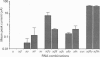Abstract
1. Nicotinic acetylcholine (ACh) receptors in developing vertebrate skeletal muscle exhibit functional heterogeneity in both conductance and kinetics. To assess the contributions of receptors differing in subunit composition to the heterogeneity, various combinations of mouse alpha beta delta gamma epsilon subunit RNAs were tested for the ability to express functional receptors in Xenopus oocytes. 2. Two combinations of dual-subunit RNAs (alpha delta and alpha gamma) resulted in detectable ACh-activated currents and six different combinations of three or more subunit RNAs produced significant numbers of functional channels. The order of combinations yielding the greatest amount of current was alpha beta gamma > alpha beta delta = alpha delta epsilon > alpha delta gamma > alpha delta > alpha gamma. 3. The extent to which a channel type with three different subunits was expressed was highly dependent upon the ratios of RNAs coding for the different subunits. For alpha beta delta receptors the efficiency of expression was alpha: beta: delta (1/5:1/5:1) >> (1:1:1) >> (1/5:1:1/5) > (1:1/5:1/5). 4. The level of expression of three-subunit combinations was also critically dependent upon the order of RNAs injected. When alpha delta or alpha gamma RNA combinations were co-injected 2 days prior to the injection of beta RNA, the expression was 2-5 times greater than when alpha beta injection was followed by injection of delta or gamma RNA. 5. Single-channel measurements revealed that alpha beta delta channels were not expressed in the presence of alpha beta delta epsilon RNAs, even under conditions when the amount of delta RNA injected was 5-fold higher than the amount of epsilon RNA. 6. These data indicate that the functional expression of subunit-omitted receptors depends critically upon the relative amounts of the five different subunit RNAs. Receptors composed of three different subunits express in the presence of the subunit RNAs characteristic of embryonic muscle (alpha beta delta gamma), but are not observed with the combination of RNAs characteristic of adult muscle (alpha beta delta epsilon).
Full text
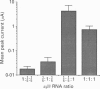
PDF














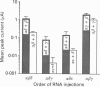
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. N., Albuquerque E. X. Characteristics of acetylcholine-activated channels of innervated and chronically denervated skeletal muscles. Exp Neurol. 1986 Mar;91(3):532–545. doi: 10.1016/0014-4886(86)90050-6. [DOI] [PubMed] [Google Scholar]
- Baldwin T. J., Yoshihara C. M., Blackmer K., Kintner C. R., Burden S. J. Regulation of acetylcholine receptor transcript expression during development in Xenopus laevis. J Cell Biol. 1988 Feb;106(2):469–478. doi: 10.1083/jcb.106.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P., Merlie J. P. Mutational analysis of muscle nicotinic acetylcholine receptor subunit assembly. J Cell Biol. 1990 Dec;111(6 Pt 1):2613–2622. doi: 10.1083/jcb.111.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P., Smith M. M., Merlie J. P. Assembly intermediates of the mouse muscle nicotinic acetylcholine receptor in stably transfected fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 1):2601–2611. doi: 10.1083/jcb.111.6.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Henderson L. Regulation of acetylcholine receptor channel function during development of skeletal muscle. Dev Biol. 1988 Sep;129(1):1–11. doi: 10.1016/0012-1606(88)90156-x. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P. Resolving the structural basis for developmental changes in muscle ACh receptor function: it takes nerve. Trends Neurosci. 1989 May;12(5):174–177. doi: 10.1016/0166-2236(89)90064-7. [DOI] [PubMed] [Google Scholar]
- Buller A. L., White M. M. Functional acetylcholine receptors expressed in Xenopus oocytes after injection of Torpedo beta, gamma, and delta subunit RNAs are a consequence of endogenous oocyte gene expression. Mol Pharmacol. 1990 Mar;37(3):423–428. [PubMed] [Google Scholar]
- Camacho P., Liu Y., Mandel G., Brehm P. The epsilon subunit confers fast channel gating on multiple classes of acetylcholine receptors. J Neurosci. 1993 Feb;13(2):605–613. doi: 10.1523/JNEUROSCI.13-02-00605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Lester H. A. Structure of the gamma-less nicotinic acetylcholine receptor: learning from omission. Mol Pharmacol. 1992 Apr;41(4):708–717. [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Rogers S. W., Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991 Jan;12(1):34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Kojima H., Carr J. A., Colquhoun D. Expression of cloned receptor subunits produces multiple receptors. Proc Biol Sci. 1990 Nov 22;242(1304):108–112. doi: 10.1098/rspb.1990.0112. [DOI] [PubMed] [Google Scholar]
- Golino M. D., Hamill O. P. Subunit requirements for Torpedo AChR channel expression: a specific role for the delta-subunit in voltage-dependent gating. J Membr Biol. 1992 Sep;129(3):297–309. doi: 10.1007/BF00232911. [DOI] [PubMed] [Google Scholar]
- Gu Y., Forsayeth J. R., Verrall S., Yu X. M., Hall Z. W. Assembly of the mammalian muscle acetylcholine receptor in transfected COS cells. J Cell Biol. 1991 Aug;114(4):799–807. doi: 10.1083/jcb.114.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Characterization of acetylcholine receptor subunits in developing and in denervated mammalian muscle. J Biol Chem. 1988 Sep 15;263(26):12878–12885. [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Henderson L. P., Lechleiter J. D., Brehm P. Single channel properties of newly synthesized acetylcholine receptors following denervation of mammalian skeletal muscle. J Gen Physiol. 1987 Jun;89(6):999–1014. doi: 10.1085/jgp.89.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Imoto K., Mishina M., Konno T., Numa S., Sakmann B. Spontaneous and agonist-induced openings of an acetylcholine receptor channel composed of bovine muscle alpha-, beta- and delta-subunits. Pflugers Arch. 1990 Oct;417(2):129–135. doi: 10.1007/BF00370689. [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Schuetze S. M. Kinetic differences between embryonic- and adult-type acetylcholine receptors in rat myotubes. J Physiol. 1988 Feb;396:267–296. doi: 10.1113/jphysiol.1988.sp016962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R., Owens J. L., Camacho P., Mandel G., Brehm P. Multiple conductance classes of mouse nicotinic acetylcholine receptors expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2067–2071. doi: 10.1073/pnas.87.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Fukuda K., Konno T., Mori Y., Tanaka K., Mishina M., Numa S. Functional properties of nicotinic acetylcholine receptor subunits expressed in various combinations. FEBS Lett. 1987 Apr 20;214(2):253–258. doi: 10.1016/0014-5793(87)80065-0. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Nakajima S., Nakajima Y., Takahashi T. Differential development of two classes of acetylcholine receptors in Xenopus muscle in culture. Science. 1984 Oct 5;226(4670):55–57. doi: 10.1126/science.6474189. [DOI] [PubMed] [Google Scholar]
- Lo D. C., Pinkham J. L., Stevens C. F. Influence of the gamma subunit and expression system on acetylcholine receptor gating. Neuron. 1990 Dec;5(6):857–866. doi: 10.1016/0896-6273(90)90345-g. [DOI] [PubMed] [Google Scholar]
- Martinou J. C., Merlie J. P. Nerve-dependent modulation of acetylcholine receptor epsilon-subunit gene expression. J Neurosci. 1991 May;11(5):1291–1299. doi: 10.1523/JNEUROSCI.11-05-01291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. In vivo development of nicotinic acetylcholine receptor channels in Xenopus myotomal muscle. J Neurosci. 1989 Mar;9(3):1018–1028. doi: 10.1523/JNEUROSCI.09-03-01018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. Three conductance classes of nicotinic acetylcholine receptors are expressed in developing amphibian skeletal muscle. J Neurosci. 1989 Jul;9(7):2575–2580. doi: 10.1523/JNEUROSCI.09-07-02575.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. L., Boulter J., Patrick J., Heinemann S. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 1989 Nov;3(5):589–596. doi: 10.1016/0896-6273(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Rohrbough J., Kidokoro Y. Changes in kinetics of acetylcholine receptor channels after initial expression in Xenopus myocyte culture. J Physiol. 1990 Jun;425:245–269. doi: 10.1113/jphysiol.1990.sp018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozental R. In vitro denervation of frog skeletal muscle: expression of several conductance classes of nicotinic receptors. Neurosci Lett. 1991 Nov 25;133(1):65–67. doi: 10.1016/0304-3940(91)90058-2. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Trautmann A., Koenig J. Single acetylcholine-activated channel currents in developing muscle cells. Dev Biol. 1984 Aug;104(2):366–379. doi: 10.1016/0012-1606(84)90092-7. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Claudio T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. J Biol Chem. 1991 Oct 15;266(29):19369–19377. [PubMed] [Google Scholar]
- Steinbach J. H. Structural and functional diversity in vertebrate skeletal muscle nicotinic acetylcholine receptors. Annu Rev Physiol. 1989;51:353–365. doi: 10.1146/annurev.ph.51.030189.002033. [DOI] [PubMed] [Google Scholar]
- White M. M., Mayne K. M., Lester H. A., Davidson N. Mouse-Torpedo hybrid acetylcholine receptors: functional homology does not equal sequence homology. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4852–4856. doi: 10.1073/pnas.82.14.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Criado M., Stein E., Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989 Jan 2;242(2):419–424. doi: 10.1016/0014-5793(89)80514-9. [DOI] [PubMed] [Google Scholar]
- Yu L., Leonard R. J., Davidson N., Lester H. A. Single-channel properties of mouse-Torpedo acetylcholine receptor hybrids expressed in Xenopus oocytes. Brain Res Mol Brain Res. 1991 Jun;10(3):203–211. doi: 10.1016/0169-328x(91)90062-3. [DOI] [PubMed] [Google Scholar]