Abstract
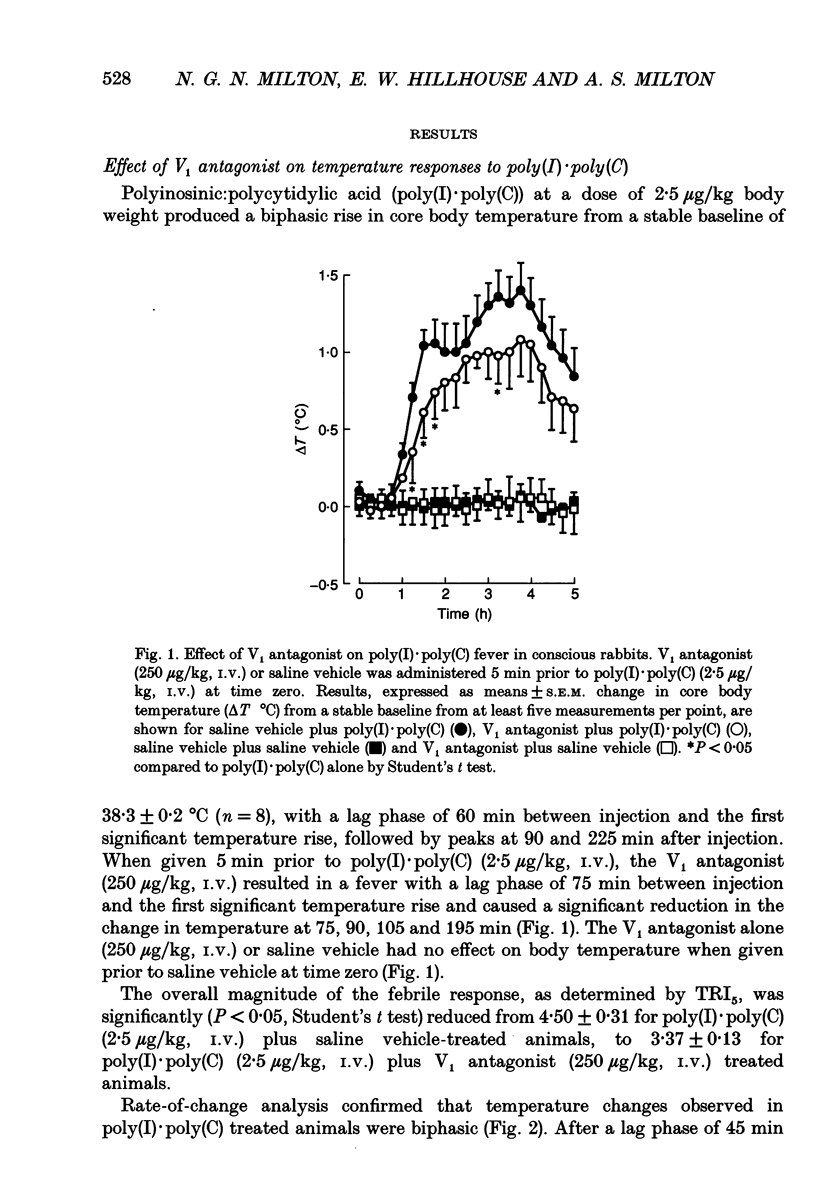
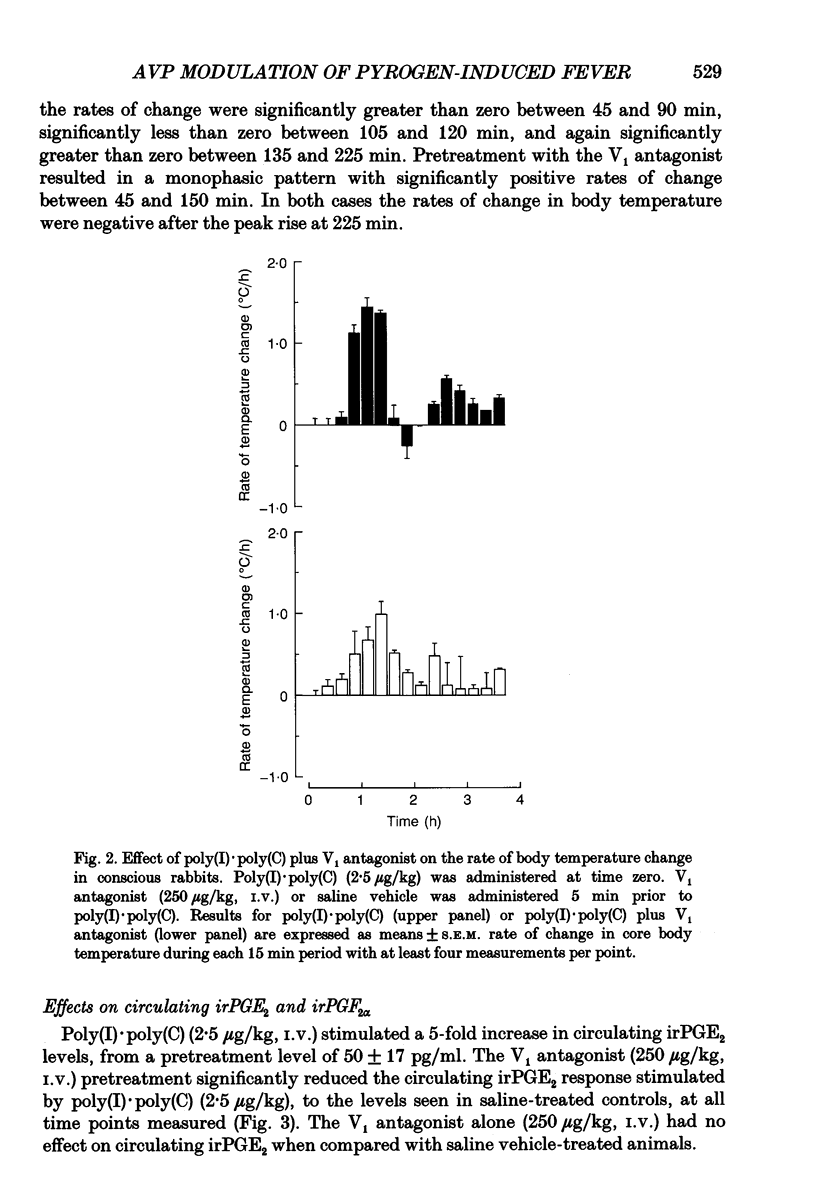
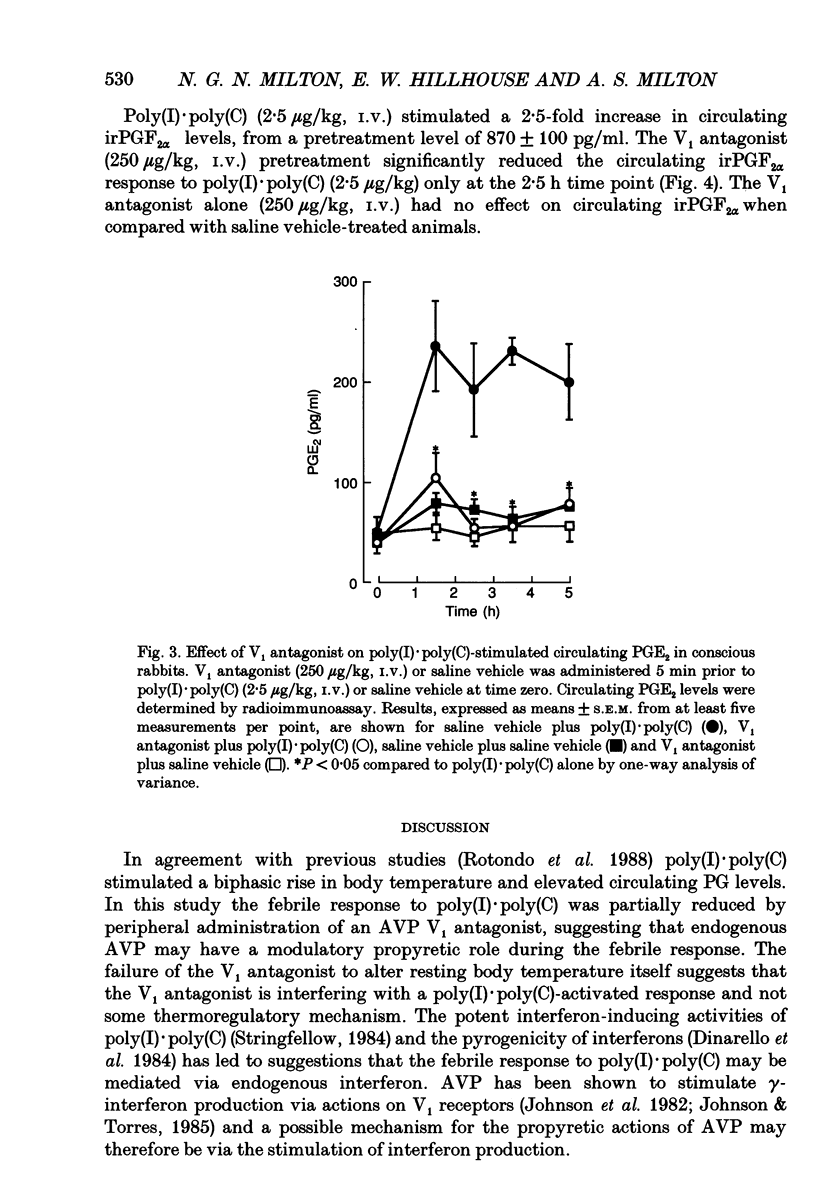
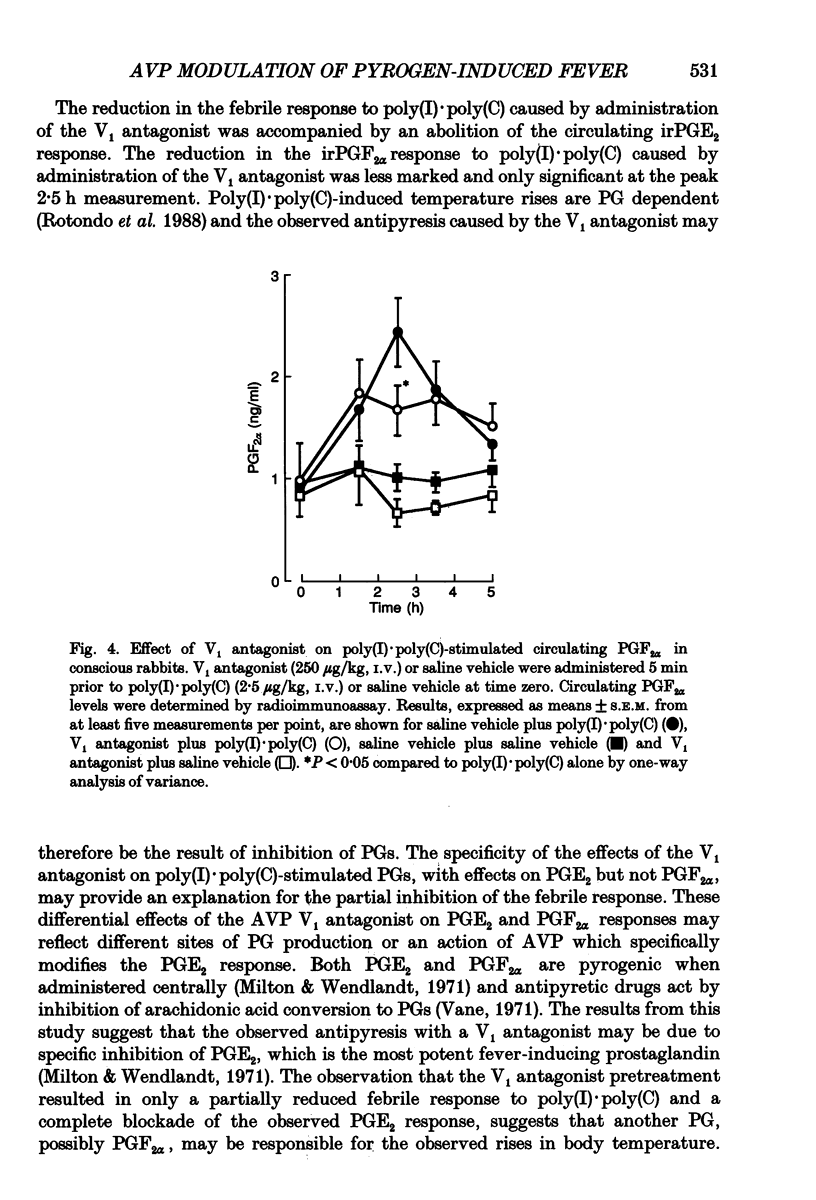
1. The actions of peripheral arginine vasopressin (AVP) on the febrile responses of conscious rabbits induced by peripherally administered polyinosinic:polycytidylic acid (poly(I).poly(C)) have been studied using an AVP V1 receptor antagonist ([deamino-Pen1, O-Me-Tyr2, Arg8]-vasopressin). 2. Temperature responses were monitored continuously using rectal thermistor probes. Test substances were administered intravenously (i.v.). Blood samples were taken at timed intervals from a marginal ear vein and plasma PGE2 and PGF2 alpha levels determined by radioimmunoassay. 3. Poly(I).poly(C) (2.5 micrograms/kg) stimulated a reproducible biphasic rise in body temperature with a lag phase of 45-60 min and peaks at 90 and 225 min. The febrile response was accompanied by a 5-fold rise in circulating immunoreactive (ir) PGE2, which peaked after 90 min and remained elevated up to 300 min. Poly(I).poly(C) also stimulated a 2.5-fold rise in circulating irPGF2 alpha, which peaked after 150 min and was followed by a return to basal levels after 300 min. 4. The overall magnitude of the febrile response to poly(I).poly(C) (2.5 micrograms/kg, i.v.) was significantly antagonized by the AVP V1 receptor antagonist (250 micrograms/kg, i.v.) administered 5 min prior to the pyrogen. 5. The irPGE2 response to poly(I).poly(C) (2.5 micrograms/kg, i.v.) was significantly antagonized by the AVP V1 receptor antagonist (250 micrograms/kg, i.v.) administered 5 min prior to the pyrogen. The irPGF2 alpha response was only reduced at the peak 150 min time point measurement. 6. In conclusion, these results show a modulatory role for a peripherally administered AVP V1 antagonist in the febrile responses to poly(I).poly(C), suggesting a possible propyretic role for endogenous peripheral AVP. This modulatory role appears to be mediated via actions on prostaglandin E2.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardini G. L., Richards D. B., Lipton J. M. Antipyretic effect of centrally administered CRF. Peptides. 1984 Jan-Feb;5(1):57–59. doi: 10.1016/0196-9781(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Cooper K. E., Cranston W. I., Honour A. J. Observations on the site & mode of action of pyrogens in the rabbit brain. J Physiol. 1967 Jul;191(2):325–337. doi: 10.1113/jphysiol.1967.sp008253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E., Kasting N. W., Lederis K., Veale W. L. Evidence supporting a role for endogenous vasopressin in natural suppression of fever in the sheep. J Physiol. 1979 Oct;295:33–45. doi: 10.1113/jphysiol.1979.sp012953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E., Naylor A. M., Veale W. L. Evidence supporting a role for endogenous vasopressin in fever suppression in the rat. J Physiol. 1987 Jun;387:163–172. doi: 10.1113/jphysiol.1987.sp016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascombe M. J., Milton A. S. Study on the possible entry of bacterial endotoxin and prostaglandin E2 into the central nervous system from the blood. Br J Pharmacol. 1979 Aug;66(4):565–572. doi: 10.1111/j.1476-5381.1979.tb13695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Bernheim H. A., Duff G. W., Le H. V., Nagabhushan T. L., Hamilton N. C., Coceani F. Mechanisms of fever induced by recombinant human interferon. J Clin Invest. 1984 Sep;74(3):906–913. doi: 10.1172/JCI111508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Farrar W. L., Torres B. A. Vasopressin replacement of interleukin 2 requirement in gamma interferon production: lymphokine activity of a neuroendocrine hormone. J Immunol. 1982 Sep;129(3):983–986. [PubMed] [Google Scholar]
- Kasting N. W., Carr D. B., Martin J. B., Blume H., Bergland R. Changes in cerebrospinal fluid and plasma vasopressin in the febrile sheep. Can J Physiol Pharmacol. 1983 Apr;61(4):427–431. doi: 10.1139/y83-064. [DOI] [PubMed] [Google Scholar]
- Kluger M. J. Fever: role of pyrogens and cryogens. Physiol Rev. 1991 Jan;71(1):93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács G. L., De Wied D. Hormonally active arginine-vasopressin suppresses endotoxin-induced fever in rats: lack of effect of oxytocin and a behaviorally active vasopressin fragment. Neuroendocrinology. 1983 Oct;37(4):258–261. doi: 10.1159/000123554. [DOI] [PubMed] [Google Scholar]
- Kozak W., Milton A. S., Abul H., Davidson J., Rotondo D. Lipopolysaccharide, muramyl dipeptide and polyinosinic: polycytidylic acid induce the accumulation of inositol phosphates in blood monocytes and lymphocytes. Cell Signal. 1989;1(4):345–356. doi: 10.1016/0898-6568(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Malkinson T. J., Bridges T. E., Lederis K., Veale W. L. Perfusion of the septum of the rabbit with vasopressin antiserum enhances endotoxin fever. Peptides. 1987 Mar-Apr;8(2):385–389. doi: 10.1016/0196-9781(87)90115-x. [DOI] [PubMed] [Google Scholar]
- Mens W. B., Witter A., van Wimersma Greidanus T. B. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983 Feb 28;262(1):143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Milton A. S., Wendlandt S. Effects on body temperature of prostaglandins of the A, E and F series on injection into the third ventricle of unanaesthetized cats and rabbits. J Physiol. 1971 Oct;218(2):325–336. doi: 10.1113/jphysiol.1971.sp009620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton N. G., Hillhouse E. W., Milton A. S. A possible role for endogenous peripheral corticotrophin-releasing factor-41 in the febrile response of conscious rabbits. J Physiol. 1993 Jun;465:415–425. doi: 10.1113/jphysiol.1993.sp019684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton N. G., Hillhouse E. W., Milton A. S. Activation of the hypothalamo-pituitary-adrenocortical axis in the conscious rabbit by the pyrogen polyinosinic:polycytidylic acid is dependent on corticotrophin-releasing factor-41. J Endocrinol. 1992 Oct;135(1):69–75. doi: 10.1677/joe.0.1350069. [DOI] [PubMed] [Google Scholar]
- Naylor A. M., Ruwe W. D., Veale W. L. Antipyretic action of centrally administered arginine vasopressin but not oxytocin in the cat. Brain Res. 1986 Oct 15;385(1):156–160. doi: 10.1016/0006-8993(86)91558-1. [DOI] [PubMed] [Google Scholar]
- Opp M., Obál F., Jr, Krueger J. M. Corticotropin-releasing factor attenuates interleukin 1-induced sleep and fever in rabbits. Am J Physiol. 1989 Sep;257(3 Pt 2):R528–R535. doi: 10.1152/ajpregu.1989.257.3.R528. [DOI] [PubMed] [Google Scholar]
- Rotondo D., Abul H. T., Milton A. S., Davidson J. Pyrogenic immunomodulators increase the level of prostaglandin E2 in the blood simultaneously with the onset of fever. Eur J Pharmacol. 1988 Sep 13;154(2):145–152. doi: 10.1016/0014-2999(88)90091-x. [DOI] [PubMed] [Google Scholar]
- Veale W. L., Cooper K. E., Ruwe W. D. Vasopressin: its role in antipyresis and febrile convulsion. Brain Res Bull. 1984 Feb;12(2):161–165. doi: 10.1016/0361-9230(84)90184-9. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M., Hertting G., Knepel W. Adrenocorticotropin and beta-endorphin release from rat adenohypophysis in vitro: inhibition by prostaglandin E2 formed locally in response to vasopressin and corticotropin-releasing factor. Endocrinology. 1984 Sep;115(3):895–903. doi: 10.1210/endo-115-3-895. [DOI] [PubMed] [Google Scholar]
- Wang B. C., Share L., Crofton J. T., Kimura T. Changes in vasopressin concentration in plasma and cerebrospinal fluid in response to hemorrhage in anesthetized dogs. Neuroendocrinology. 1981 Aug;33(2):61–66. doi: 10.1159/000123203. [DOI] [PubMed] [Google Scholar]
- Wuthrich R. P., Vallotton M. B. Prostaglandin E2 and cyclic AMP response to vasopressin in renal medullary tubular cells. Am J Physiol. 1986 Sep;251(3 Pt 2):F499–F505. doi: 10.1152/ajprenal.1986.251.3.F499. [DOI] [PubMed] [Google Scholar]


