Abstract
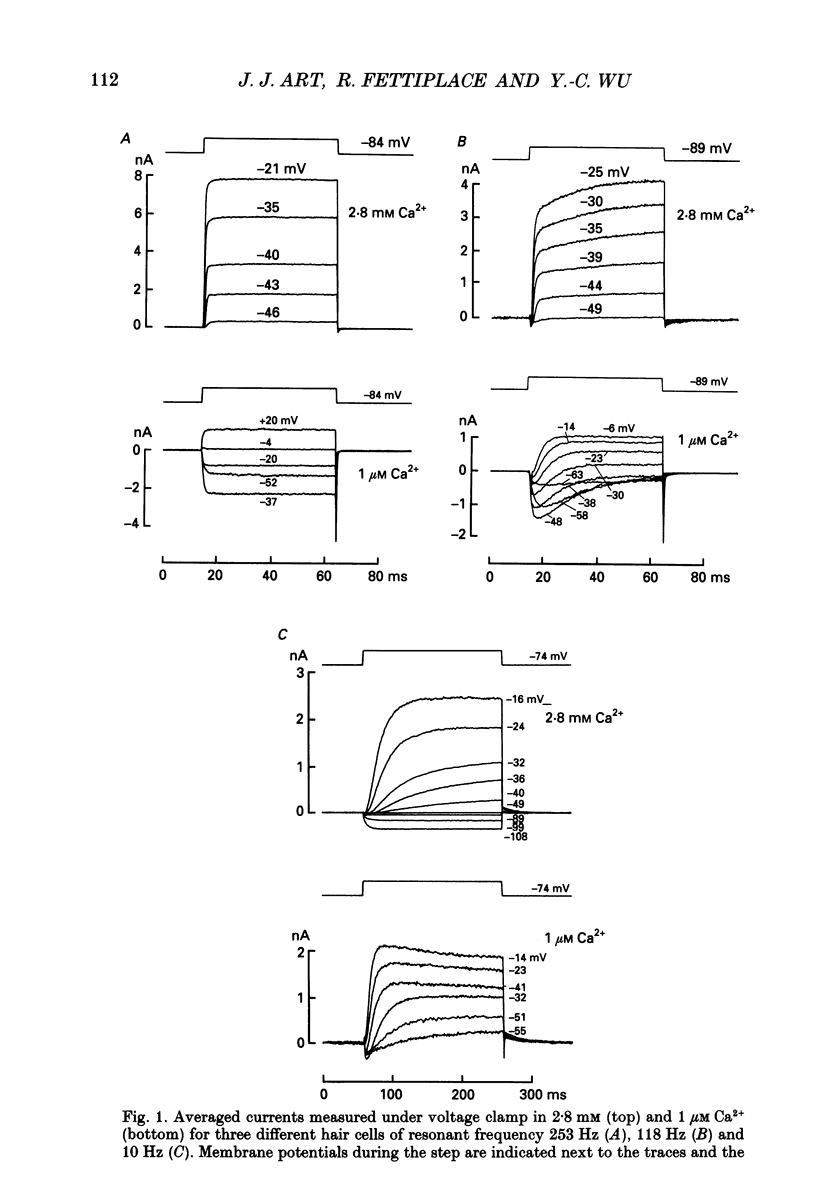
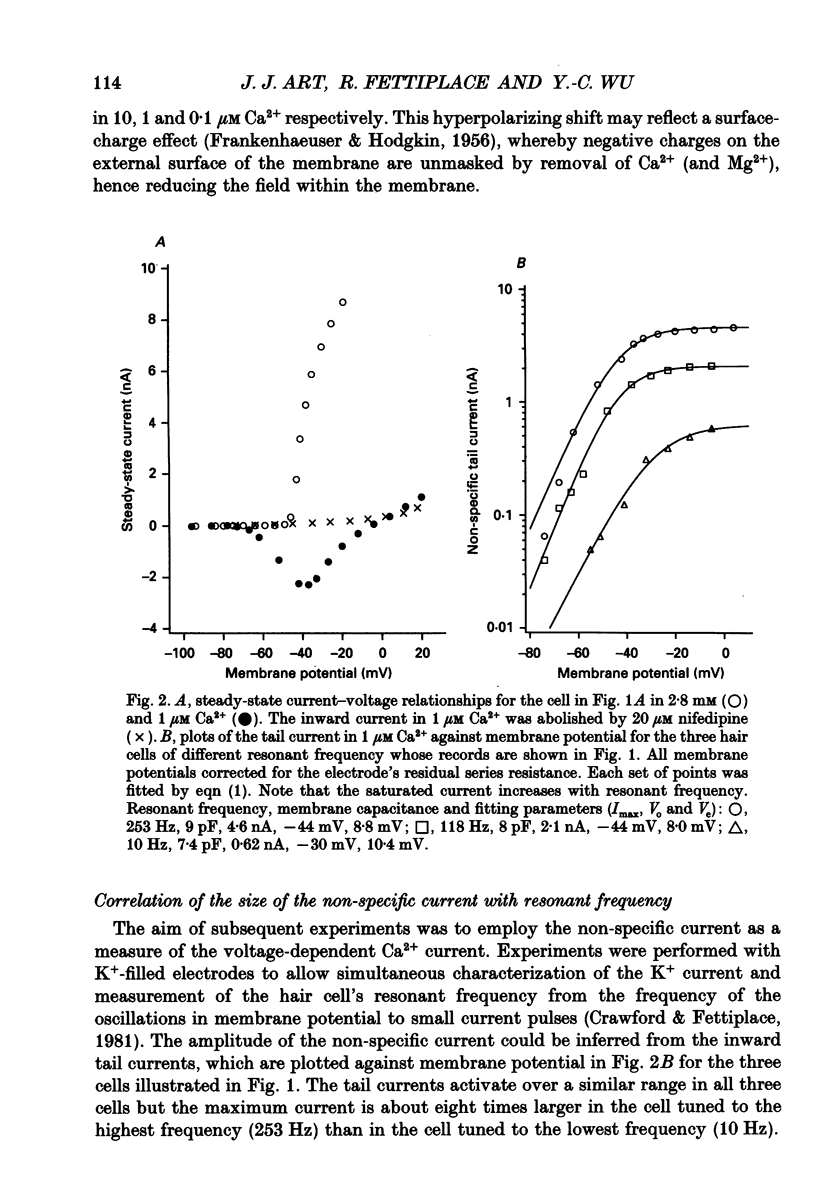
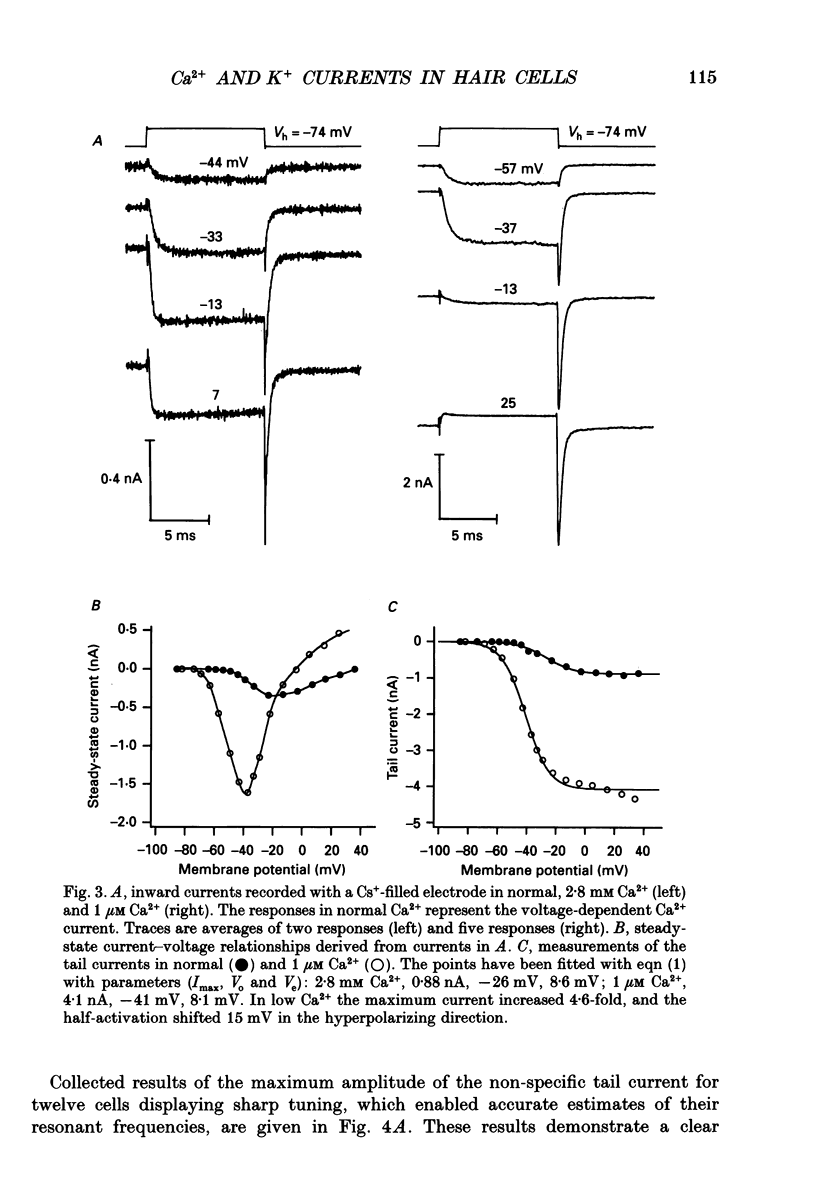
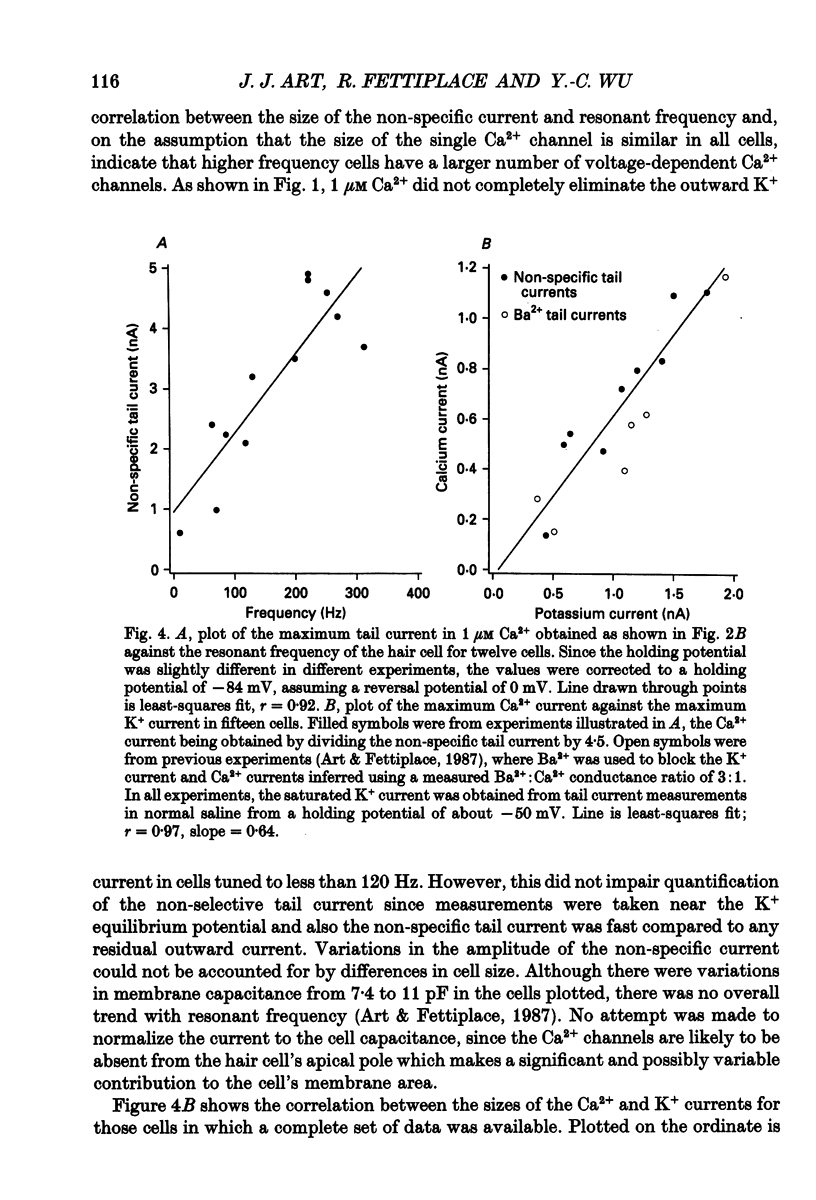
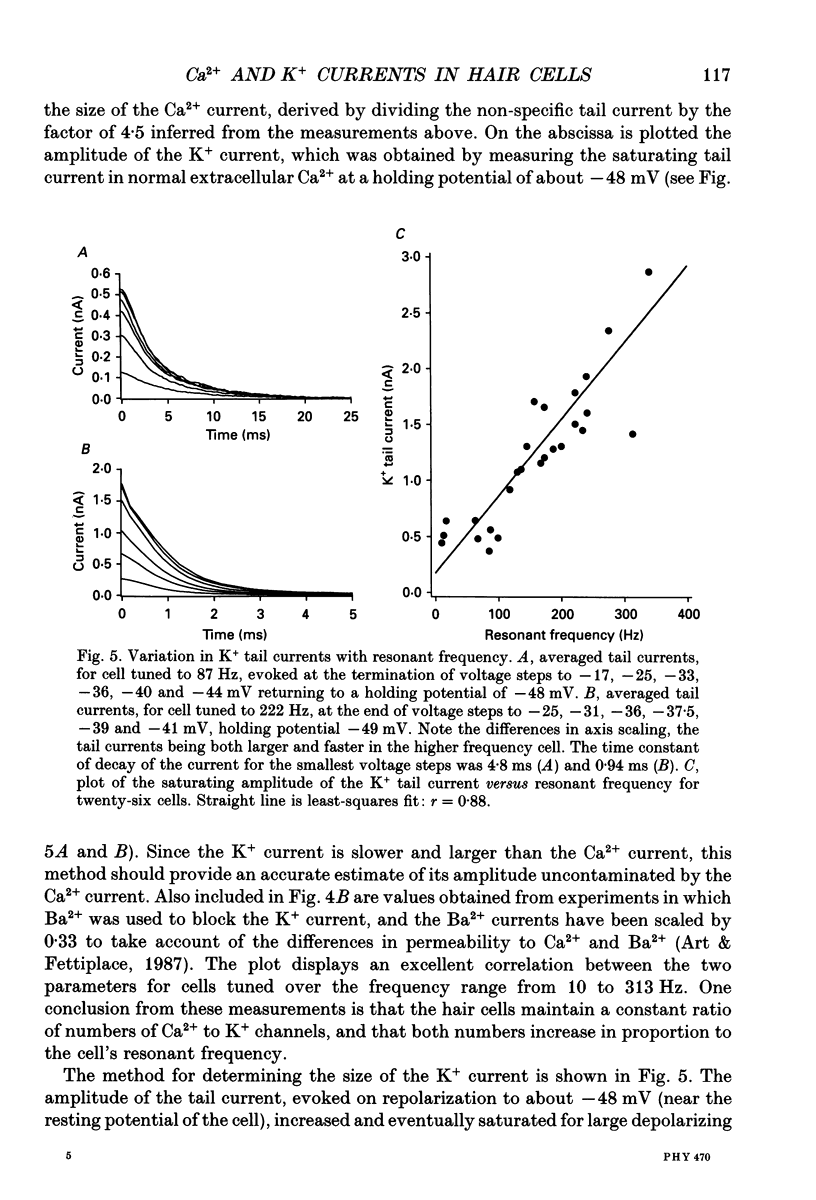
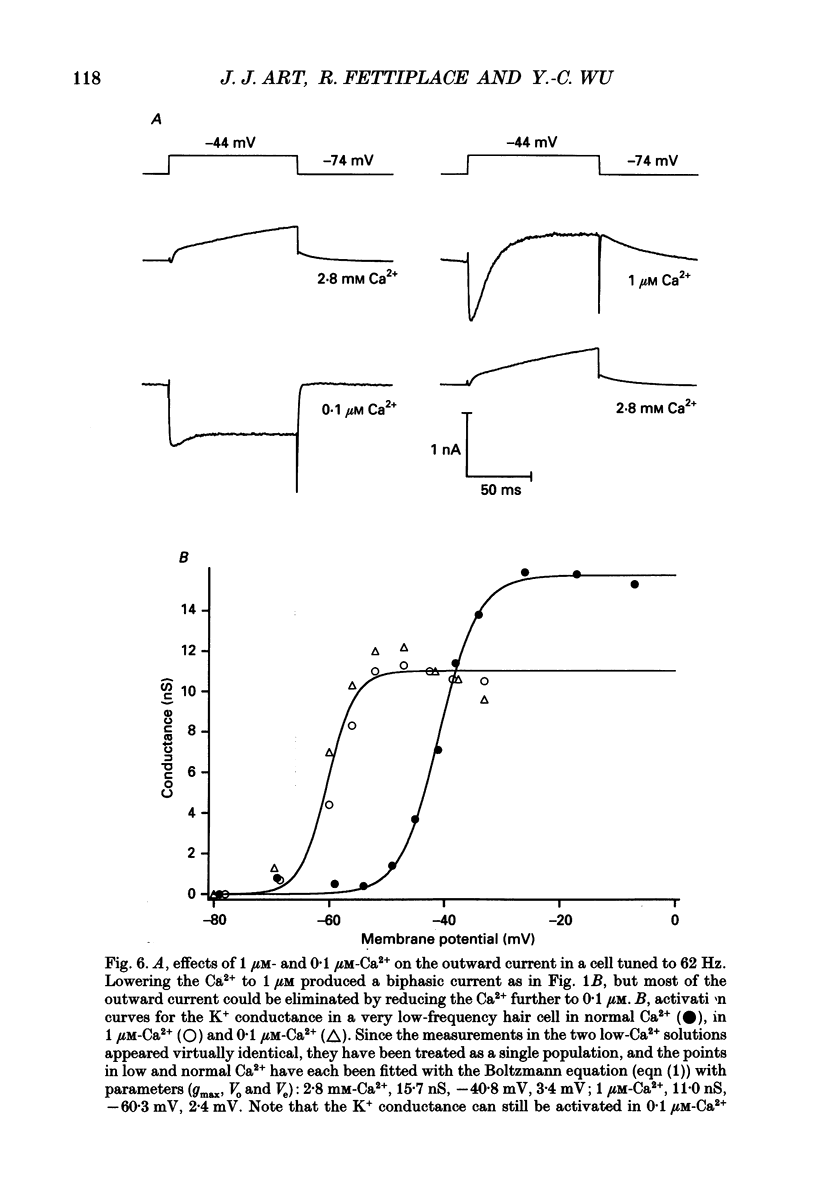
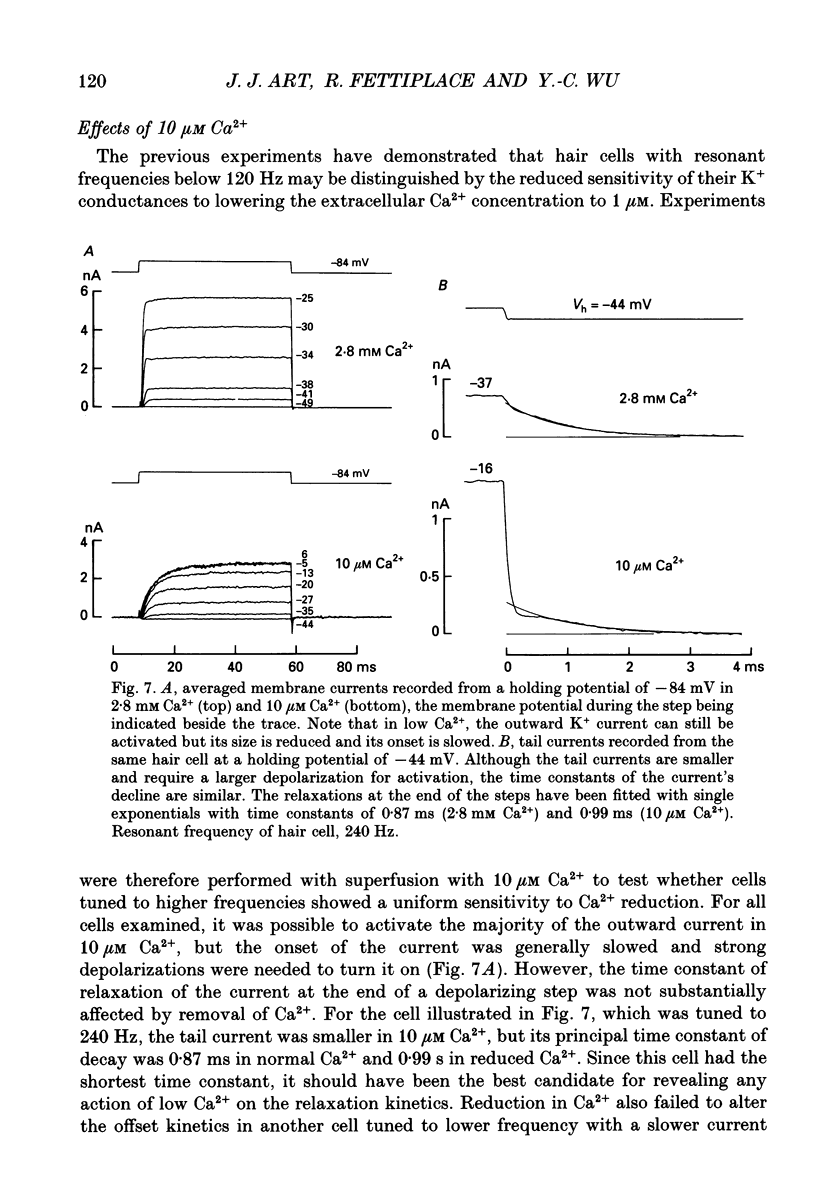
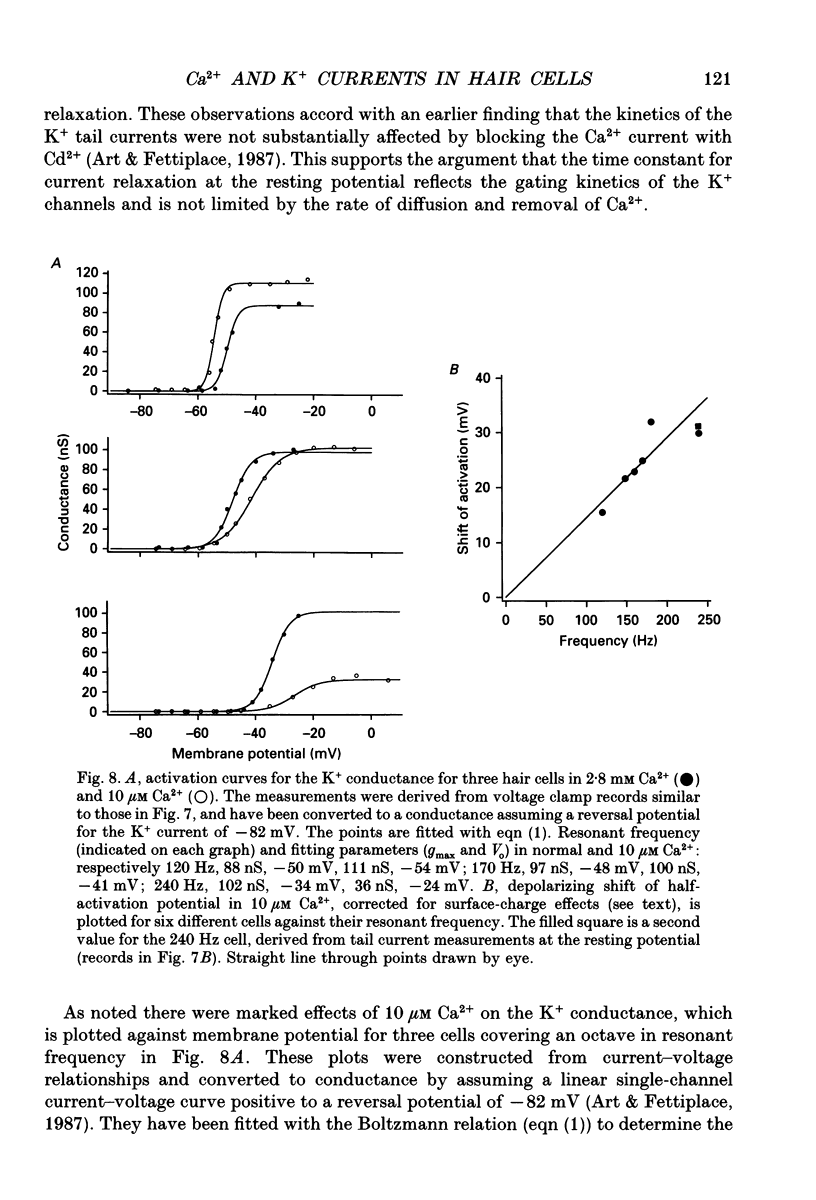
1. The voltage-dependent conductances of turtle cochlear hair cells of known resonant frequency were characterized by tight-seal, whole-cell recording during superfusion with solutions containing normal (2.8 mM) and reduced (0.1-10 microM) Ca2+. 2. In 1 microM Ca2+, the current flowing through the voltage-dependent Ca2+ channels was increased roughly fivefold and had a reversal potential near 0 mV. This observation may be explained by the Ca2+ channels becoming non-selectively permeable to monovalent cations in low-Ca2+ solutions. Lowering the Ca2+ further to 0.1 microM produced little increase in the current. 3. The size of the non-selective current increased systematically with the resonant frequency of the hair cell over the range from 10 to 320 Hz. This suggests that hair cells tuned to higher frequencies contain more voltage-dependent Ca2+ channels. 4. There was a good correlation between the amplitudes of the non-selective current and the K+ current which underlies electrical tuning of these hair cells. The amplitude of the K+ current also increased systematically with resonant frequency. 5. In cells with resonant frequencies between 120 and 320 Hz, the K+ current was completely abolished in 1 microM Ca2+, consistent with prior evidence that this current flows through Ca2+ activated K+ channels. In a majority of cells tuned between 50 and 120 Hz, the K+ current was incompletely blocked in 1 microM Ca2+, but was eliminated in 0.1 microM Ca2+. In all hair cells the K+ current was abolished by 25 mM tetraethylammonium chloride. 6. In cells tuned to 10-20 Hz, the K+ current was not substantially diminished even in 0.1 microM Ca2+, which argues that it may not be Ca2+ activated. 7. In cells tuned to frequencies above 100 Hz, the K+ current could still be evoked by depolarization during superfusion with 10 microM Ca2+. However, its half-activation voltage was shifted to more depolarized levels and its maximum amplitude was systematically reduced with increasing resonant frequency. 8. These observations are consistent with the notion that in cells tuned to more than 50 Hz, there is a fixed ratio of the number of voltage-dependent Ca2+ channels to Ca(2+)-activated K+ channels, the numbers of each increasing in proportion to resonant frequency. The results also provide indirect evidence that the Ca(2+)-activated K+ channels in cells tuned to higher frequencies may be less sensitive to intracellular Ca2+.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Miller C. Do voltage-dependent K+ channels require Ca2+? A critical test employing a heterologous expression system. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7579–7582. doi: 10.1073/pnas.87.19.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art J. J., Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987 Apr;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina V., Gericke M., Bregestovski P. Kinetic differences between Ca(2+)-dependent K+ channels in smooth muscle cells isolated from normal and atherosclerotic human aorta. Proc Biol Sci. 1991 Apr 22;244(1309):51–55. doi: 10.1098/rspb.1991.0050. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Evans M. G., Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol. 1991 Mar;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981 Mar;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980 Sep;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G., Murrow B. W. Calcium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:553–568. doi: 10.1113/jphysiol.1990.sp018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G. Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:529–551. doi: 10.1113/jphysiol.1990.sp018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Nagai T., Evans M. G. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci. 1988 Jul;8(7):2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Carbone E., Zucker H. Block of Na+ ion permeation and selectivity of Ca channels. Ann N Y Acad Sci. 1989;560:94–102. doi: 10.1111/j.1749-6632.1989.tb24083.x. [DOI] [PubMed] [Google Scholar]
- Nerbonne J. M., Gurney A. M. Blockade of Ca2+ and K+ currents in bag cell neurons of Aplysia californica by dihydropyridine Ca2+ antagonists. J Neurosci. 1987 Mar;7(3):882–893. doi: 10.1523/JNEUROSCI.07-03-00882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. The hair cell as a presynaptic terminal. Ann N Y Acad Sci. 1991;635:221–233. doi: 10.1111/j.1749-6632.1991.tb36494.x. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992 Jun;12(6):870-4, 876-9. [PubMed] [Google Scholar]
- Sneary M. G. Auditory receptor of the red-eared turtle: II. Afferent and efferent synapses and innervation patterns. J Comp Neurol. 1988 Oct 22;276(4):588–606. doi: 10.1002/cne.902760411. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


