Abstract
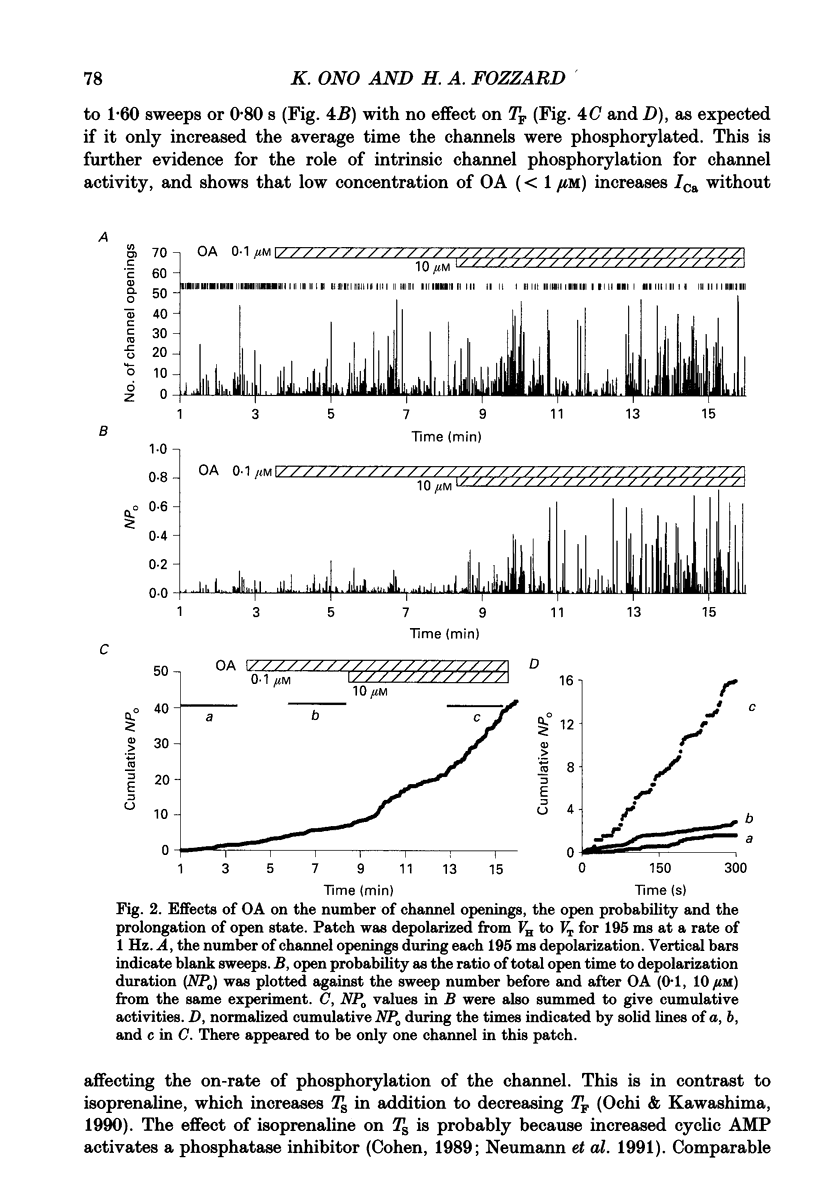
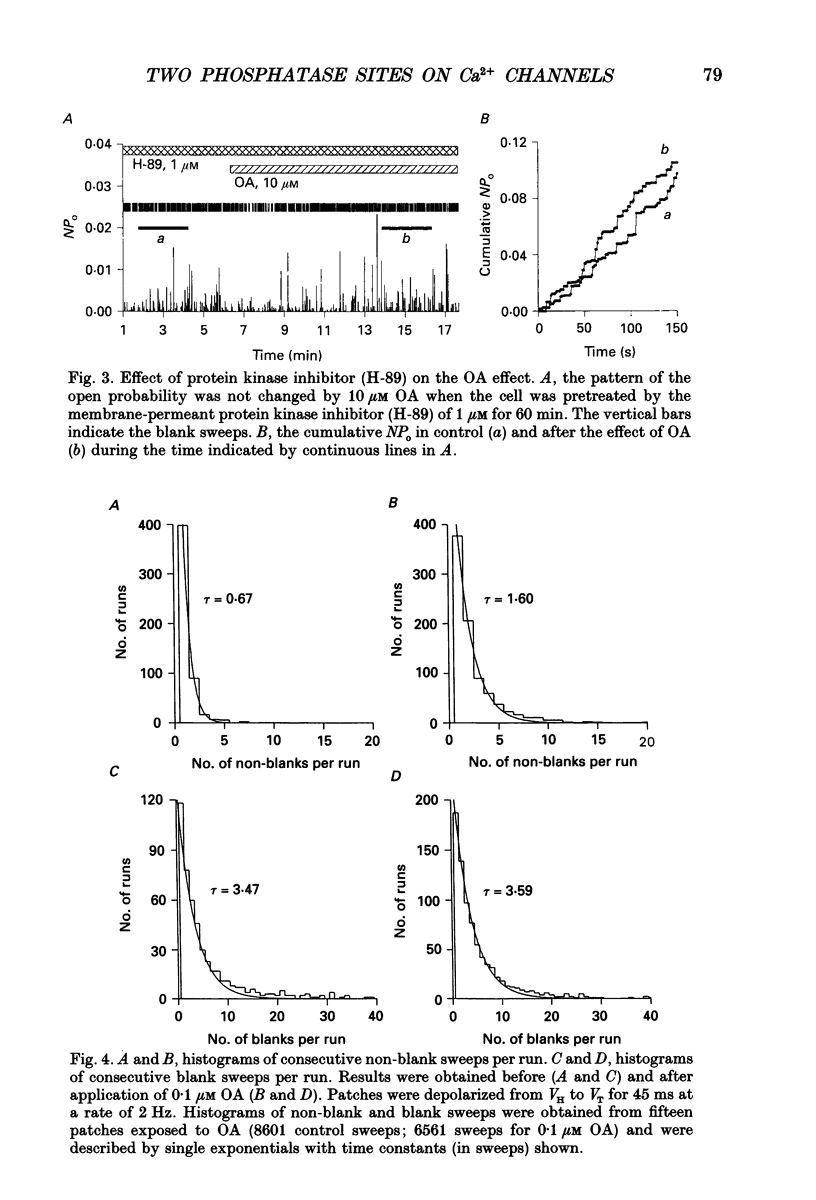
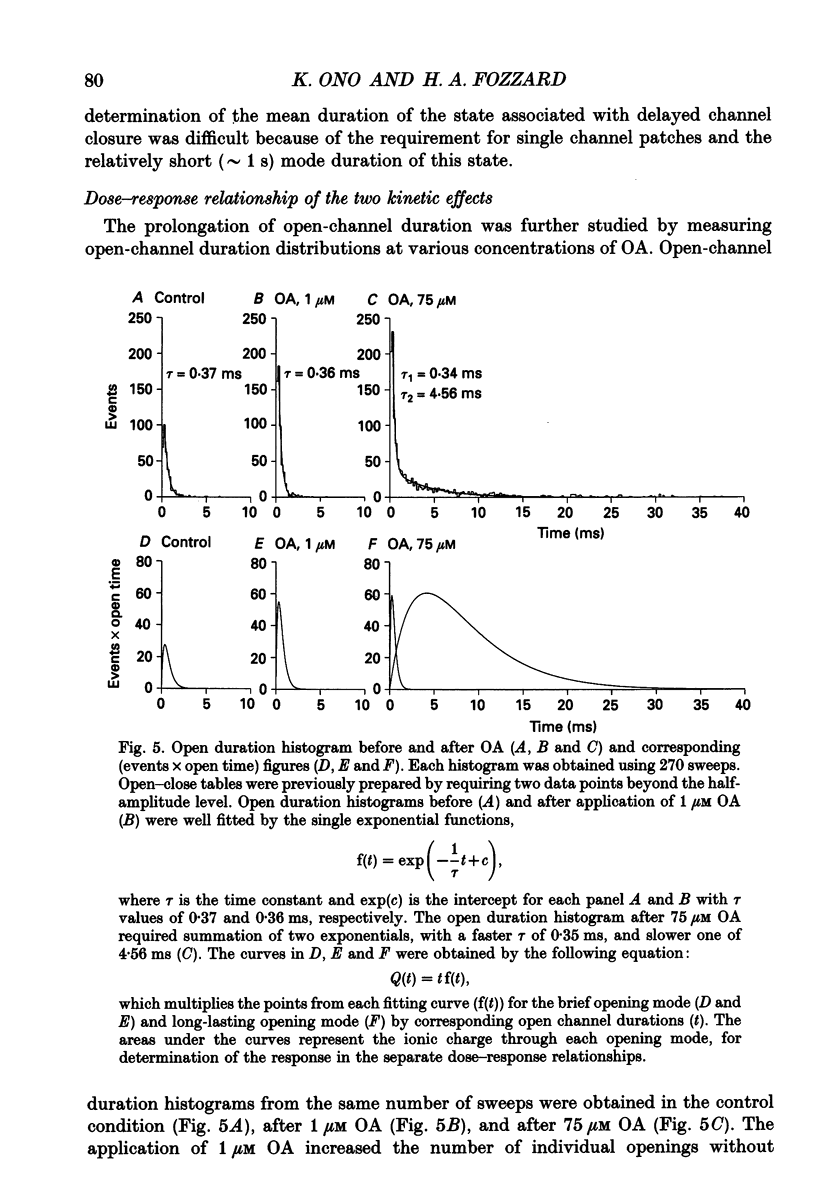
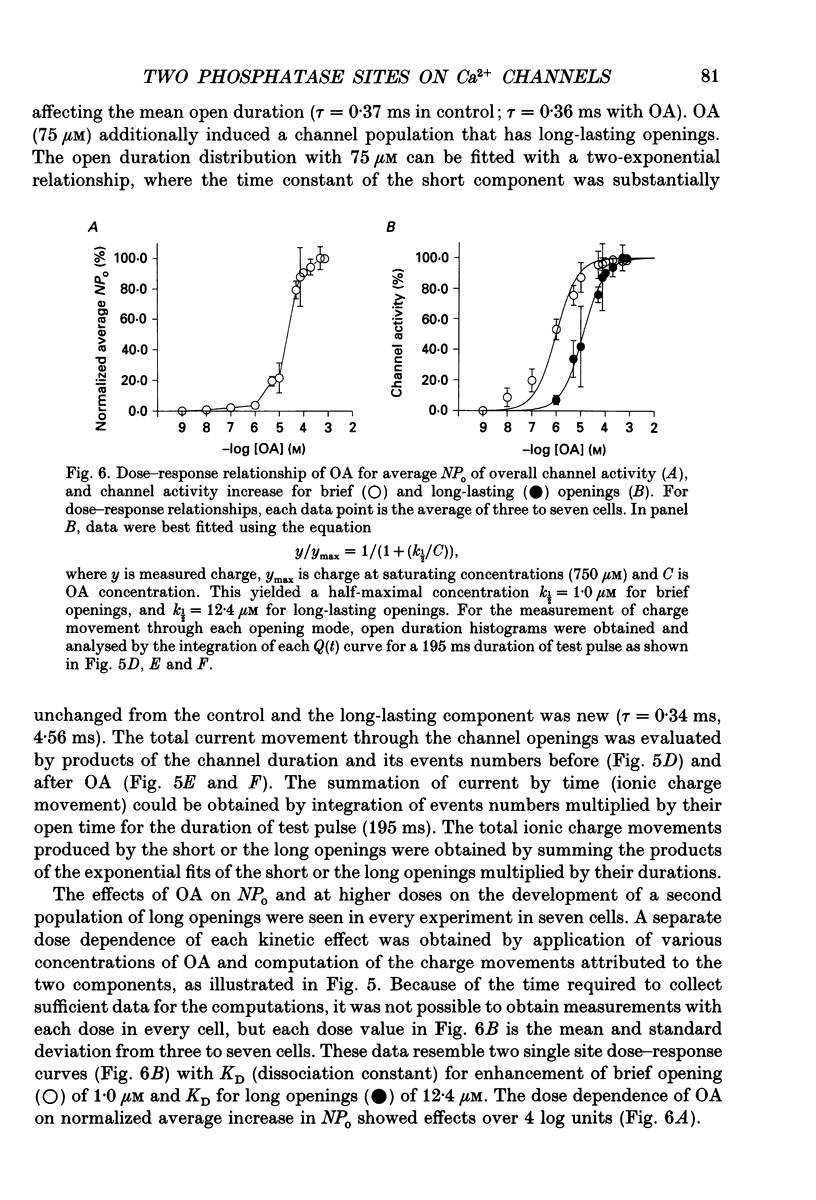
1. Changes in dihydropyridine-sensitive (L-type) Ca2+ channel kinetics were studied after prolongation of intrinsic phosphorylated time by the phosphatase inhibitor okadaic acid (OA) in cell-attached patches made from single isolated rabbit ventricular myocytes, using barium as the charge carrier. 2. At low concentrations (0.001-1 microM), OA decreased the number of sweeps without openings, while open duration was not changed. However, when cells were pretreated by a membrane-permeant cyclic AMP, 0.1 microM OA induced long-lasting channel openings as well. 3. At high concentrations (10-750 microM), OA additionally induced long-lasting openings, resulting in open time distributions that were best fitted by two exponentials. 4. The durations of an available state (TS) and an unavailable state (TF) were estimated by the numbers of non-blank sweeps per run and blank sweeps per run by applying repetitive 45 ms steps at 2 Hz to 0 mV from holding potentials of -80 mV. TS was well fitted by an exponential curve, of which the time constant was increased from 0.67 to 1.60 sweeps by 0.1 microM OA, while TF was 0.347 sweeps and remained unchanged. 5. OA activated brief openings and long-lasting, wide openings in a concentration-dependent manner. Namely, we find different dose-response relationships for the two kinetic effects of increased opening probability (mode 1) and prolongation of opening (mode 2). This behaviour suggests that there are at least two modulatory phosphorylation sites that are dephosphorylated by different phosphatases.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2528–2532. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W., Mieskes G., Söling H. D. Regulation of the cardiac calcium channel by protein phosphatases. Eur J Biochem. 1987 Jun 1;165(2):261–266. doi: 10.1111/j.1432-1033.1987.tb11436.x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Barhanin J., Schmid A., Vandaele S., Ptasienski J., O'Callahan C., Cooper C., Lazdunski M. Photoaffinity labelling and phosphorylation of a 165 kilodalton peptide associated with dihydropyridine and phenylalkylamine-sensitive calcium channels. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1137–1145. doi: 10.1016/s0006-291x(87)80188-2. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Mundiña-Weilenmann C., Ma J., Ríos E., Hosey M. M. Dihydropyridine-sensitive skeletal muscle Ca channels in polarized planar bilayers. 2. Effects of phosphorylation by cAMP-dependent protein kinase. Biophys J. 1991 Oct;60(4):902–909. doi: 10.1016/S0006-3495(91)82124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastainczyk W., Röhrkasten A., Sieber M., Rudolph C., Schächtele C., Marmè D., Hofmann F. Phosphorylation of the purified receptor for calcium channel blockers by cAMP kinase and protein kinase C. Eur J Biochem. 1987 Nov 16;169(1):137–142. doi: 10.1111/j.1432-1033.1987.tb13590.x. [DOI] [PubMed] [Google Scholar]
- Neumann J., Gupta R. C., Schmitz W., Scholz H., Nairn A. C., Watanabe A. M. Evidence for isoproterenol-induced phosphorylation of phosphatase inhibitor-1 in the intact heart. Circ Res. 1991 Dec;69(6):1450–1457. doi: 10.1161/01.res.69.6.1450. [DOI] [PubMed] [Google Scholar]
- O'Callahan C. M., Hosey M. M. Multiple phosphorylation sites in the 165-kilodalton peptide associated with dihydropyridine-sensitive calcium channels. Biochemistry. 1988 Aug 9;27(16):6071–6077. doi: 10.1021/bi00416a036. [DOI] [PubMed] [Google Scholar]
- Ochi R., Kawashima Y. Modulation of slow gating process of calcium channels by isoprenaline in guinea-pig ventricular cells. J Physiol. 1990 May;424:187–204. doi: 10.1113/jphysiol.1990.sp018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Phosphorylation restores activity of L-type calcium channels after rundown in inside-out patches from rabbit cardiac cells. J Physiol. 1992 Aug;454:673–688. doi: 10.1113/jphysiol.1992.sp019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]


