Abstract
1. The outward rectifying K+ conductance and underlying single channel behaviour in mouse small intestine (MSI) smooth muscle cells was studied using microelectrode impalement and the patch clamp technique. 2. At 37 degrees C, smooth muscle cells in MSI explants had a resting membrane potential around -65 mV and showed spontaneous electrical and mechanical activity. 3. Under whole-cell voltage clamp, depolarization of smooth muscle cells in the explants evoked a methoxyverapamil (D600)-sensitive, partially inactivating inward current and a non-inactivating outward current. The outward current was also observed in enzymatically dispersed cells from neonatal mouse small intestine. 4. The reversal potential of the outward current as established in tail current experiments was -70.2 mV. Tail currents could be fitted with a single exponential, suggesting the participation of only one population of channels. 5. The outward current was sensitive to 4-aminopyridine (10(-4) M), Ba2+ (1 mM) and to the presence of Cs+ in the pipette, but not to D600 (10(-6) M), or the presence of ATP (1 mM) in the pipette. 6. In the cell-attached patch configuration, a unitary outward current was observed that showed increased activity upon depolarization of the patch. The current-voltage relationship was close to linear with a slope conductance of 186 pS. 7. With normal K+ (6 mM) in the pipette, the extrapolated reversal potential for the unitary current was around -75 mV, while with high K+ (120 mM) the reversal potential was close to 0 mV. 8. Averaging single channel traces recorded under a depolarizing pulse protocol resulted in a trace with similar time characteristics as the outward current observed in the whole-cell configuration. 9. The burst behaviour of the channel was described by a simple model consisting of two closed states, Cf (intraburst closed state) and Cs (interburst closed state) and an open state (O). The rate constants in the model showed differential sensitivity to potential changes, channel blockade by Ba2+ and equimolar K+ conditions. 10. It was concluded that the outward rectifying potassium current in MSI smooth muscle cells is mediated by a 186 pS bursting channel. Voltage dependency and Ba2+ blockade are mainly reflected by changes in the transition rate from the open channel state to the interburst closed state.
Full text
PDF

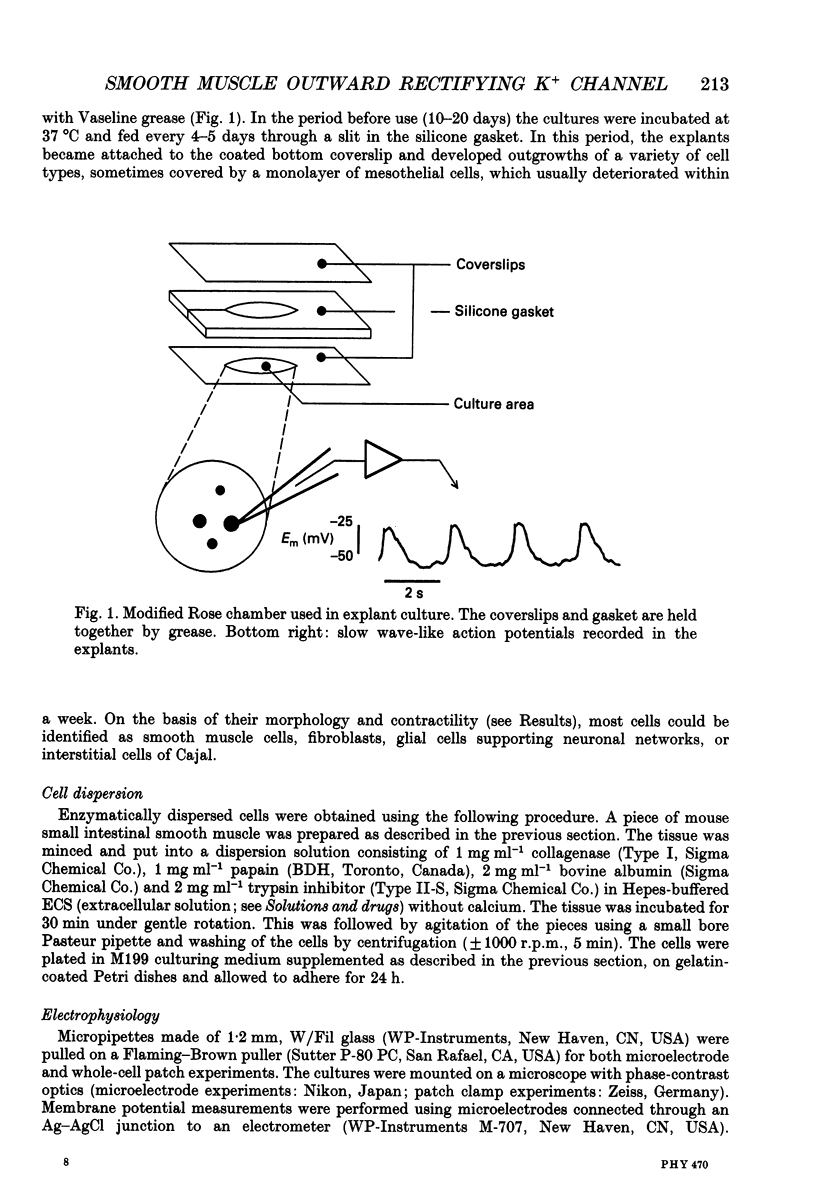


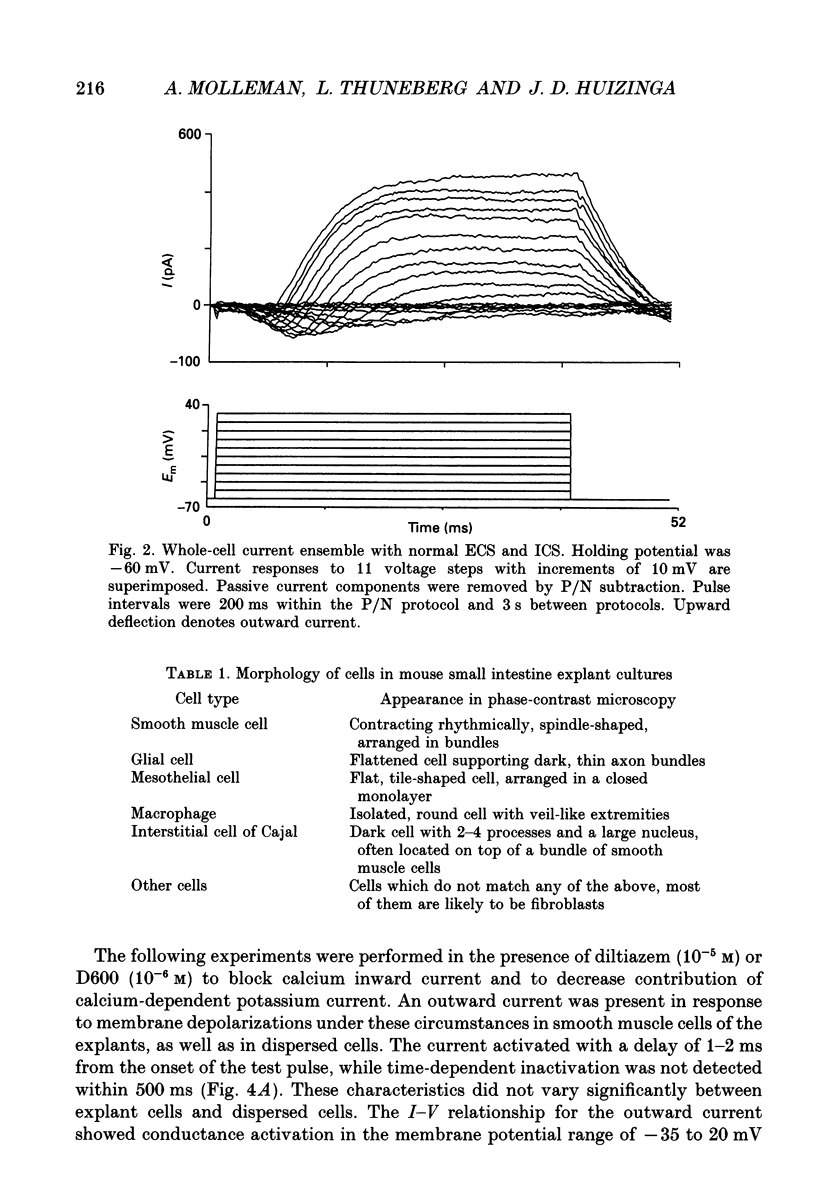
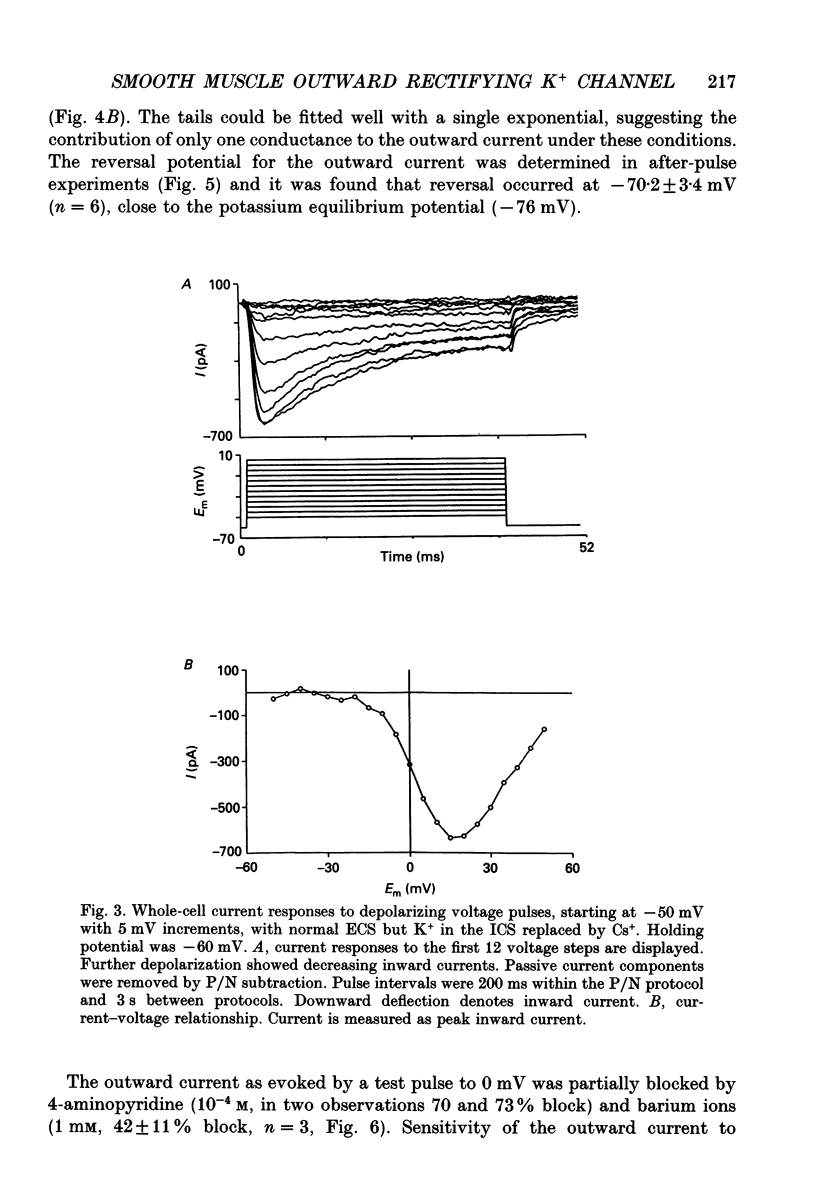
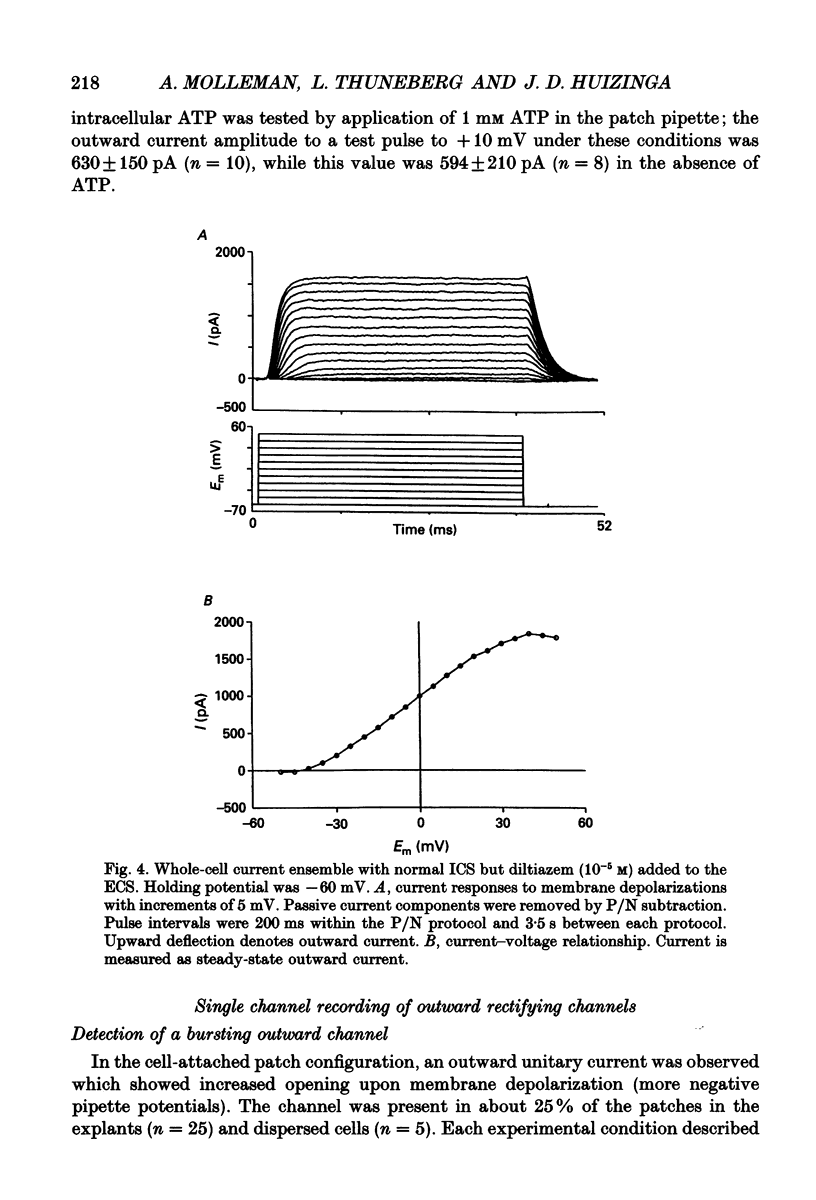
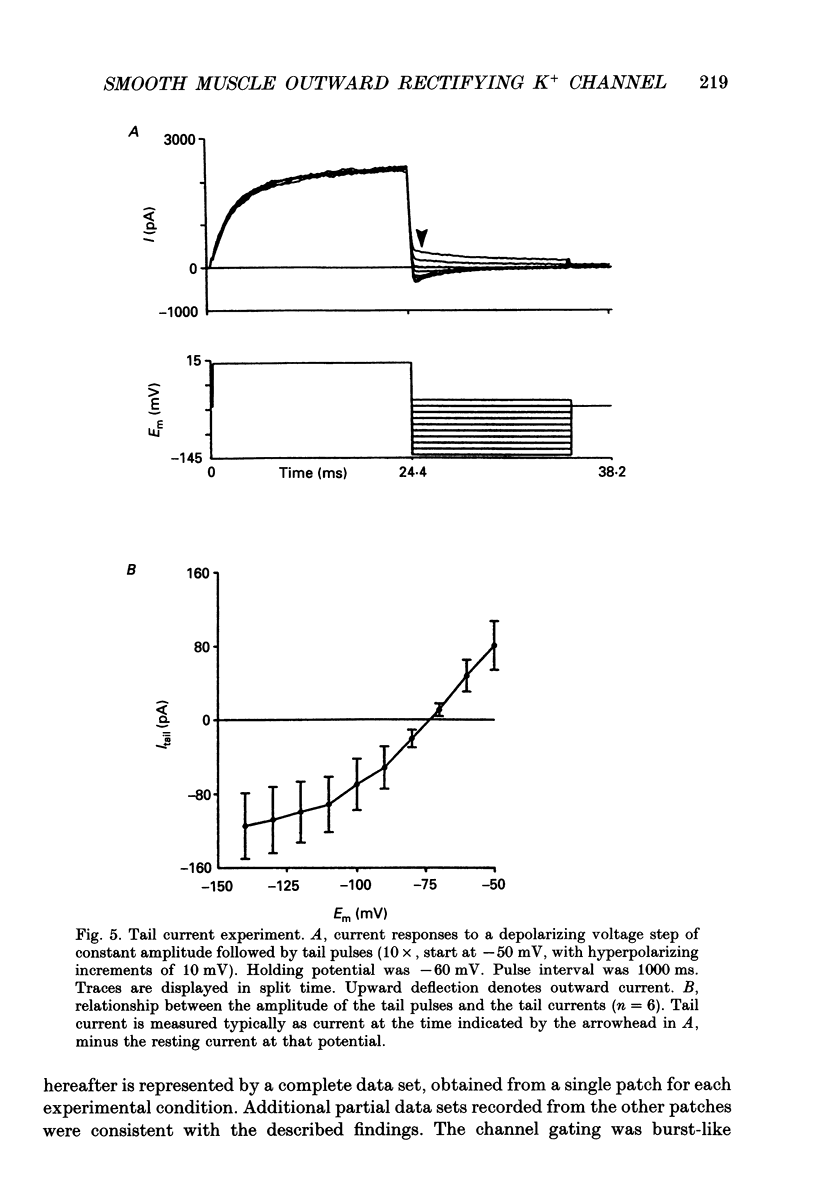
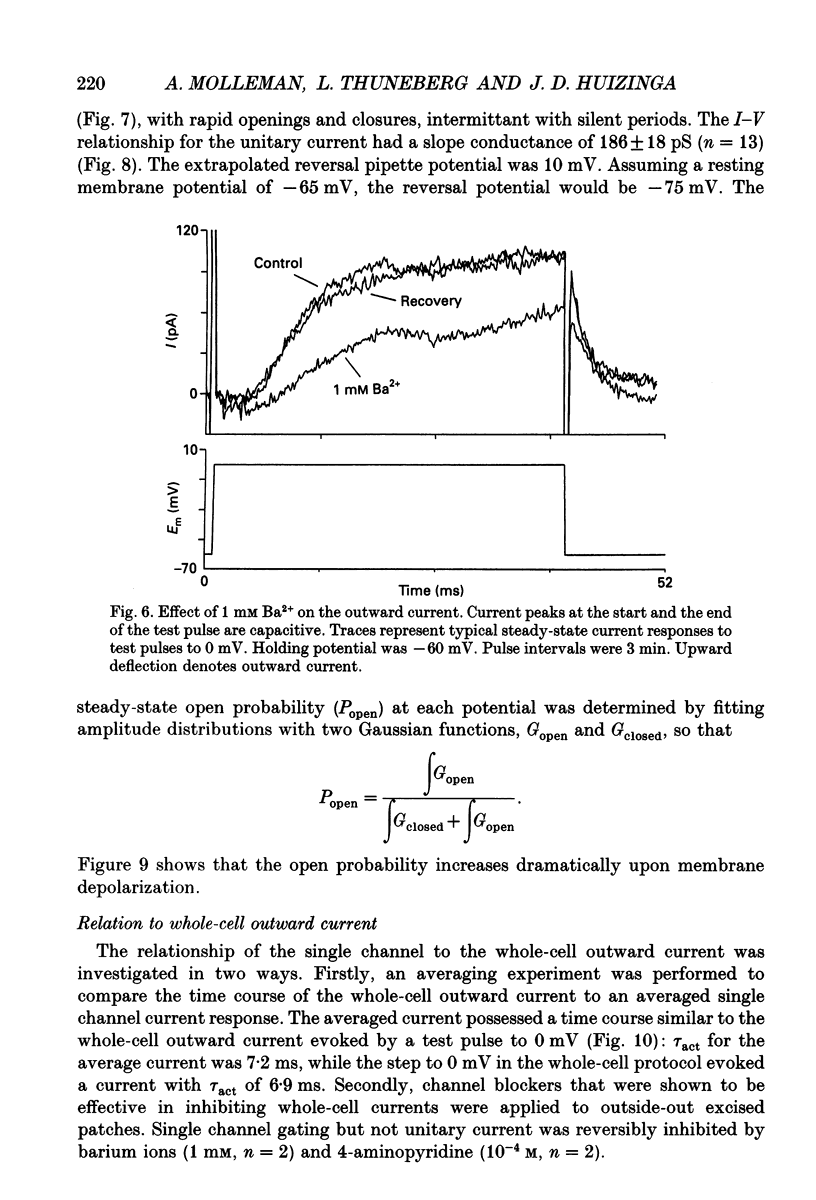
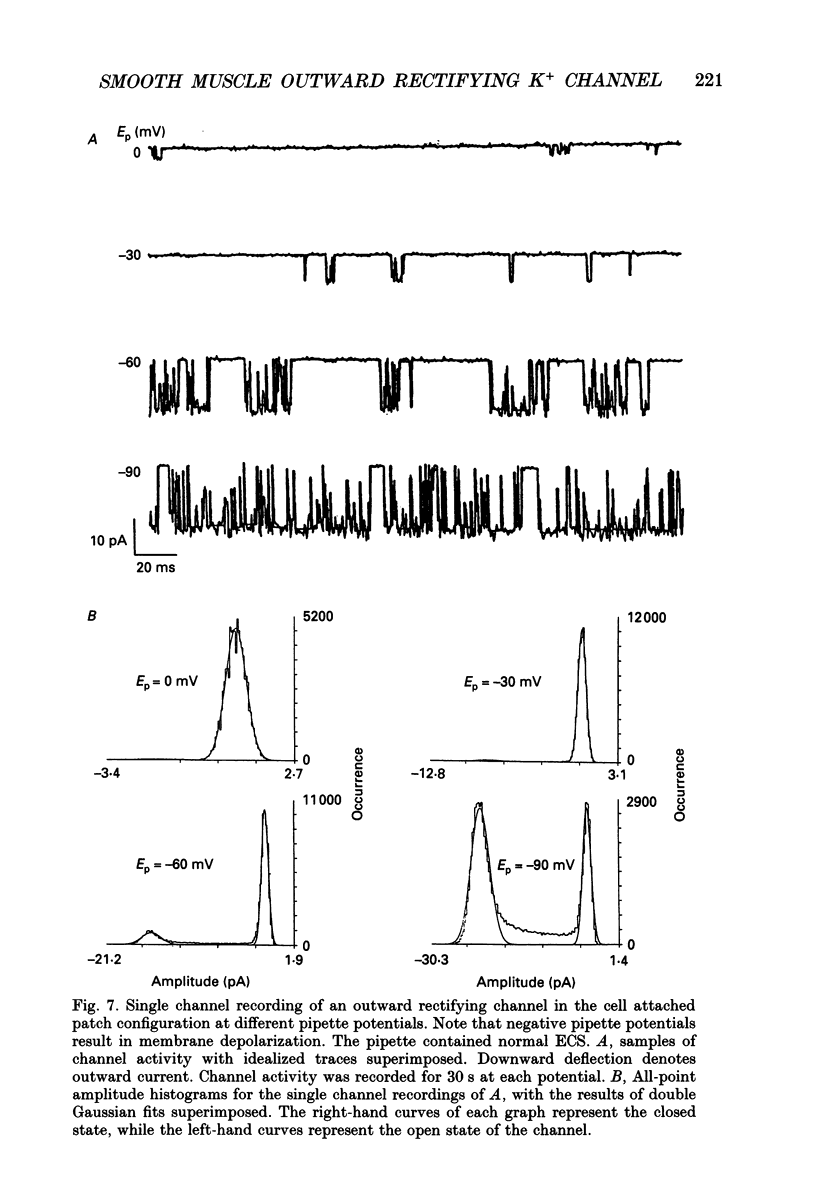
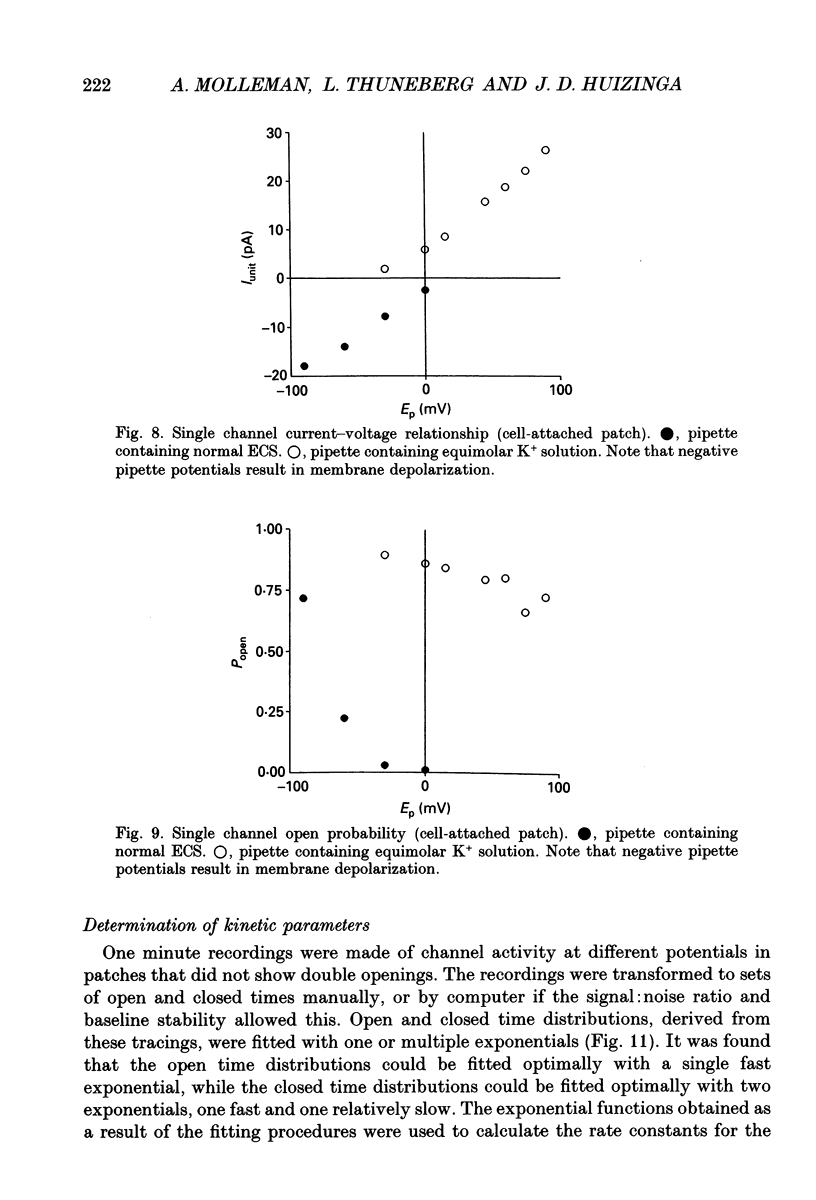




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Barry P. H., Lynch J. W. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991 Apr;121(2):101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
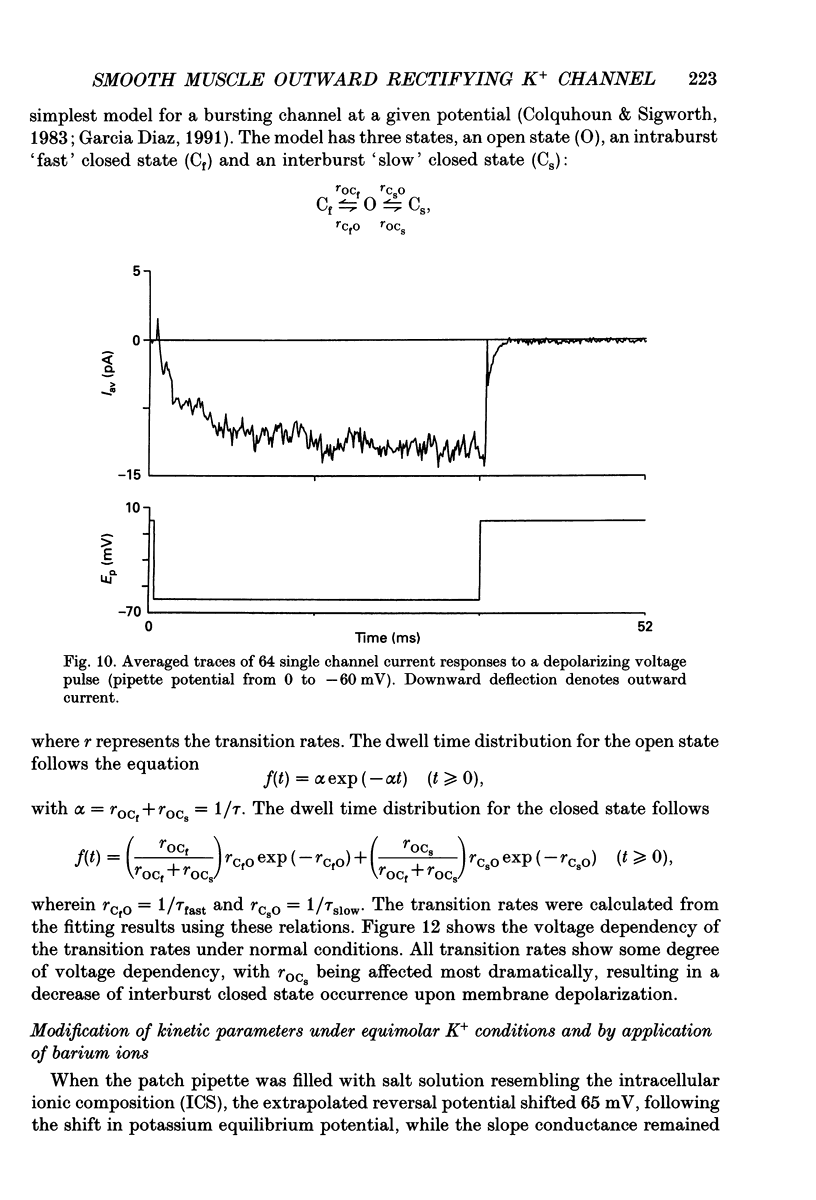
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
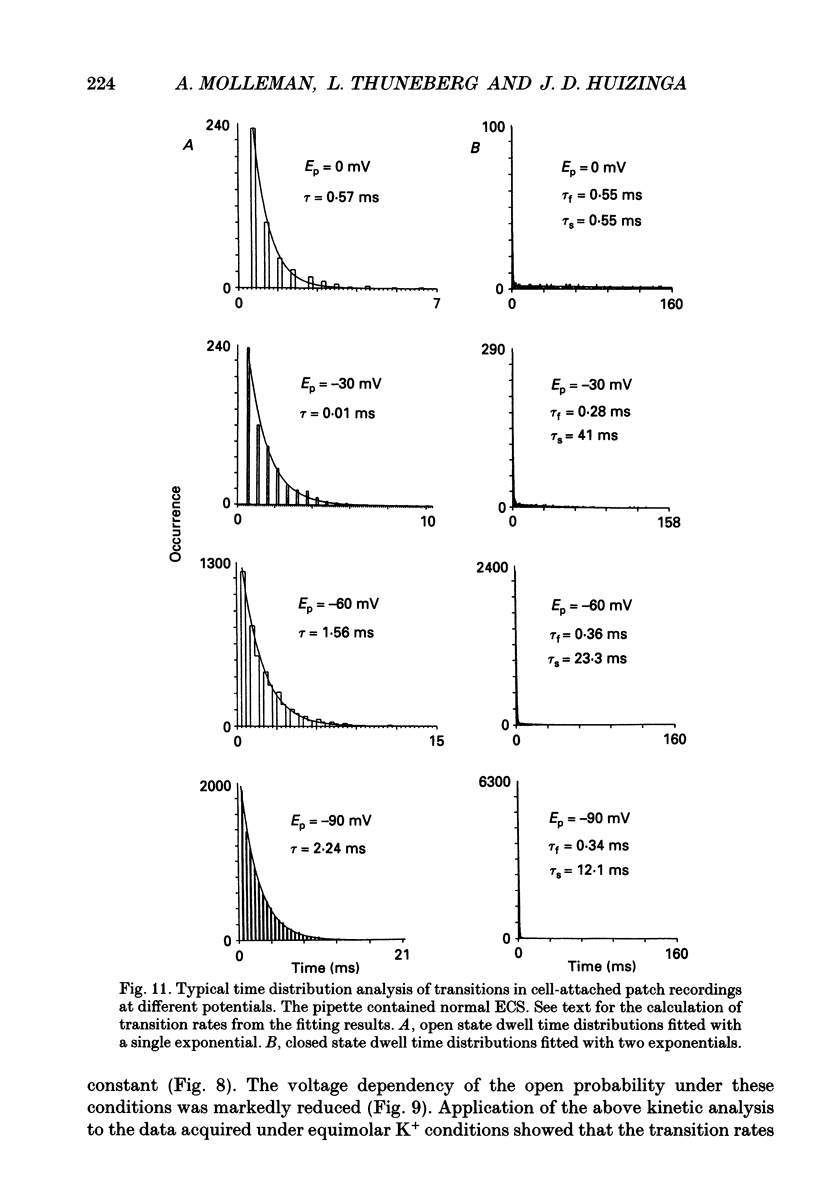
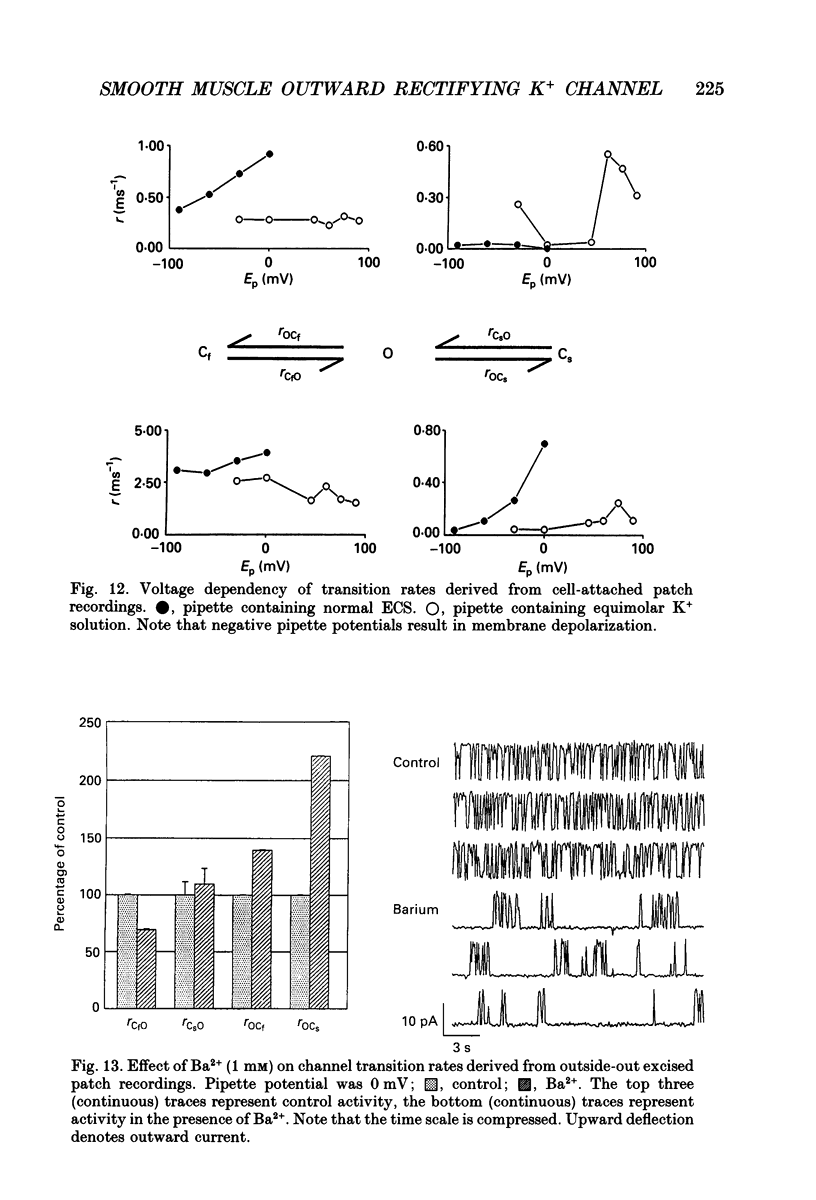
- Benham C. D., Bolton T. B. Patch-clamp studies of slow potential-sensitive potassium channels in longitudinal smooth muscle cells of rabbit jejunum. J Physiol. 1983 Jul;340:469–486. doi: 10.1113/jphysiol.1983.sp014774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G., Caillé J. P., Payet M. D., Peyrow M., Sauvé R., Renaud J. F., Sperelakis N. Bethanidine increases one type of potassium current and relaxes aortic muscle. Can J Physiol Pharmacol. 1988 Jun;66(6):731–736. doi: 10.1139/y88-116. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Sanders K. M. Characterization of macroscopic outward currents of canine colonic myocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C461–C469. doi: 10.1152/ajpcell.1989.257.3.C461. [DOI] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- García-Díaz J. F. Whole-cell and single channel K+ and Cl- currents in epithelial cells of frog skin. J Gen Physiol. 1991 Jul;98(1):131–161. doi: 10.1085/jgp.98.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lynch C., 3rd Ionic conductances in frog short skeletal muscle fibres with slow delayed rectifier currents. J Physiol. 1985 Nov;368:359–378. doi: 10.1113/jphysiol.1985.sp015862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Latorre R., Reisin I. Coupling of voltage-dependent gating and Ba++ block in the high-conductance, Ca++-activated K+ channel. J Gen Physiol. 1987 Sep;90(3):427–449. doi: 10.1085/jgp.90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman A., Nelemans A., Den Hertog A. P2-purinoceptor-mediated membrane currents in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1989 Oct 4;169(1):167–174. doi: 10.1016/0014-2999(89)90829-7. [DOI] [PubMed] [Google Scholar]
- Molleman A., Nelemans A., van den Akker J., Duin M., den Hertog A. Voltage-dependent sodium and potassium, but no calcium conductances in DDT1 MF-2 smooth muscle cells. Pflugers Arch. 1991 Jan;417(5):479–484. doi: 10.1007/BF00370943. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuenkel E. L. Single potassium channels recorded from vascular smooth muscle cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H760–H769. doi: 10.1152/ajpheart.1989.257.3.H760. [DOI] [PubMed] [Google Scholar]
- Tagami M., Nara Y., Kubota A., Sunaga T., Maezawa H., Fujino H., Yamori Y. Morphological and functional differentiation of cultured vascular smooth-muscle cells. Cell Tissue Res. 1986;245(2):261–266. doi: 10.1007/BF00213930. [DOI] [PubMed] [Google Scholar]
- Terada K., Kitamura K., Kuriyama H. Different inhibitions of the voltage-dependent K+ current by Ca2+ antagonists in the smooth muscle cell membrane of rabbit small intestine. Pflugers Arch. 1987 May;408(6):558–564. doi: 10.1007/BF00581156. [DOI] [PubMed] [Google Scholar]
- Volk K. A., Matsuda J. J., Shibata E. F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J Physiol. 1991 Aug;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Identification and characterization of major ionic currents in isolated smooth muscle cells using the voltage-clamp technique. Pflugers Arch. 1987 Feb;408(2):83–97. doi: 10.1007/BF00581336. [DOI] [PubMed] [Google Scholar]


