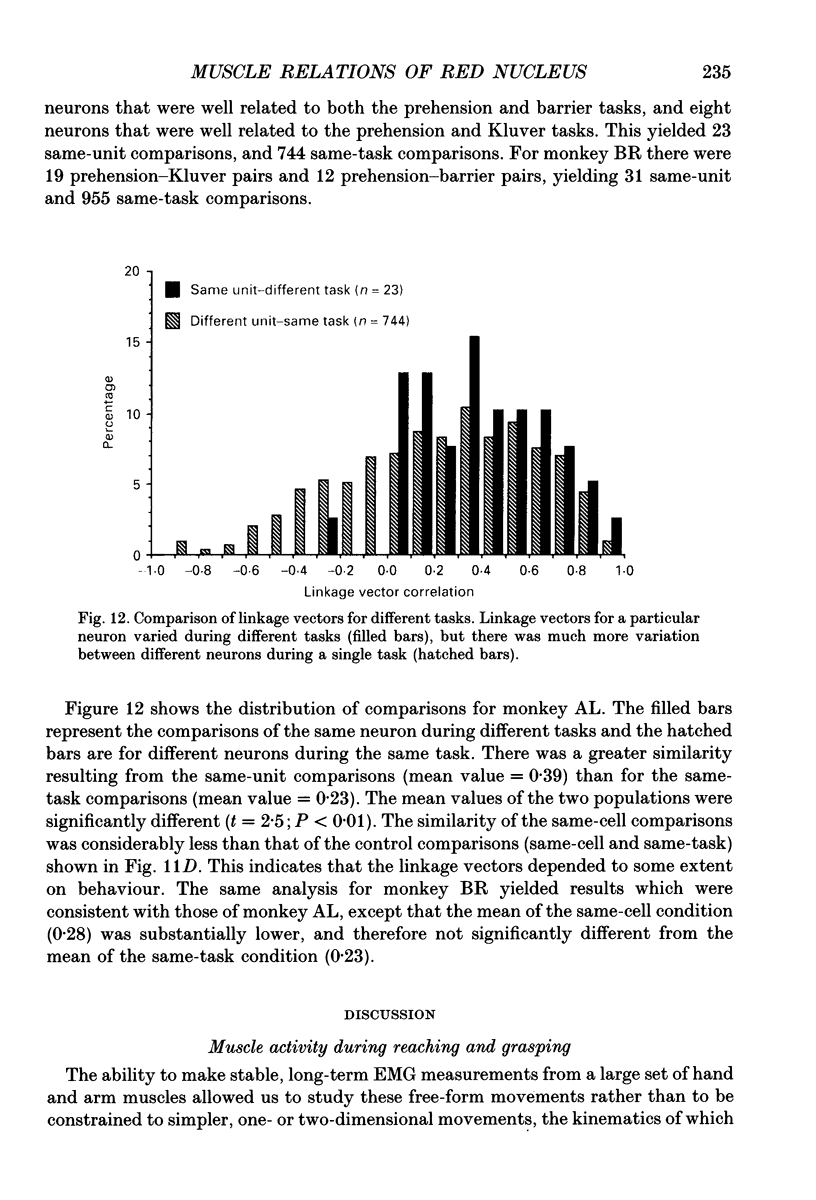








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Alstermark B., Lundberg A., Norrsell U., Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981;42(3-4):299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Mewes K., Fetz E. E. Encoding of motor parameters by corticomotoneuronal (CM) and rubromotoneuronal (RM) cells producing postspike facilitation of forelimb muscles in the behaving monkey. Behav Brain Res. 1988 Apr-May;28(1-2):181–191. doi: 10.1016/0166-4328(88)90095-2. [DOI] [PubMed] [Google Scholar]
- Fortier P. A., Kalaska J. F., Smith A. M. Cerebellar neuronal activity related to whole-arm reaching movements in the monkey. J Neurophysiol. 1989 Jul;62(1):198–211. doi: 10.1152/jn.1989.62.1.198. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A. P., Kalaska J. F., Caminiti R., Massey J. T. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982 Nov;2(11):1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A. R., Houk J. C., Kohlerman N. J. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. J Physiol. 1985 Jan;358:527–549. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A. R., Houk J. C., Kohlerman N. J. Relation between red nucleus discharge and movement parameters in trained macaque monkeys. J Physiol. 1985 Jan;358:551–570. doi: 10.1113/jphysiol.1985.sp015566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Anatomical evidence for an ipsilateral rubrospinal pathway and for direct rubrospinal projections to motoneurons in the cat. Neurosci Lett. 1987 Mar 9;74(3):269–274. doi: 10.1016/0304-3940(87)90308-9. [DOI] [PubMed] [Google Scholar]
- Houk J. C., Dessem D. A., Miller L. E., Sybirska E. H. Correlation and spectral analysis of relations between single unit discharge and muscle activities. J Neurosci Methods. 1987 Oct;21(2-4):201–224. doi: 10.1016/0165-0270(87)90117-8. [DOI] [PubMed] [Google Scholar]
- Houk J. C., Keifer J., Barto A. G. Distributed motor commands in the limb premotor network. Trends Neurosci. 1993 Jan;16(1):27–33. doi: 10.1016/0166-2236(93)90049-r. [DOI] [PubMed] [Google Scholar]
- Hunter I. W., Kearney R. E. NEXUS: a computer language for physiological systems and signal analysis. Comput Biol Med. 1984;14(4):385–401. doi: 10.1016/0010-4825(84)90039-8. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The timing of natural prehension movements. J Mot Behav. 1984 Sep;16(3):235–254. doi: 10.1080/00222895.1984.10735319. [DOI] [PubMed] [Google Scholar]
- Kalaska J. F., Cohen D. A., Prud'homme M., Hyde M. L. Parietal area 5 neuronal activity encodes movement kinematics, not movement dynamics. Exp Brain Res. 1990;80(2):351–364. doi: 10.1007/BF00228162. [DOI] [PubMed] [Google Scholar]
- Lamarre Y., Spidalieri G., Busby L., Lund J. P. Programming of initiation and execution of ballistic arm movements in the monkey. Prog Brain Res. 1980;54:157–169. doi: 10.1016/S0079-6123(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Lamarre Y., Spidalieri G., Lund J. P. Patterns of muscular and motor cortical activity during a simple arm movement in the monkey. Can J Physiol Pharmacol. 1981 Jul;59(7):748–756. doi: 10.1139/y81-111. [DOI] [PubMed] [Google Scholar]
- Lawrence D. G., Kuypers H. G. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968 Mar;91(1):15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Marple-Horvat D. E., Stein J. F. Cerebellar neuronal activity related to arm movements in trained rhesus monkeys. J Physiol. 1987 Dec;394:351–366. doi: 10.1113/jphysiol.1987.sp016874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy M. L., Hansma D. I., Houk J. C., Gibson A. R. Selective projections from the cat red nucleus to digit motor neurons. J Comp Neurol. 1987 Nov 15;265(3):367–379. doi: 10.1002/cne.902650306. [DOI] [PubMed] [Google Scholar]
- Mewes K., Cheney P. D. Facilitation and suppression of wrist and digit muscles from single rubromotoneuronal cells in the awake monkey. J Neurophysiol. 1991 Dec;66(6):1965–1977. doi: 10.1152/jn.1991.66.6.1965. [DOI] [PubMed] [Google Scholar]
- Soechting J. F., Burton J. E., Onoda N. Relationships between sensory input, motor output and unit activity in interpositus and red nuclei during intentional movement. Brain Res. 1978 Aug 18;152(1):65–79. doi: 10.1016/0006-8993(78)90134-8. [DOI] [PubMed] [Google Scholar]
- Sterling P., Kuypers H. G. Anatomical organization of the brachial spinal cord of the cat. II. The motoneuron plexus. Brain Res. 1967 Feb;4(1):16–32. doi: 10.1016/0006-8993(67)90145-x. [DOI] [PubMed] [Google Scholar]
- Sybirska E., Górska T. Effects of red nucleus lesions on forelimb movements in the cat. Acta Neurobiol Exp (Wars) 1980;40(5):821–841. [PubMed] [Google Scholar]
- Thach W. T. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol. 1978 May;41(3):654–676. doi: 10.1152/jn.1978.41.3.654. [DOI] [PubMed] [Google Scholar]



