Abstract
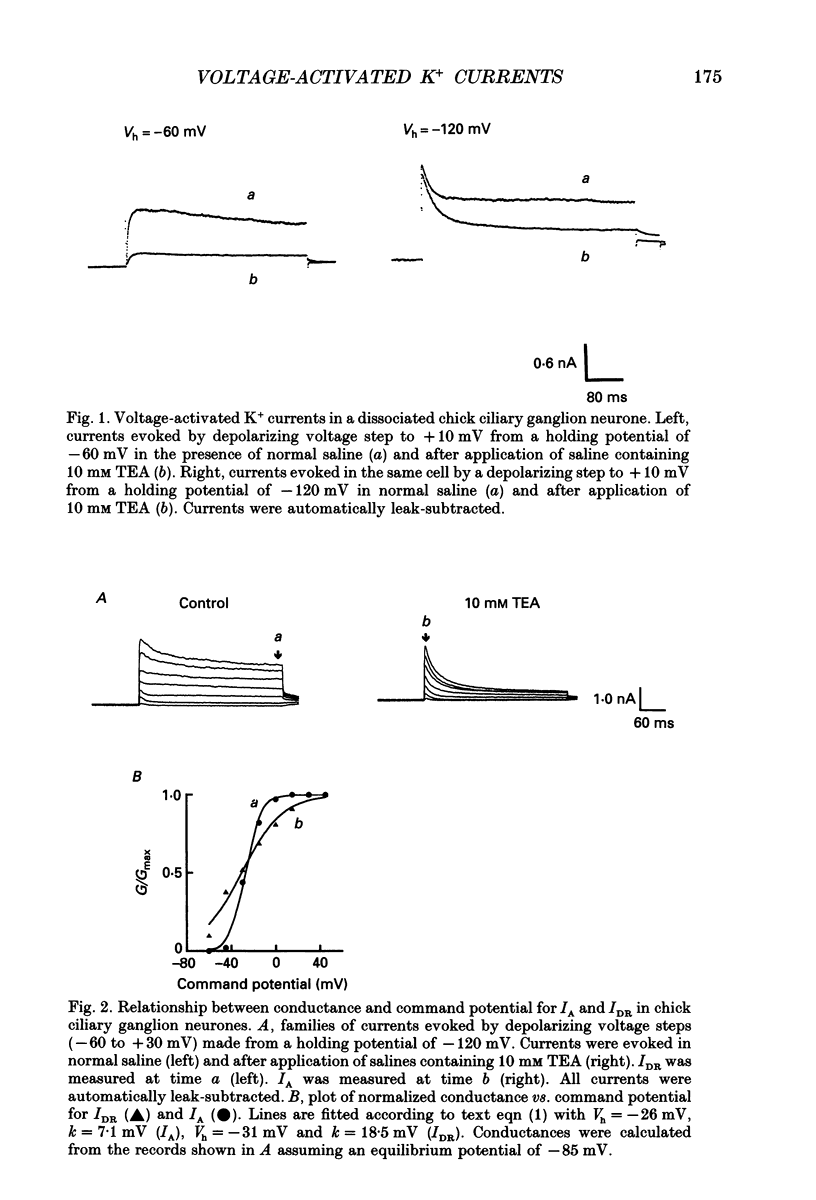
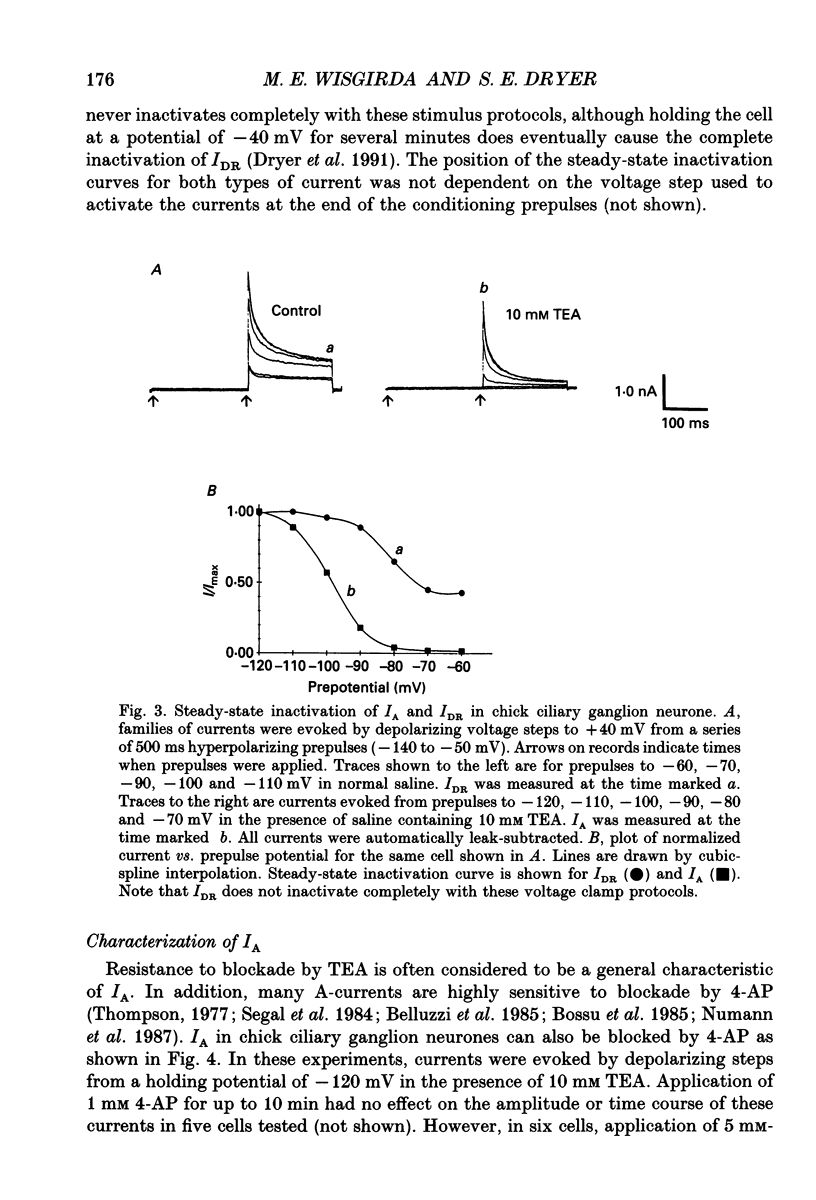
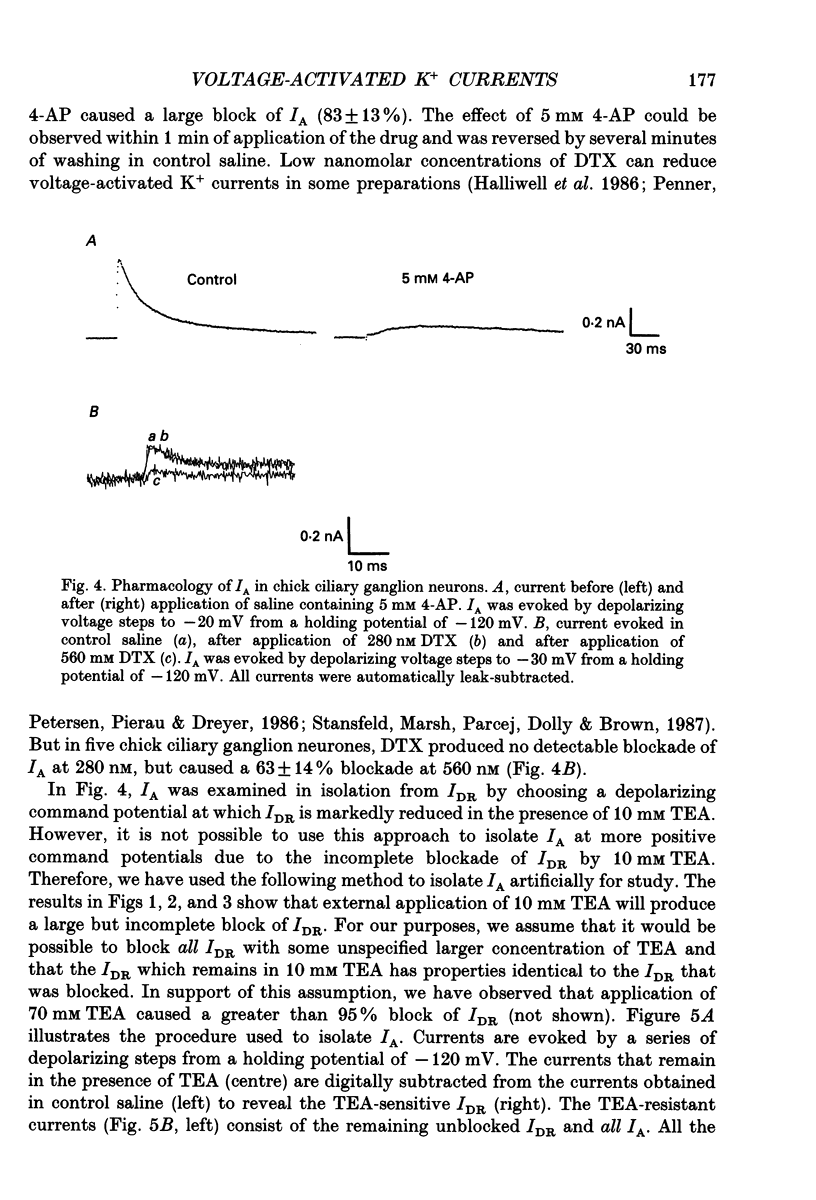
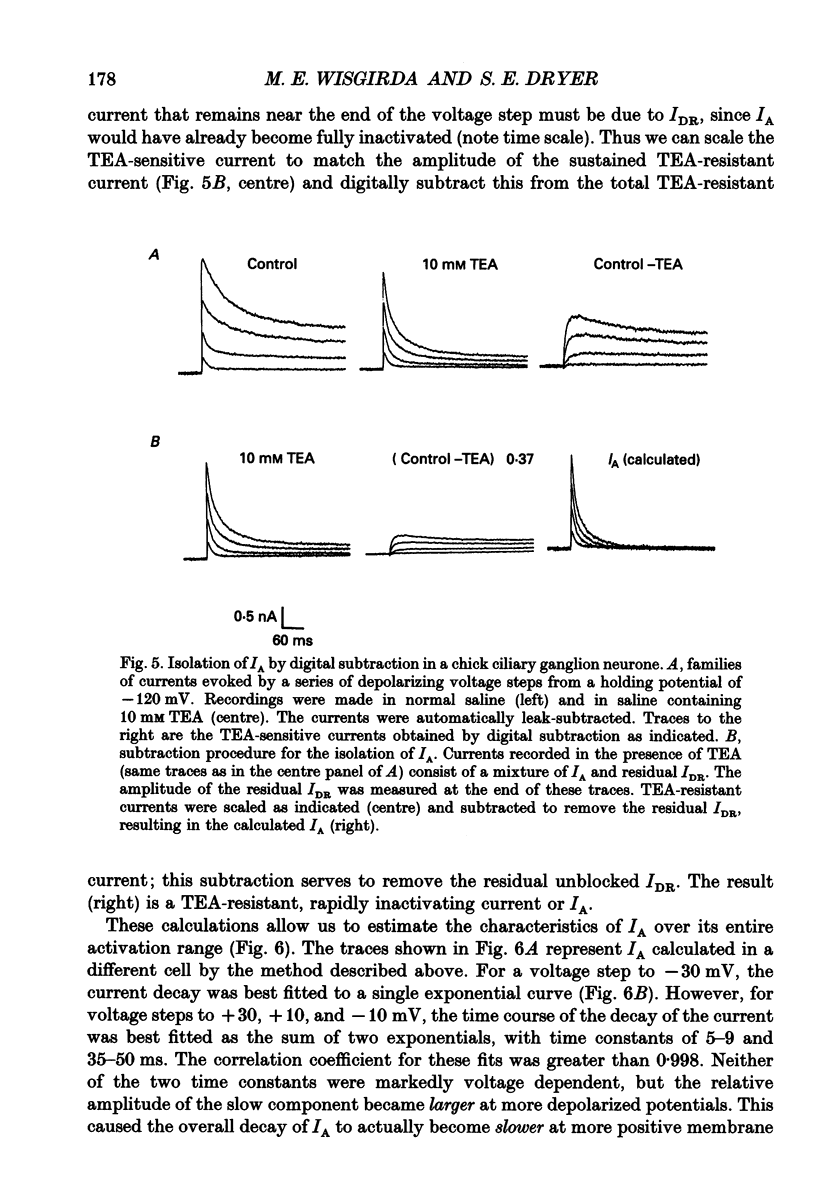
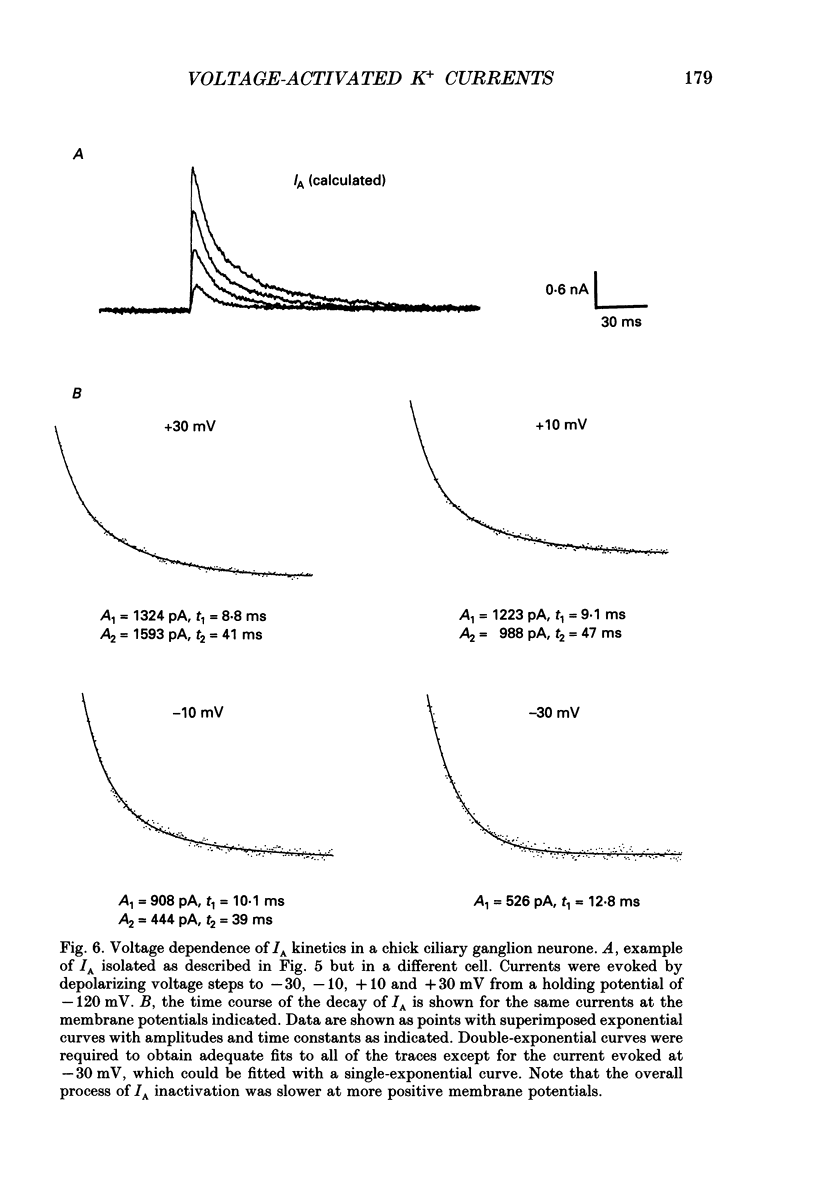
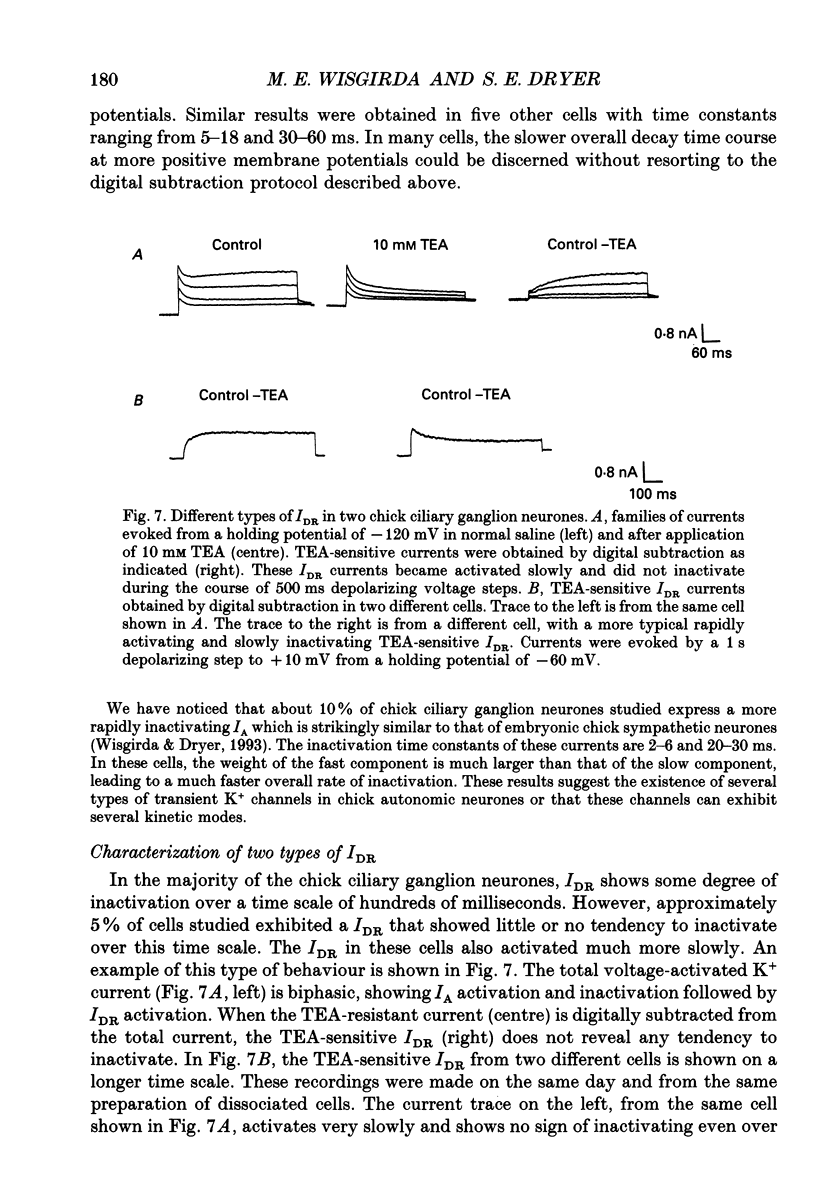
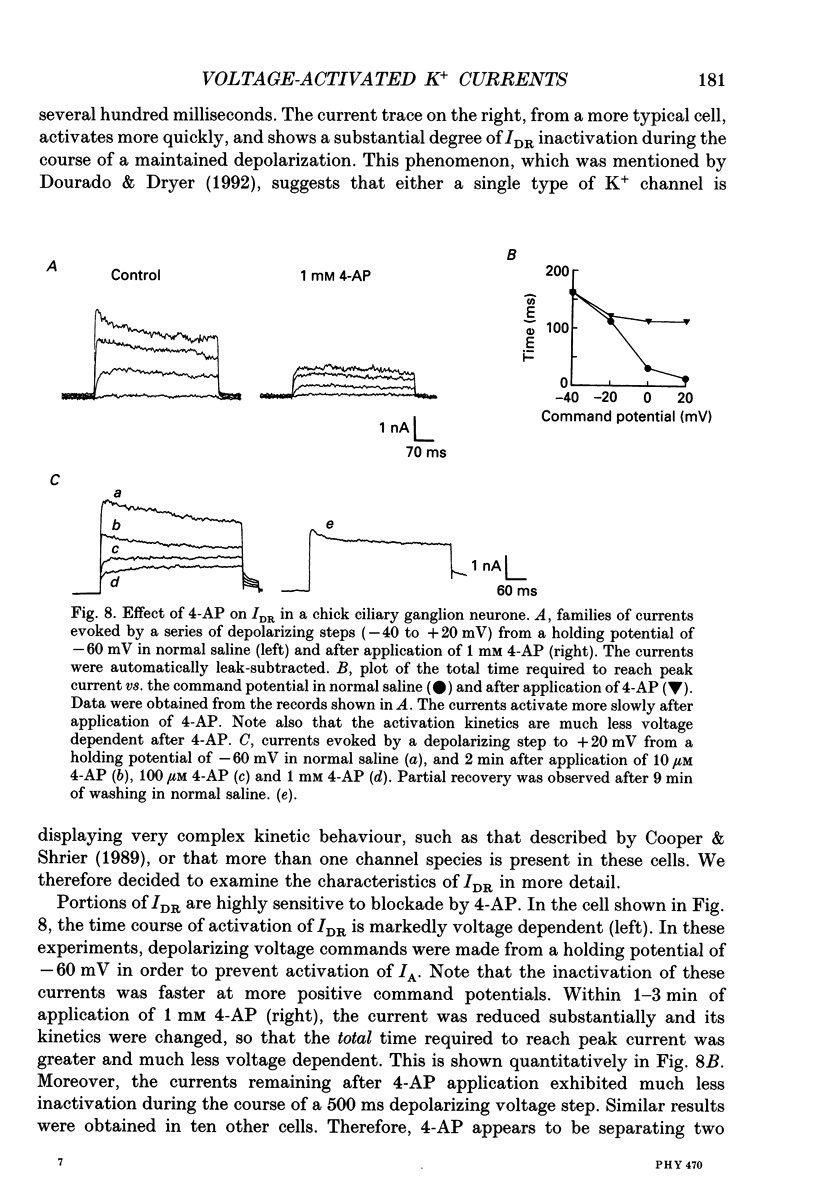
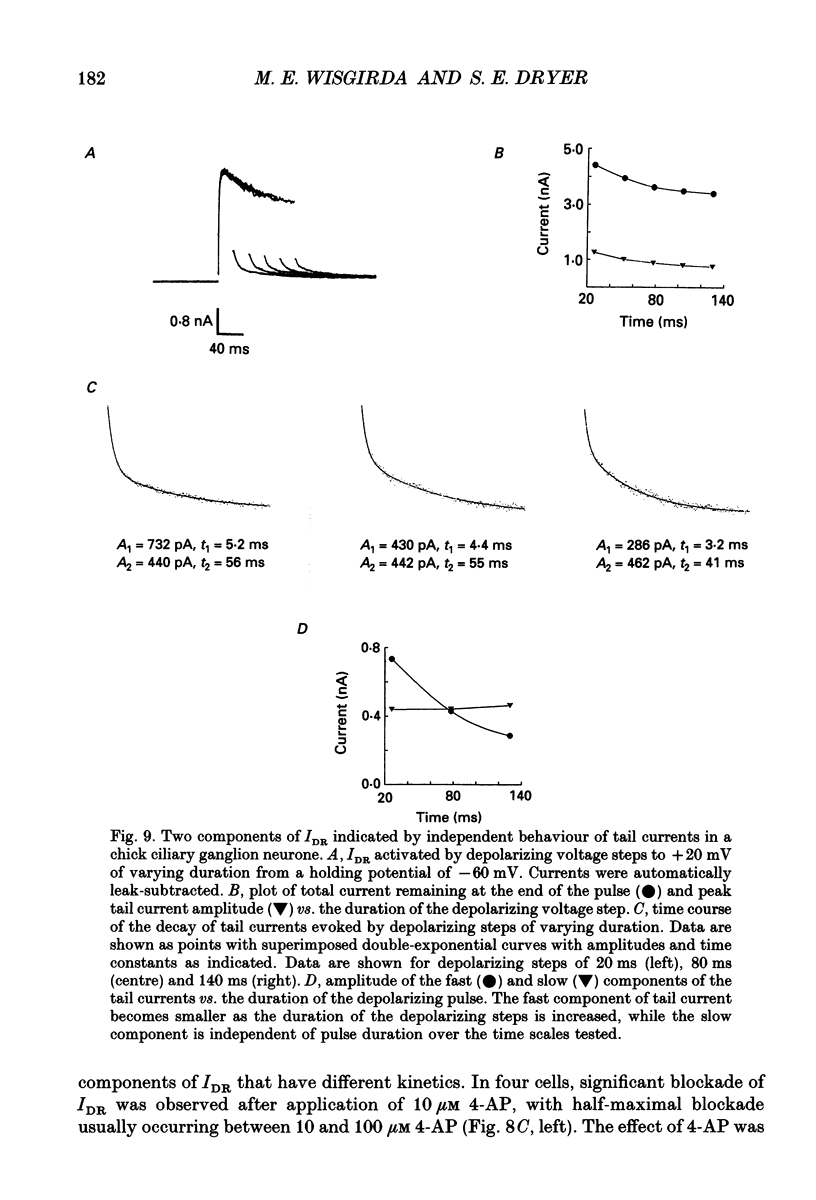
1. The properties of voltage-activated K+ currents were examined using whole-cell recording techniques in acutely isolated chick ciliary ganglion neurones. 2. Application of depolarizing voltage pulses from a holding potential of -60 mV evoked sustained outward currents that inactivated with time constants of hundreds of milliseconds (IDR). Bath application of 10 mM tetraethylammonium (TEA) caused a 70-90% reduction of IDR. Application of depolarizing voltage steps from a holding potential of -120 mV revealed a second class of TEA-resistant outward currents. These currents activated quickly but inactivated completely within tens of milliseconds (IA). IA activated at more negative command potentials than IDR. However, IDR exhibited a steeper voltage dependence of activation than IA. 3. The midpoint of the steady-state inactivation curve of IA was between -95 and -110 mV. By contrast the midpoint of the steady-state inactivation curve of IDR was between -80 and -90 mV. It was not possible to produce a complete inactivation of IDR using prepulses of up to 2 s duration. 4. The time course of IA inactivation could only be fitted with double-exponential curves with time constants of 5-18 ms and 30-60 ms at membrane potentials positive to -30 mV. The inactivation of IA was slower at more positive membrane potentials because of a greater contribution of the long time constant. The individual time constants were not markedly voltage dependent. 5. Bath application of 5 mM 4-aminopyridine (4-AP) caused a 70-100% block of IA whereas 1 mM 4-AP was ineffective. Bath application of 560 nM alpha-dendrotoxin (DTX) produced a 50-70% reduction of IA, but application of 280 nM DTX had no effect on IA. 6. Application of 1 mM 4-AP produced a reversible 55-80% block of IDR measured at the end of a 500 ms depolarizing pulse. The 4-AP-sensitive components of IDR activated rapidly and exhibited a gradual inactivation with continued depolarization. The 4-AP-resistant components of IDR activated much more slowly and showed very little tendency to inactivate. Significant blockade of IDR was produced by 10 microM 4-AP. 7. The decay of IDR tail currents could only be fitted with double exponential curves with time constants of 3-6 and 40-60 ms, respectively. The fast and slow components of the tail currents behaved independently with respect to the duration of the depolarizing voltage step. 8. Application of 1 mM 4-AP eliminated the fast, but not the slow component of IDR tail currents.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
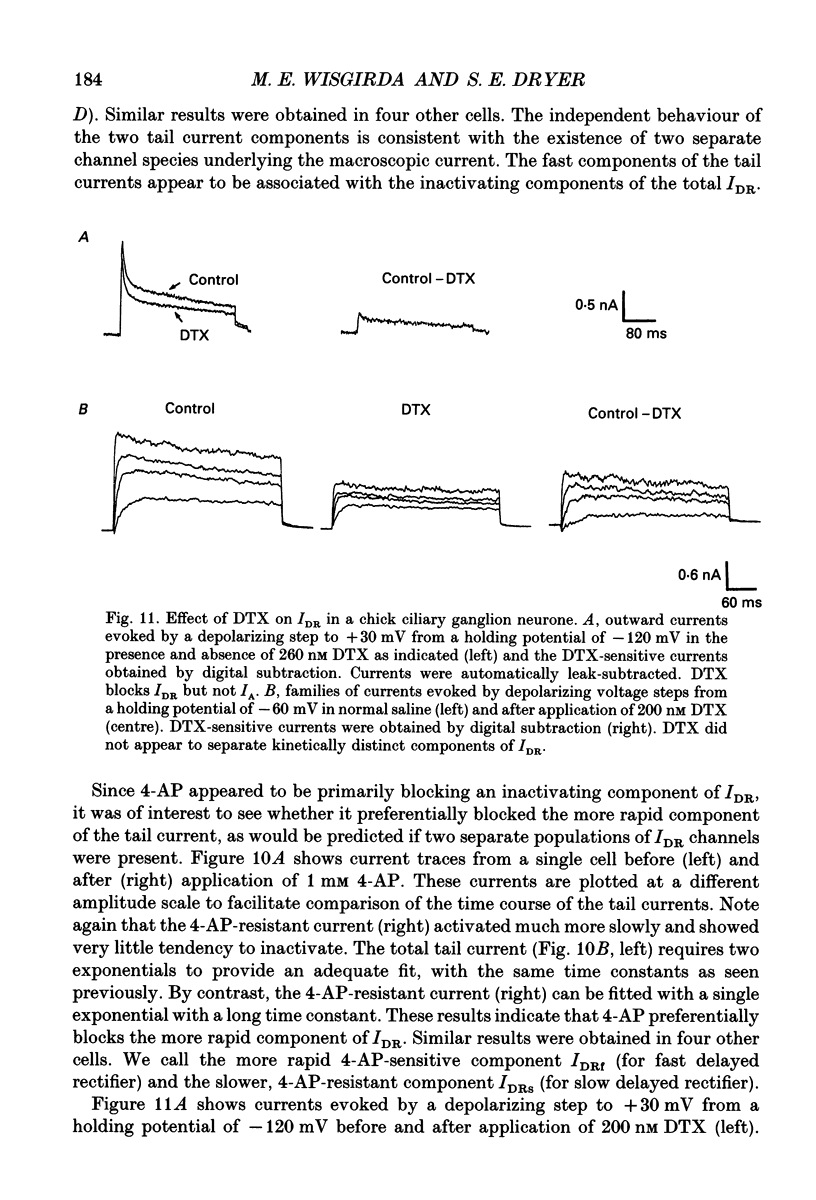
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
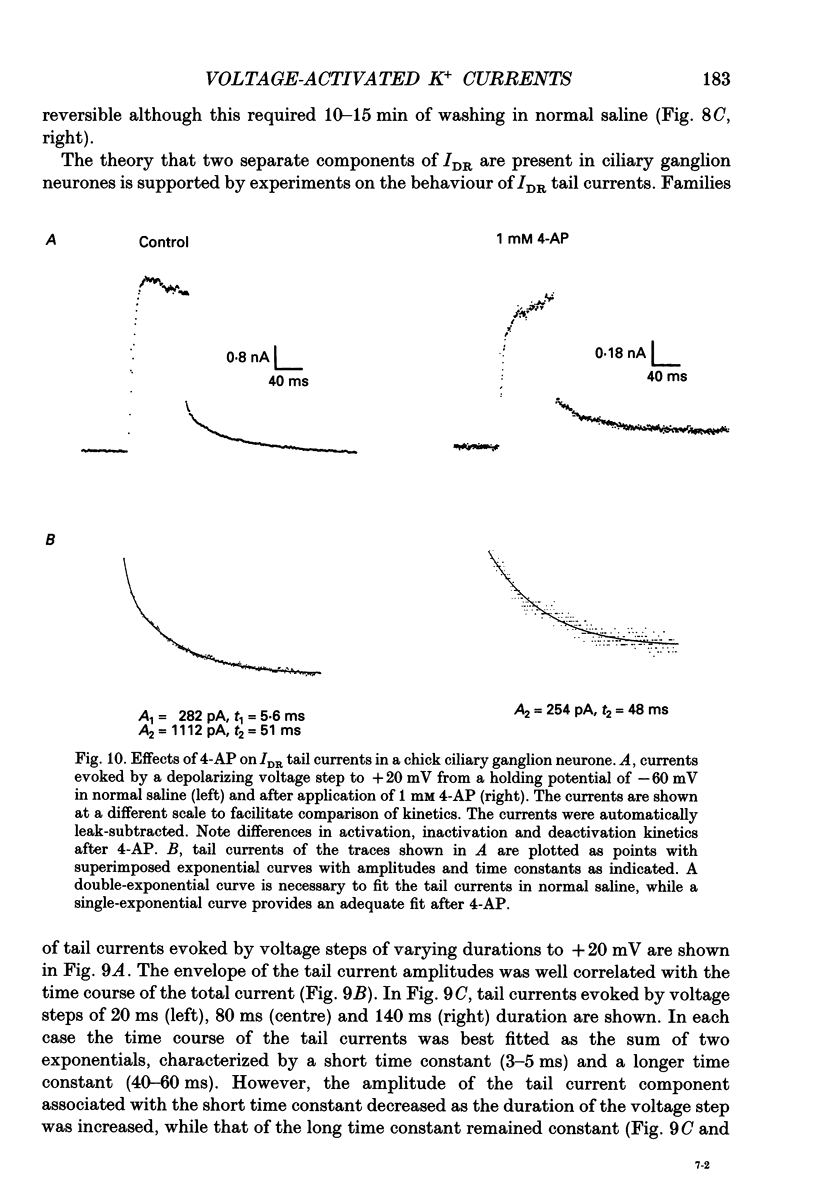
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Dupont J. L., Feltz A. IA current compared to low threshold calcium current in cranial sensory neurons. Neurosci Lett. 1985 Dec 4;62(2):249–254. doi: 10.1016/0304-3940(85)90363-5. [DOI] [PubMed] [Google Scholar]
- Christie M. J., North R. A., Osborne P. B., Douglass J., Adelman J. P. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990 Mar;4(3):405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Inactivation of A currents and A channels on rat nodose neurons in culture. J Gen Physiol. 1989 Nov;94(5):881–910. doi: 10.1085/jgp.94.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado M. M., Dryer S. E. Changes in the electrical properties of chick ciliary ganglion neurones during embryonic development. J Physiol. 1992 Apr;449:411–428. doi: 10.1113/jphysiol.1992.sp019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Dourado M. M., Wisgirda M. E. Characteristics of multiple Ca(2+)-activated K+ channels in acutely dissociated chick ciliary-ganglion neurones. J Physiol. 1991 Nov;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E. Na(+)-activated K+ channels and voltage-evoked ionic currents in brain stem and parasympathetic neurones of the chick. J Physiol. 1991 Apr;435:513–532. doi: 10.1113/jphysiol.1991.sp018522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G. H., Chiappinelli V. A. An inward rectifier is present in presynaptic nerve terminals in the chick ciliary ganglion. Brain Res. 1992 Mar 13;575(1):103–112. doi: 10.1016/0006-8993(92)90429-d. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Othman I. B., Pelchen-Matthews A., Dolly J. O. Central action of dendrotoxin: selective reduction of a transient K conductance in hippocampus and binding to localized acceptors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):493–497. doi: 10.1073/pnas.83.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. L., Henderson L. P., Spitzer N. C. Changes in densities and kinetics of delayed rectifier potassium channels during neuronal differentiation. Neuron. 1988 Oct;1(8):739–750. doi: 10.1016/0896-6273(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Luneau C. J., Williams J. B., Marshall J., Levitan E. S., Oliva C., Smith J. S., Antanavage J., Folander K., Stein R. B., Swanson R. Alternative splicing contributes to K+ channel diversity in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wagoner P. K. The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J Physiol. 1989 Mar;410:587–612. doi: 10.1113/jphysiol.1989.sp017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Petersen M., Pierau F. K., Dreyer F. Dendrotoxin: a selective blocker of a non-inactivating potassium current in guinea-pig dorsal root ganglion neurones. Pflugers Arch. 1986 Oct;407(4):365–369. doi: 10.1007/BF00652619. [DOI] [PubMed] [Google Scholar]
- Quandt F. N. Three kinetically distinct potassium channels in mouse neuroblastoma cells. J Physiol. 1988 Jan;395:401–418. doi: 10.1113/jphysiol.1988.sp016926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984 Jun;51(6):1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Halliwell J. V., Brown D. A. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurones by blocking a slowly inactivating outward current. Neurosci Lett. 1986 Mar 14;64(3):299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Parcej D. N., Dolly J. O., Brown D. A. Mast cell degranulating peptide and dendrotoxin selectively inhibit a fast-activating potassium current and bind to common neuronal proteins. Neuroscience. 1987 Dec;23(3):893–902. doi: 10.1016/0306-4522(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Marshall J., Smith J. S., Williams J. B., Boyle M. B., Folander K., Luneau C. J., Antanavage J., Oliva C., Buhrow S. A. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990 Jun;4(6):929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J. C., Tseng G. N., Schwartz A., Tanouye M. A. Molecular cloning and functional expression of a potassium channel cDNA isolated from a rat cardiac library. FEBS Lett. 1990 Jul 30;268(1):63–68. doi: 10.1016/0014-5793(90)80973-m. [DOI] [PubMed] [Google Scholar]
- Wei A., Covarrubias M., Butler A., Baker K., Pak M., Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990 May 4;248(4955):599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]


