Abstract
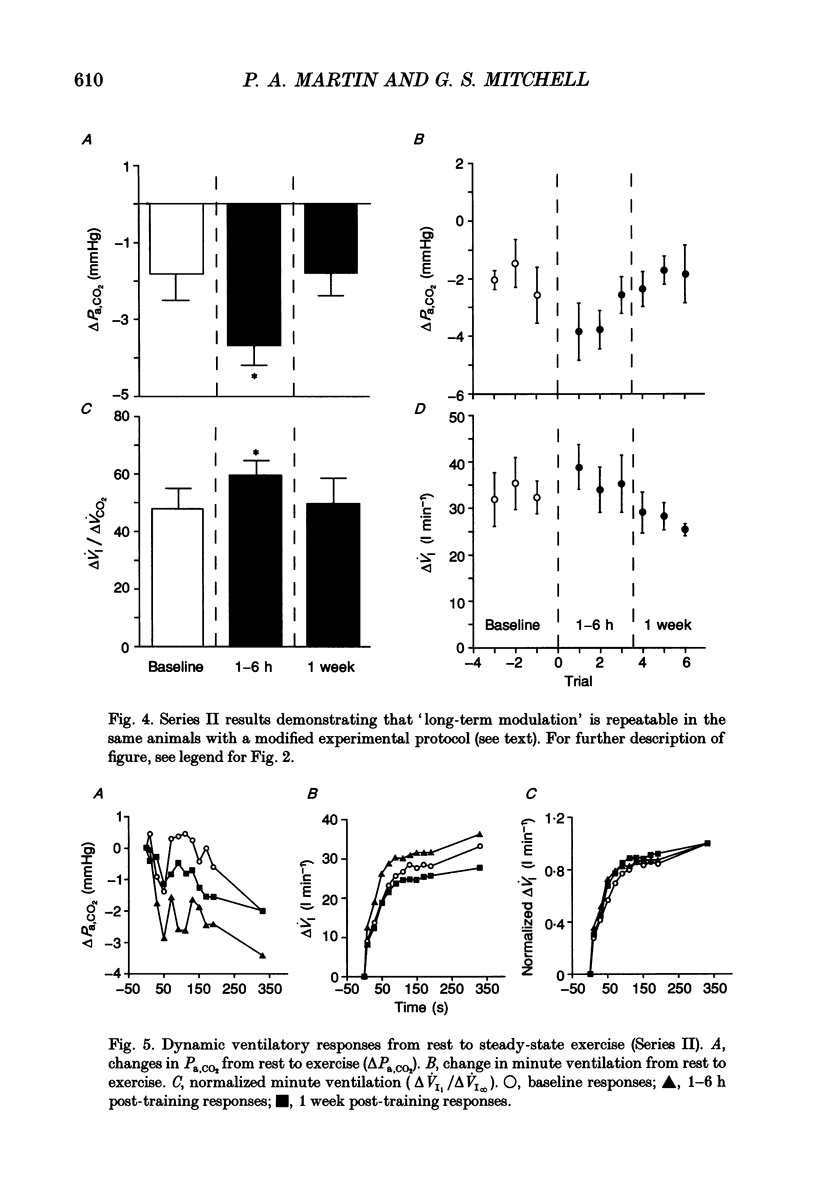
1. To test the hypothesis that repeated associations of exercise and increased respiratory dead space elicit mechanisms that augment future ventilatory responses to exercise alone, experiments were conducted on normal adult goats familiarized with experimental procedures. 2. Measurements of ventilation, arterial blood gases and CO2 production were made at rest, during mild steady-state exercise (4 km h-1; 5% grade) and with increased dead space at rest in seven goats before and after training. In Series I experiments, training consisted of fourteen to twenty exercise trials explicitly paired with increased dead space (0.8 l) over 2 days. Increased dead space predominantly represents a CO2 chemoreceptor stimulus with only mild hypoxic stimulation. Post-training measurements were made 1-6 h and 1 week after training was completed. 3. The same goats repeated a slightly modified protocol several months later (Series II; 6 trials per day for 4 days) to determine if responses were both repeatable and reversible, and to investigate training effects on dynamic ventilatory responses at the onset of exercise. 4. In Series I experiments, resting minute ventilation and breathing frequency were elevated 1-6 h post-training compared to baseline (44 and 74% respectively), whereas resting tidal volume decreased (14%). One week post-training, resting values had returned to baseline. Series II training had no significant effects on resting measurements. 5. Relative to baseline, arterial partial pressure of CO2 (Pa,CO2) values decreased significantly more from rest to exercise 1-6 h post-training in both Series I (2.7 +/- 0.2 vs. 1.8 +/- 0.9 mmHg) and Series II (3.4 +/- 0.6 vs. 2.0 +/- 0.6 mmHg). The exercise ventilatory response increased 25-28% 1-6 h post-training (both series), largely due to a greater exercise frequency response, but returned to baseline 1 week post-training. Training had no effect on ventilatory responses to CO2 at rest, suggesting that decreases in CO2 chemoreceptor responsiveness did not cause the greater exercise ventilatory response. Model estimates indicate that the net feedforward exercise ventilatory stimulus was increased 40-50% by training. 6. Training had no discernable effects on ventilatory dynamics at the onset of exercise. However, post-training differences in Pa,CO2 regulation and ventilation were established early in exercise, prior to steady state. 7. Collectively, these experiments suggest a previously unsuspected degree of repeatable and reversible plasticity in the control system subserving the exercise ventilatory response. Such plasticity may contribute to the development of normal exercise hyperpnoea and to adaptive responses of the ventilatory control system in adult animals.
Full text
PDF











Selected References
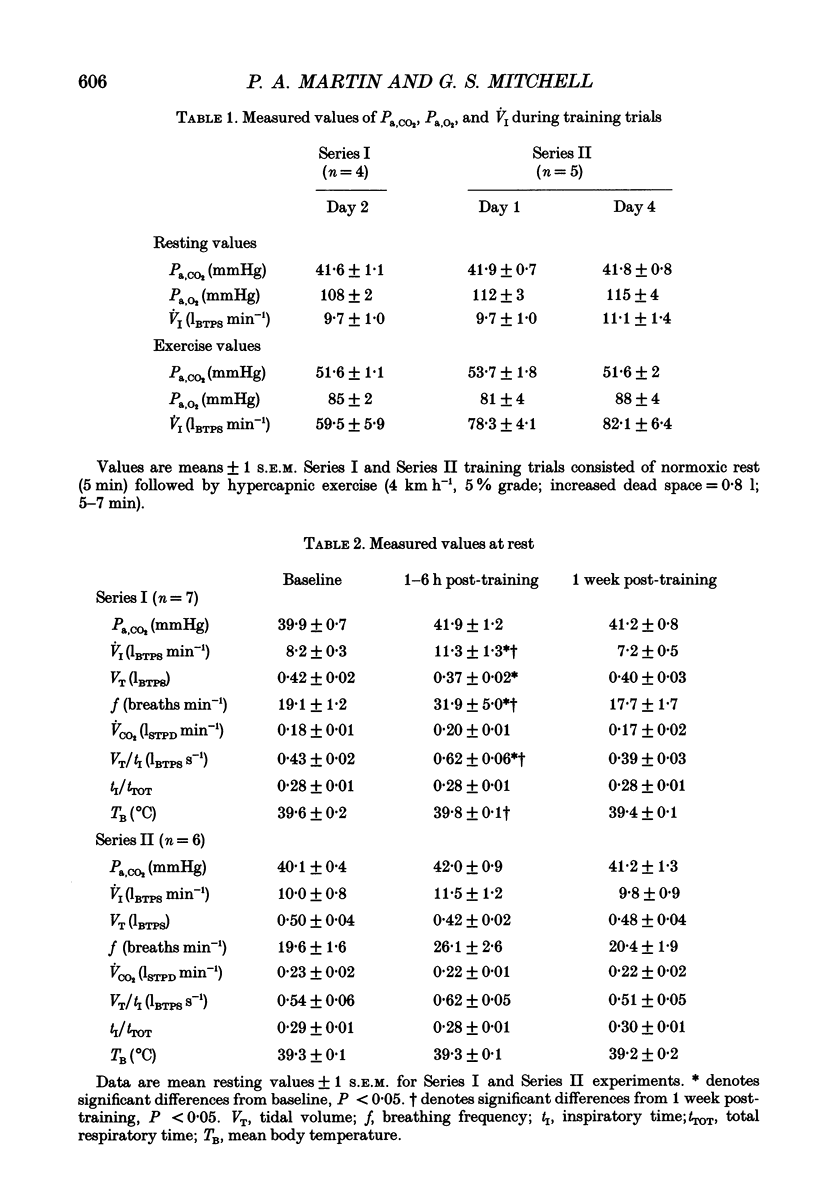
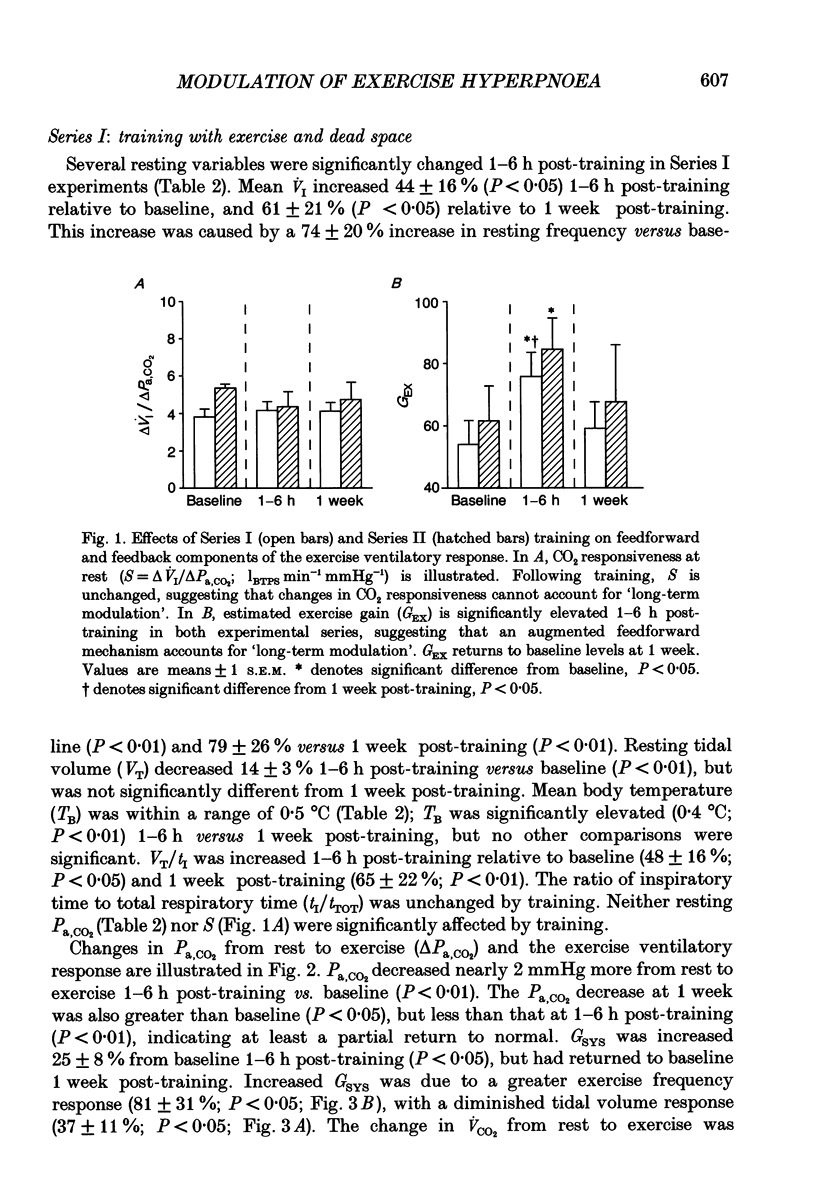
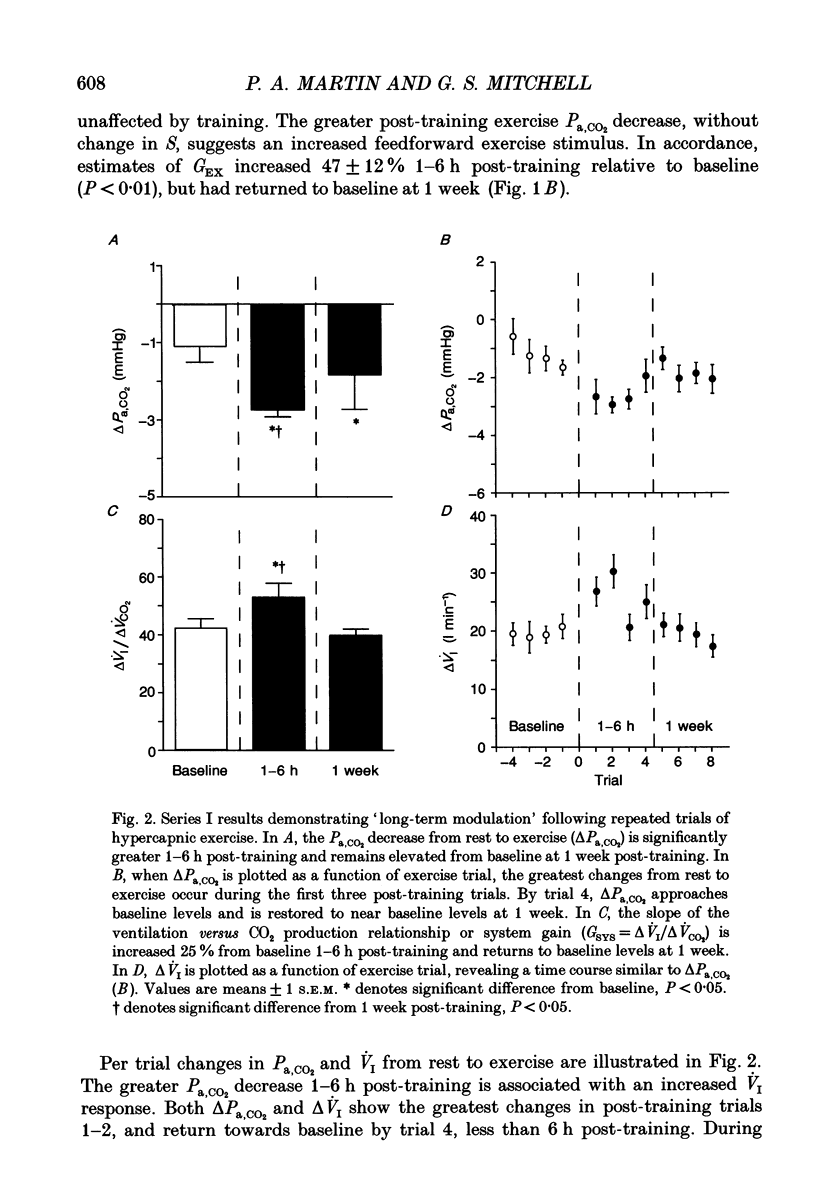
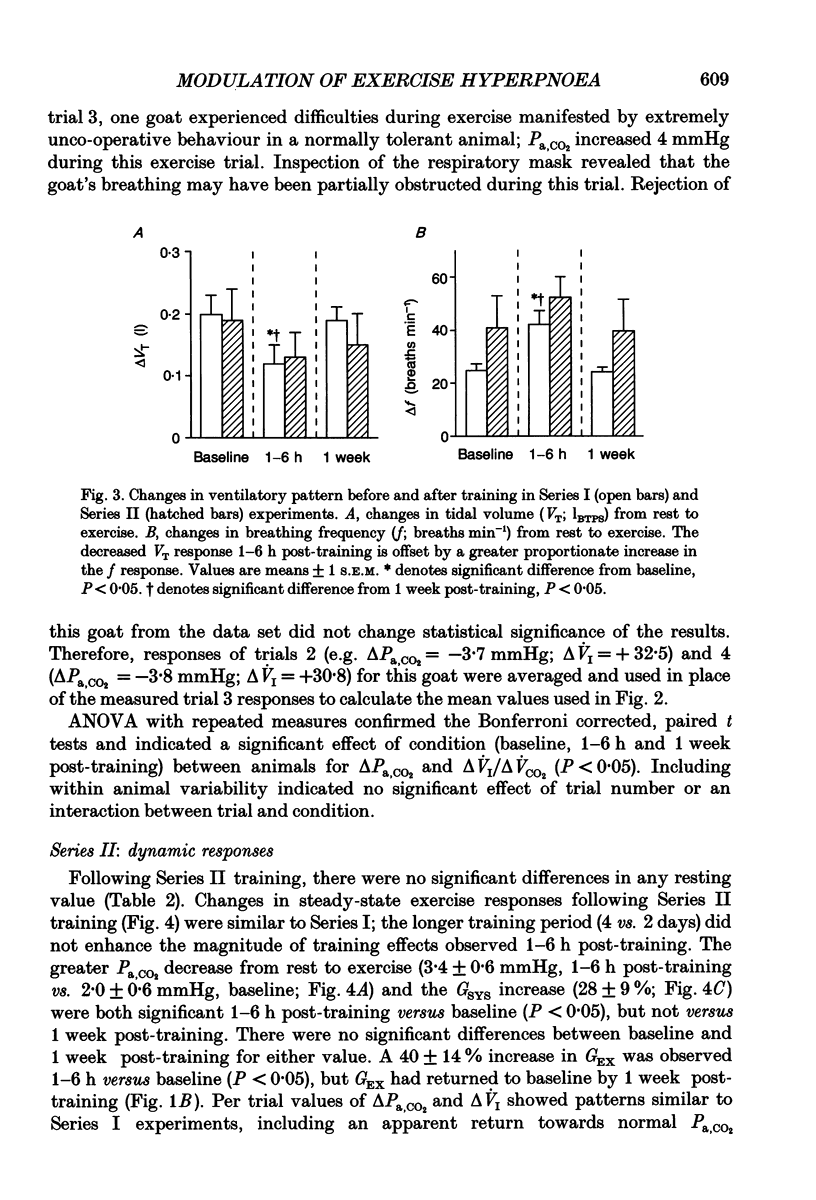
These references are in PubMed. This may not be the complete list of references from this article.
- Bach K. B., Lutcavage M. E., Mitchell G. S. Serotonin is necessary for short-term modulation of the exercise ventilatory response. Respir Physiol. 1993 Jan;91(1):57–70. doi: 10.1016/0034-5687(93)90089-s. [DOI] [PubMed] [Google Scholar]
- Baker M. A. Effects of dehydration and rehydration on thermoregulatory sweating in goats. J Physiol. 1989 Oct;417:421–435. doi: 10.1113/jphysiol.1989.sp017810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett F. M., Fordyce W. E. Characteristics of the ventilatory exercise stimulus. Respir Physiol. 1985 Jan;59(1):55–63. doi: 10.1016/0034-5687(85)90018-0. [DOI] [PubMed] [Google Scholar]
- Bennett F. M., Fordyce W. E. Gain of the ventilatory exercise stimulus: definition and meaning. J Appl Physiol (1985) 1988 Nov;65(5):2011–2017. doi: 10.1152/jappl.1988.65.5.2011. [DOI] [PubMed] [Google Scholar]
- Bisgard G. E., Forster H. V., Mesina J., Sarazin R. G. Role of the carotid body in hyperpnea of moderate exercise in goats. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1216–1222. doi: 10.1152/jappl.1982.52.5.1216. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Forster H. V. Mediation of Ventilatory Adaptations. Physiol Rev. 1982 Jan;62(1):262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Mitchell G. S., Smith C. A. Exercise and chemoreception. Am Rev Respir Dis. 1984 Feb;129(2 Pt 2):S31–S34. doi: 10.1164/arrd.1984.129.2P2.S31. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Vidruk E. H., Mitchell G. S. Pulmonary control systems in exercise: update. Fed Proc. 1985 Apr;44(7):2260–2270. [PubMed] [Google Scholar]
- Gallego J., Perruchet P. Classical conditioning of ventilatory responses in humans. J Appl Physiol (1985) 1991 Feb;70(2):676–682. doi: 10.1152/jappl.1991.70.2.676. [DOI] [PubMed] [Google Scholar]
- Goode R. C., Brown E. B., Jr, Howson M. G., Cunningham D. J. Respiratory effects of breathing down a tube. Respir Physiol. 1969 Apr;6(3):343–359. doi: 10.1016/0034-5687(69)90033-4. [DOI] [PubMed] [Google Scholar]
- Houk J. C. Control strategies in physiological systems. FASEB J. 1988 Feb;2(2):97–107. doi: 10.1096/fasebj.2.2.3277888. [DOI] [PubMed] [Google Scholar]
- Levy M., Susswein A. J. Learned changes of respiratory pump rate in response to lowered pH in Aplysia. Behav Neural Biol. 1990 Nov;54(3):218–233. doi: 10.1016/0163-1047(90)90606-7. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Douse M. A., Foley K. T. Receptor interactions in modulating ventilatory activity. Am J Physiol. 1990 Nov;259(5 Pt 2):R911–R920. doi: 10.1152/ajpregu.1990.259.5.R911. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Osborne J. L. Avian intrapulmonary chemoreceptors: respiratory response to a step decrease in PCO2. Respir Physiol. 1978 May;33(2):251–261. doi: 10.1016/0034-5687(78)90074-9. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Smith C. A., Dempsey J. A. Changes in the VI-VCO2 relationship during exercise in goats: role of carotid bodies. J Appl Physiol Respir Environ Exerc Physiol. 1984 Dec;57(6):1894–1900. doi: 10.1152/jappl.1984.57.6.1894. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S. Ventilatory control during exercise with increased respiratory dead space in goats. J Appl Physiol (1985) 1990 Aug;69(2):718–727. doi: 10.1152/jappl.1990.69.2.718. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y. Regulation of breathing during exercise. Ann Physiol Anthropol. 1990 Apr;9(2):133–138. doi: 10.2114/ahs1983.9.133. [DOI] [PubMed] [Google Scholar]
- Oliven A., Lohda S., Adams M. E., Simhai B., Kelsen S. G. Effect of fatiguing resistive loads on the level and pattern of respiratory activity in awake goats. Respir Physiol. 1988 Sep;73(3):311–324. doi: 10.1016/0034-5687(88)90053-9. [DOI] [PubMed] [Google Scholar]
- Pan L. G., Forster H. V., Bisgard G. E., Kaminski R. P., Dorsey S. M., Busch M. A. Hyperventilation in ponies at the onset of and during steady-state exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1394–1402. doi: 10.1152/jappl.1983.54.5.1394. [DOI] [PubMed] [Google Scholar]
- Poon C. S. Potentiation of exercise ventilatory response by airway CO2 and dead space loading. J Appl Physiol (1985) 1992 Aug;73(2):591–595. doi: 10.1152/jappl.1992.73.2.591. [DOI] [PubMed] [Google Scholar]
- Schaefer S. L., Mitchell G. S. Ventilatory control during exercise with peripheral chemoreceptor stimulation: hypoxia vs. domperidone. J Appl Physiol (1985) 1989 Dec;67(6):2438–2446. doi: 10.1152/jappl.1989.67.6.2438. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Mitchell G. S., Jameson L. C., Musch T. I., Dempsey J. A. Ventilatory response of goats to treadmill exercise: grade effects. Respir Physiol. 1983 Dec;54(3):331–341. doi: 10.1016/0034-5687(83)90076-2. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci. 1988 Jul;11(7):328–332. doi: 10.1016/0166-2236(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Swanson G. D. The exercise hyperpnea dilemma. Chest. 1978 Feb;73(2 Suppl):277–279. doi: 10.1378/chest.73.2.277. [DOI] [PubMed] [Google Scholar]
- Thompson R. F. The neurobiology of learning and memory. Science. 1986 Aug 29;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Tobin M. J., Perez W., Guenther S. M., D'Alonzo G., Dantzker D. R. Breathing pattern and metabolic behavior during anticipation of exercise. J Appl Physiol (1985) 1986 Apr;60(4):1306–1312. doi: 10.1152/jappl.1986.60.4.1306. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wolpaw J. R., Carp J. S. Memory traces in spinal cord. Trends Neurosci. 1990 Apr;13(4):137–142. doi: 10.1016/0166-2236(90)90005-u. [DOI] [PubMed] [Google Scholar]
- Younes M., Burks J. Breathing pattern during and after exercise of different intensities. J Appl Physiol (1985) 1985 Sep;59(3):898–908. doi: 10.1152/jappl.1985.59.3.898. [DOI] [PubMed] [Google Scholar]


