Abstract
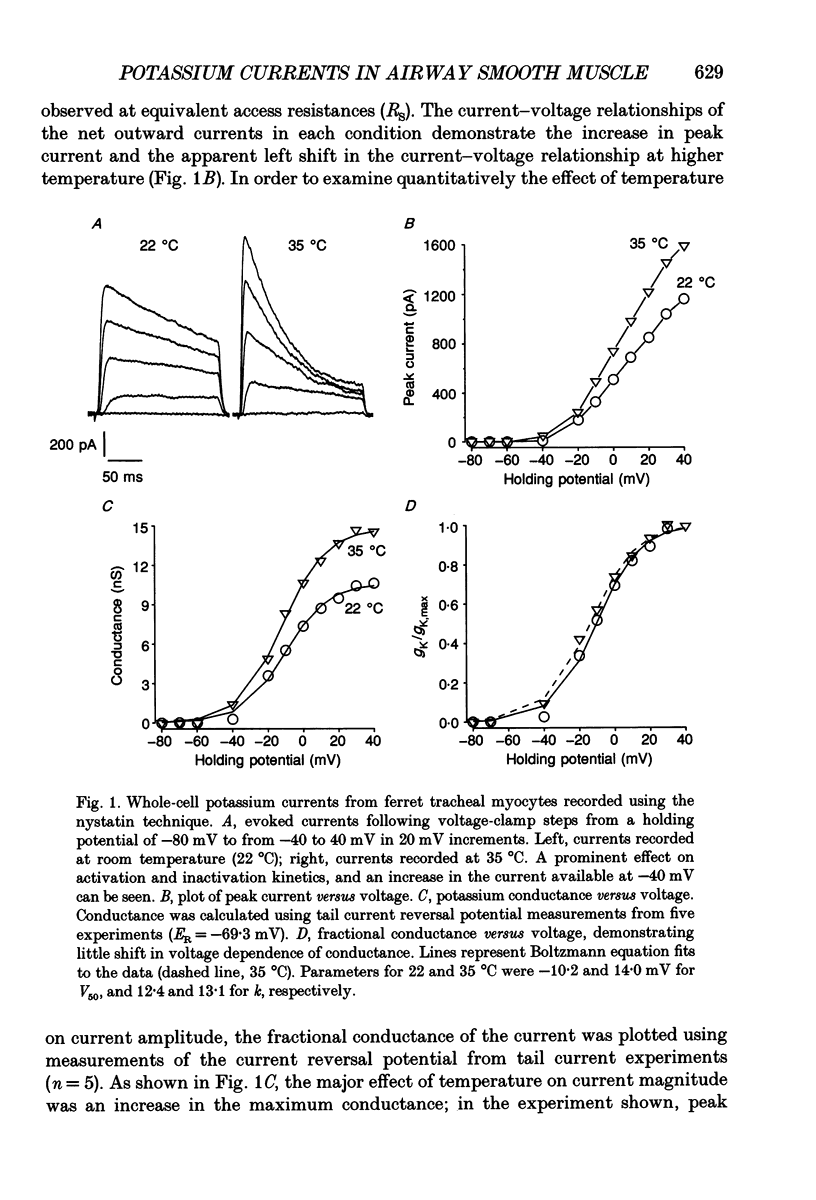
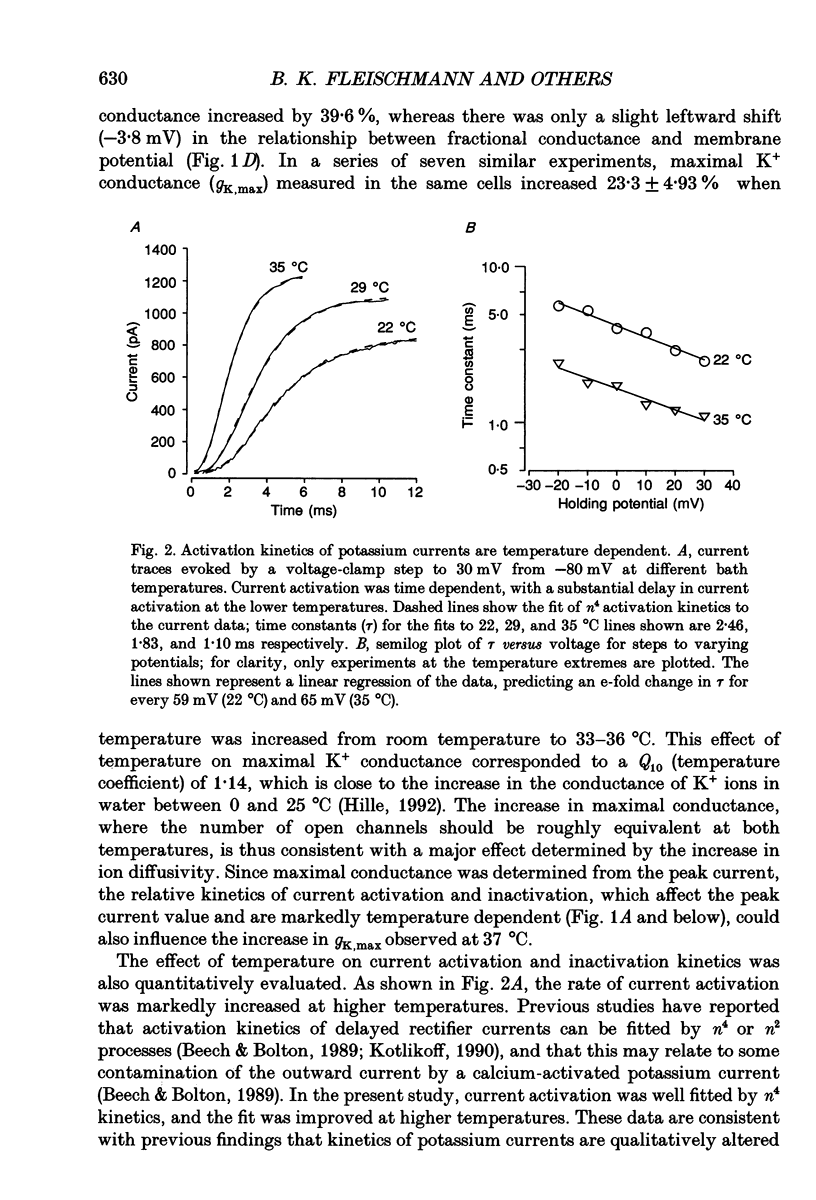
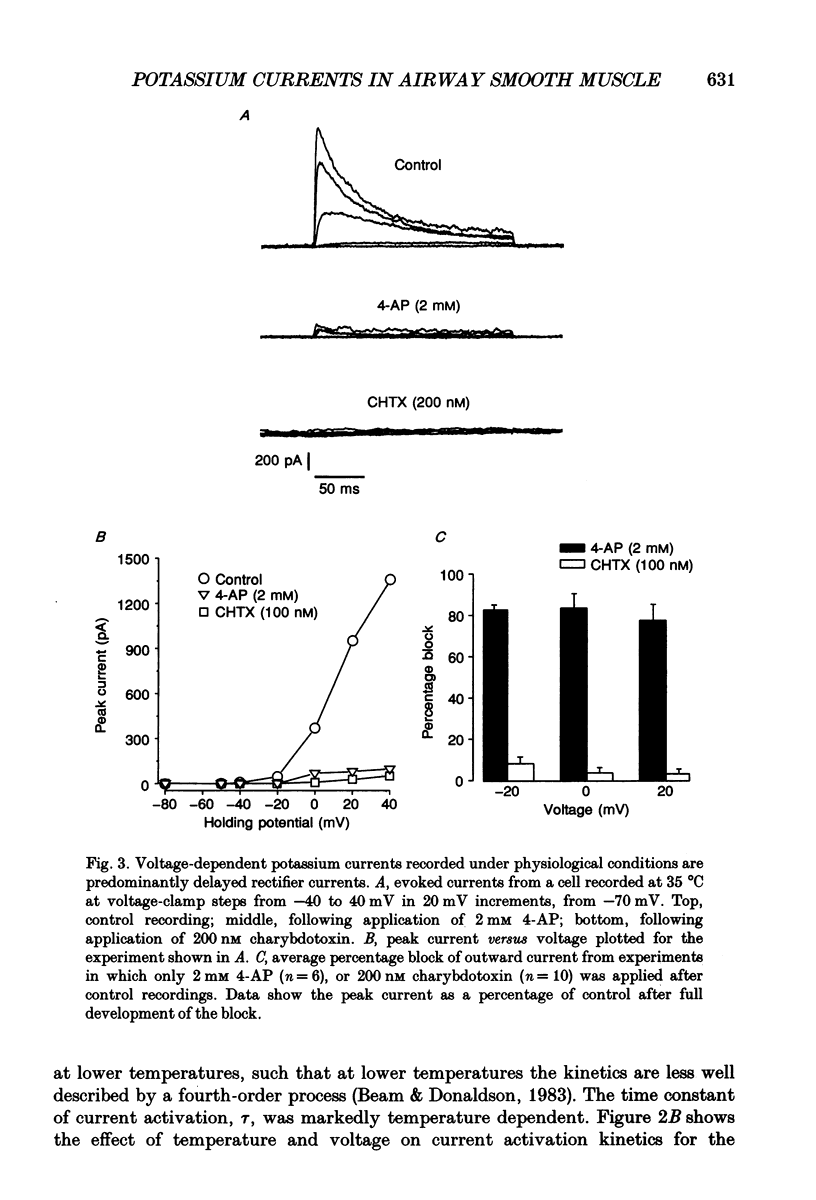
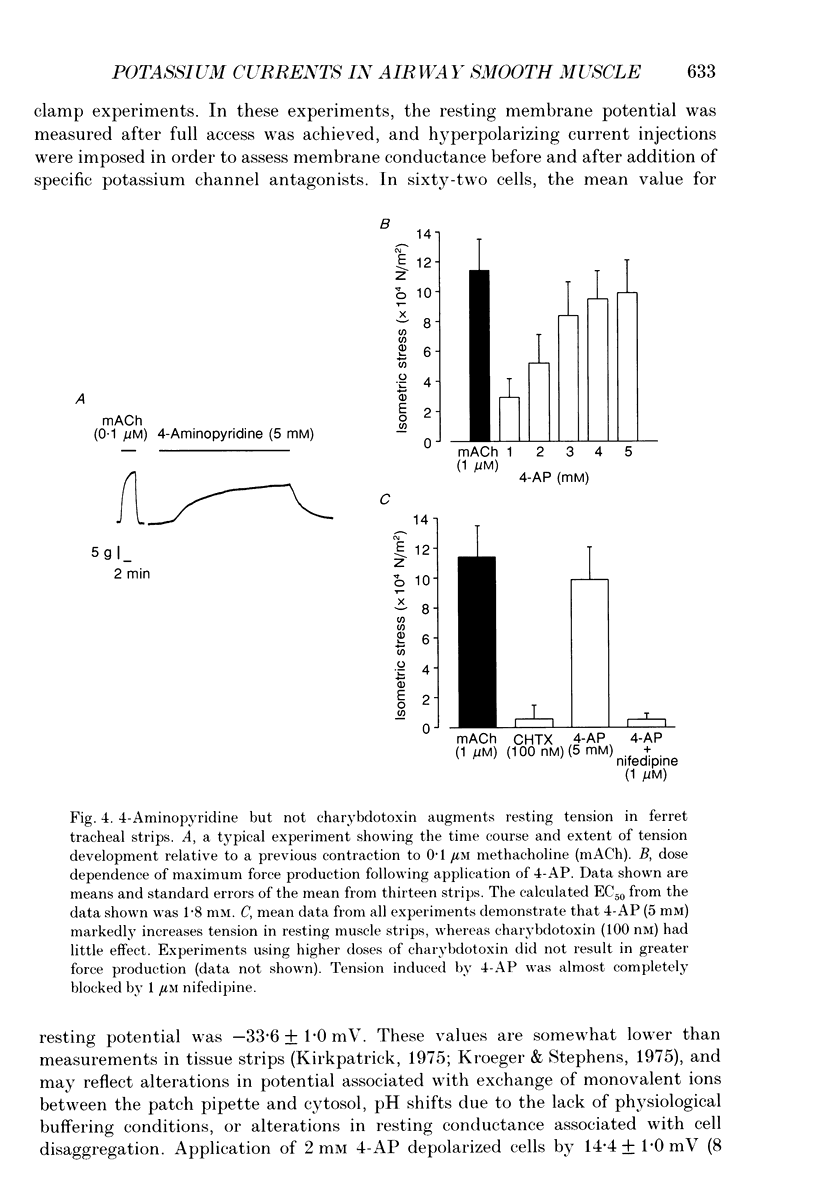
1. In order to determine the physiological role of specific potassium currents in airway smooth muscle, potassium currents were measured in freshly dissociated ferret trachealis cells using the nystatin-permeabilized, whole-cell method, at 35 degrees C. 2. The magnitude of the outward currents was markedly increased as bath temperature was increased from 22 to 35 degrees C. This increase was primarily due to the increase in maximum potassium conductance (gK,max), although there was also a small leftward shift in the relationship between gK and voltage at higher temperatures. The maximum conductance and the kinetics of current activation and inactivation were also temperature dependent. At 35 degrees C, gating of the current was steeply voltage dependent between -40 and 0 mV. Current activation was well fitted by fourth-order kinetics; the mean time constants of activation (30 mV clamp step) were 1.09 +/- 0.17 and 1.96 +/- 0.27 ms at 35 and 22 degrees C, respectively. 3. Outward currents using the nystatin method were qualitatively similar to delayed rectifier currents recorded in dialysed cells with high calcium buffering capacity solutions. 4-Aminopyridine (4-AP; 2 mM), a specific blocker of delayed rectifier potassium channels in this tissue, inhibited over 80% of the outward current evoked by voltage-clamp steps to between -10 and +20 mV (n = 6). Less than 5% of the outward current was blocked over the same voltage range by charybdotoxin (100 nM; n = 15), a specific antagonist of large-conductance, calcium-activated potassium channels in this tissue. 4. The degree to which delayed rectifier and calcium-activated potassium conductances control resting membrane potential was examined in current-clamp experiments. The resting membrane potential of current clamped cells was -33.6 +/- 1.0 mV (n = 62). Application of 4-AP (2 mM) resulted in a 14.4 +/- 1.0 mV depolarization (n = 8) and an increase in input resistance. Charybdotoxin (100 nM) had no effect on resting membrane potential (n = 6). 5. Force measurements were made in isolated strips of trachealis muscle to determine the effect of pharmacological blockade of individual potassium conductances on resting tone. In the presence of tetrodotoxin (1 microM) and atropine (1 microM), 4-AP increased baseline tension in a dose-dependent manner, with an EC50 of 1.8 mM (n = 13); application of 5 mM 4-AP increased tone to 86.8 +/- 8.1% of that produced by 1 microM methacholine, and this tone was almost completely inhibited by nifedipine (1 microM).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed F., Foster R. W., Small R. C. Some effects of nifedipine in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Apr;84(4):861–869. doi: 10.1111/j.1476-5381.1985.tb17380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Donaldson P. L. A quantitative study of potassium channel kinetics in rat skeletal muscle from 1 to 37 degrees C. J Gen Physiol. 1983 Apr;81(4):485–512. doi: 10.1085/jgp.81.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. L., Elliott K. R., Foster R. W., Green K. A., Murray M. A., Small R. C. Mechanical, biochemical and electrophysiological studies of RP 49356 and cromakalim in guinea-pig and bovine trachealis muscle. Pulm Pharmacol. 1991;4(2):91–98. doi: 10.1016/0952-0600(91)90058-b. [DOI] [PubMed] [Google Scholar]
- Boyle J. P., Tomasic M., Kotlikoff M. I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J Physiol. 1992 Feb;447:329–350. doi: 10.1113/jphysiol.1992.sp019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F. Electromechanical coupling in canine trachealis muscle: acetylcholine contractions. Am J Physiol. 1979 Mar;236(3):C177–C184. doi: 10.1152/ajpcell.1979.236.3.C177. [DOI] [PubMed] [Google Scholar]
- Green K. A., Foster R. W., Small R. C. A patch-clamp study of K(+)-channel activity in bovine isolated tracheal smooth muscle cells. Br J Pharmacol. 1991 Apr;102(4):871–878. doi: 10.1111/j.1476-5381.1991.tb12269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973 Sep;33(3):275–283. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- Hisada T., Kurachi Y., Sugimoto T. Properties of membrane currents in isolated smooth muscle cells from guinea-pig trachea. Pflugers Arch. 1990 Apr;416(1-2):151–161. doi: 10.1007/BF00370237. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J Physiol. 1981 Jul;316:33–46. doi: 10.1113/jphysiol.1981.sp013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Janssen L. J., Daniel E. E. Depolarizing agents induce oscillations in canine bronchial smooth muscle membrane potential: possible mechanisms. J Pharmacol Exp Ther. 1991 Oct;259(1):110–117. [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan M. S., Jager L. P., Daniel E. E., Garfield R. E. Effects of 4-aminopyridine and tetraethylammonium chloride on the electrical activity and cable properties of canine tracheal smooth muscle. J Pharmacol Exp Ther. 1983 Dec;227(3):706–715. [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff M. I. Calcium currents in isolated canine airway smooth muscle cells. Am J Physiol. 1988 Jun;254(6 Pt 1):C793–C801. doi: 10.1152/ajpcell.1988.254.6.C793. [DOI] [PubMed] [Google Scholar]
- Kotlikoff M. I. Potassium currents in canine airway smooth muscle cells. Am J Physiol. 1990 Dec;259(6 Pt 1):L384–L395. doi: 10.1152/ajplung.1990.259.6.L384. [DOI] [PubMed] [Google Scholar]
- Kroeger E. A., Stephens N. L. Effect of tetraethylammonium on tonic airway smooth muscle: initiation of phasic electrical activity. Am J Physiol. 1975 Feb;228(2):633–636. doi: 10.1152/ajplegacy.1975.228.2.633. [DOI] [PubMed] [Google Scholar]
- Kume H., Kotlikoff M. I. Muscarinic inhibition of single KCa channels in smooth muscle cells by a pertussis-sensitive G protein. Am J Physiol. 1991 Dec;261(6 Pt 1):C1204–C1209. doi: 10.1152/ajpcell.1991.261.6.C1204. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Nelson M. T., Huang Y., Standen N. B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991 Mar;260(3 Pt 2):H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K., Imaizumi Y., Kojima T., Kawai T., Watanabe M. Effects of tetraethylammonium and 4-aminopyridine on outward currents and excitability in canine tracheal smooth muscle cells. Br J Pharmacol. 1990 Jul;100(3):507–515. doi: 10.1111/j.1476-5381.1990.tb15838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. A., Berry J. L., Cook S. J., Foster R. W., Green K. A., Small R. C. Guinea-pig isolated trachealis: the effects of charybdotoxin on mechanical activity, membrane potential changes and the activity of plasmalemmal K(+)-channels. Br J Pharmacol. 1991 Jul;103(3):1814–1818. doi: 10.1111/j.1476-5381.1991.tb09868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Saunders H. H., Farley J. M. Pharmacological properties of potassium currents in swine tracheal smooth muscle. J Pharmacol Exp Ther. 1992 Mar;260(3):1038–1044. [PubMed] [Google Scholar]
- Savaria D., Lanoue C., Cadieux A., Rousseau E. Large conducting potassium channel reconstituted from airway smooth muscle. Am J Physiol. 1992 Mar;262(3 Pt 1):L327–L336. doi: 10.1152/ajplung.1992.262.3.L327. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Passive properties of the membrane of single freshly isolated smooth muscle cells. Am J Physiol. 1980 Nov;239(5):C153–C161. doi: 10.1152/ajpcell.1980.239.5.C153. [DOI] [PubMed] [Google Scholar]
- Small R. C. Electrical slow waves and tone of guinea-pig isolated trachealis muscle: effects of drugs and temperature changes. Br J Pharmacol. 1982 Sep;77(1):45–54. doi: 10.1111/j.1476-5381.1982.tb09267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Phillips M., Miller C. Purification of charybdotoxin, a specific inhibitor of the high-conductance Ca2+-activated K+ channel. J Biol Chem. 1986 Nov 5;261(31):14607–14613. [PubMed] [Google Scholar]
- Suarez-Kurtz G., Garcia M. L., Kaczorowski G. J. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther. 1991 Oct;259(1):439–443. [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Ca++-activated K+ channels in vertebrate smooth muscle cells. Cell Calcium. 1983 Dec;4(5-6):321–330. doi: 10.1016/0143-4160(83)90011-8. [DOI] [PubMed] [Google Scholar]


