Abstract
1. Intracellular recordings were made from submucosal neurones in guinea-pig ileum. In some animals, the extrinsic (sympathetic) nerves to the submucosal plexus were severed 5-7 days previously. The actions of somatostatin and somatostatin analogues on membrane potential, membrane current and inhibitory postsynaptic potentials (IPSPs) were examined. 2. Somatostatin, somatostatin(1-28), [D-Trp8]somatostatin and the somatostatin analogue CGP 23996 all produced equivalent maximum hyperpolarizations or outward currents; half-maximal concentrations (EC50 values) were 9-11 nM. The somatostatin analogue MK 678 had an EC50 of 0.9 nM. Extrinsic sympathectomy did not alter concentration-response relations for somatostatin or its analogues. 3. Somatostatin (> 100 nM) produced hyperpolarization or outward current that declined almost completely during superfusion for 2-4 min; decline of the somatostatin current was exponential with a time constant of 30 s in the presence of 2 microM somatostatin. Desensitization was not altered by extrinsic denervation. 4. Recovery from desensitization was rapid and followed the time course of agonist wash-out. Forskolin, phorbol esters, dithiothreitol, hydrogen peroxide, concanavalin A, or reducing temperature from 35 to 29 degrees C did not alter the time course, degree of, or recovery from desensitization. 5. The somatostatin-induced desensitization was of the homologous type; no cross-desensitization to opiate or alpha 2-adrenoceptor agonists (which activate the same potassium conductance) occurred. 6. Somatostatin desensitization did not alter the adrenergic IPSP seen in sympathetically innervated preparations but abolished the non-adrenergic IPSP recorded from normal preparations and from preparations in which the extrinsic sympathetic nerve supply had been surgically removed. 7. The selective blockade of the non-adrenergic IPSP by the homologous-type somatostatin desensitization characterized in the present study provides strong support for the hypothesis that somatostatin is the neurotransmitter underlying the non-adrenergic IPSP in both normal and extrinsically denervated submucosal neurones.
Full text
PDF


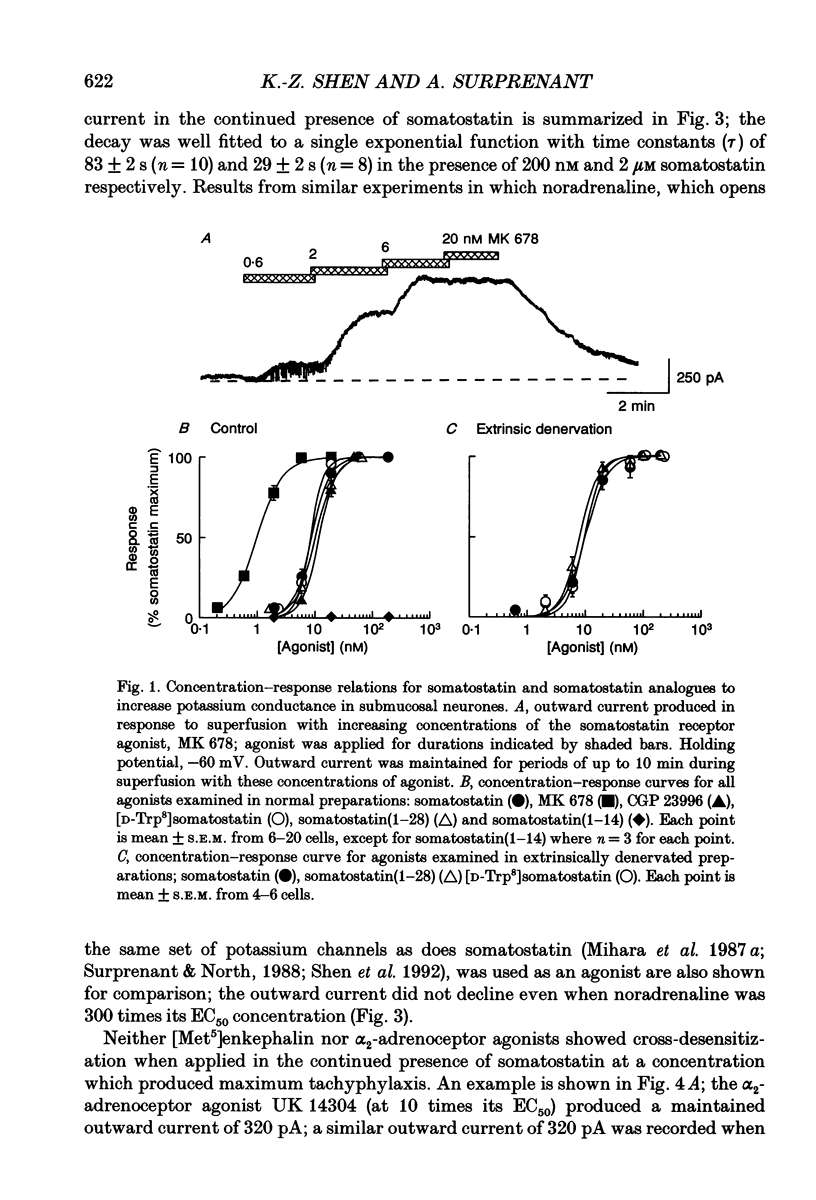
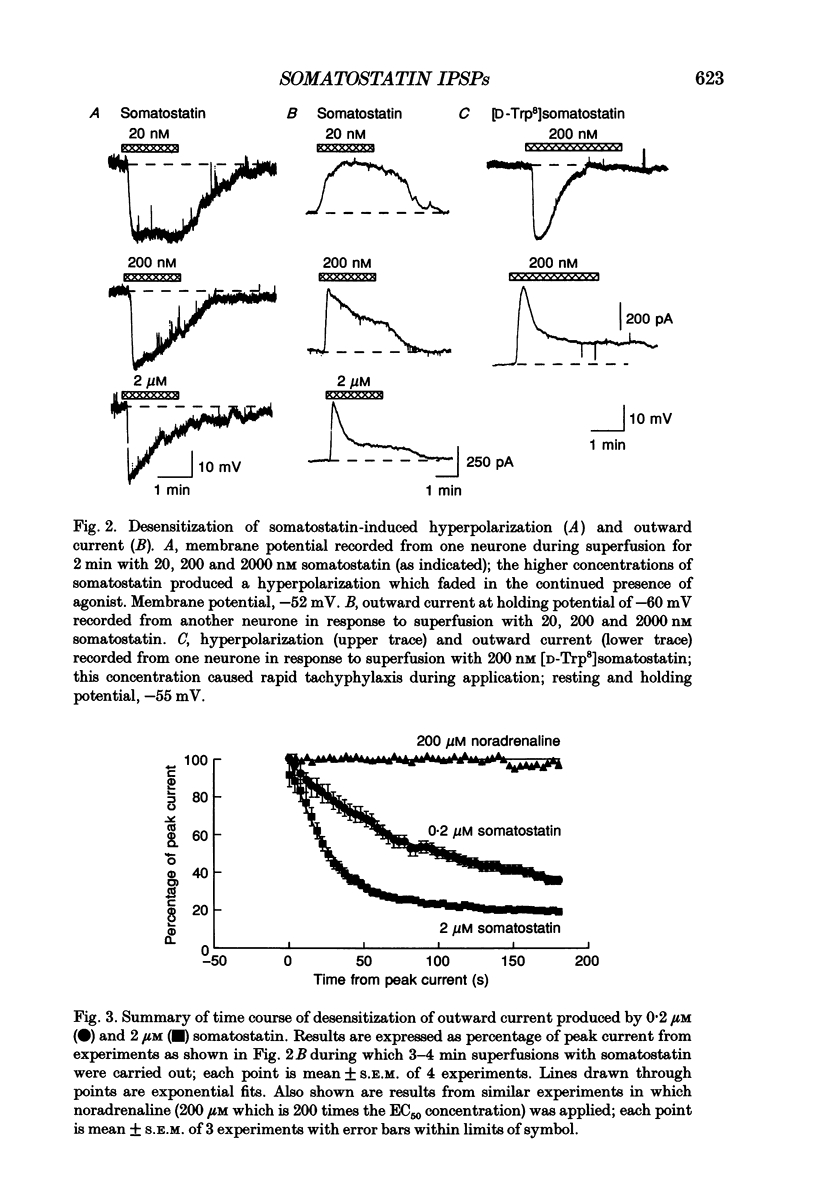

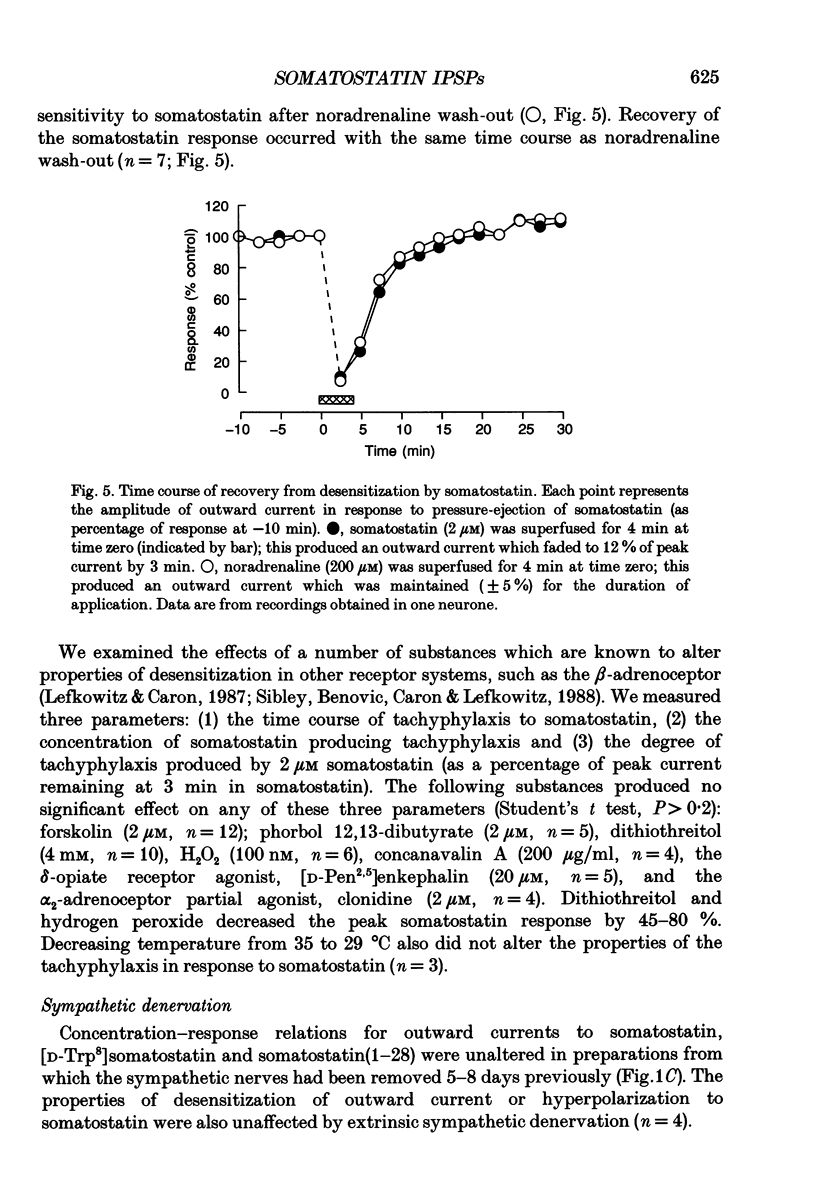
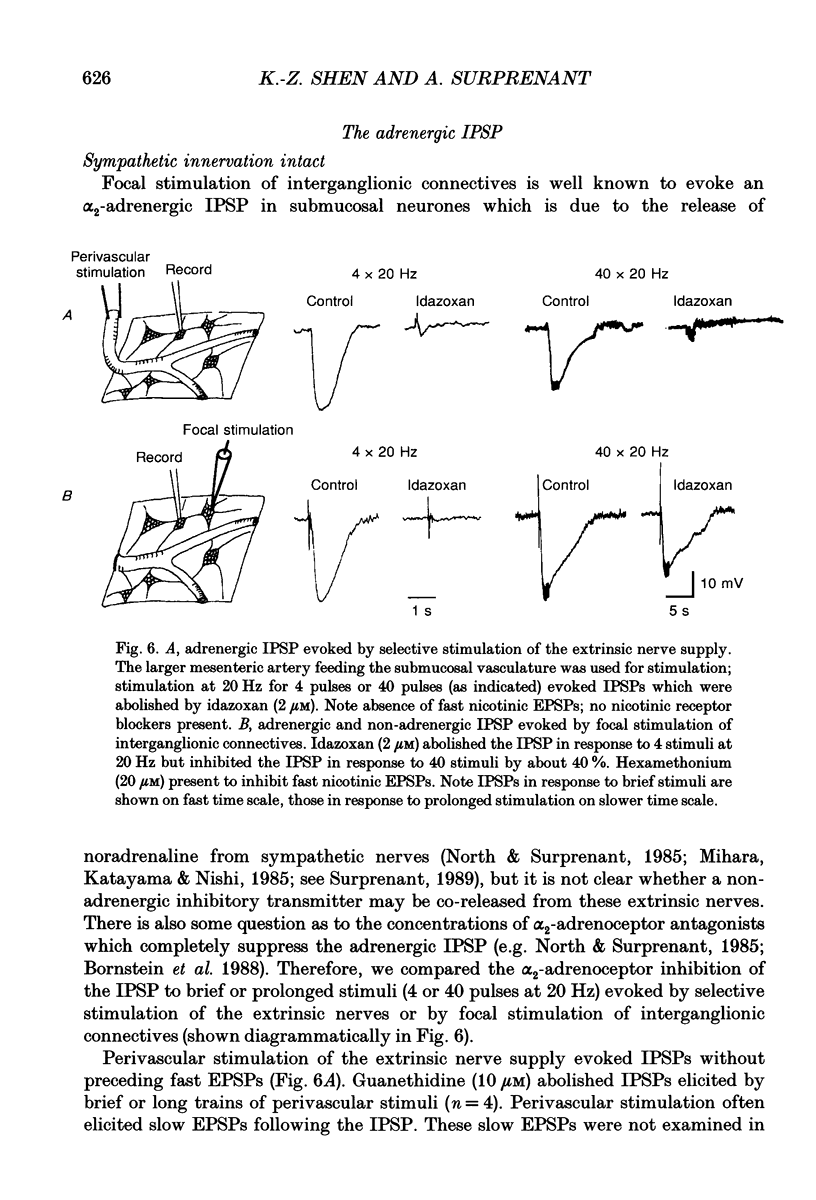






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein J. C., Costa M., Furness J. B. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol. 1988 Apr;398:371–390. doi: 10.1113/jphysiol.1988.sp017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purines and cotransmitters in adrenergic and cholinergic neurones. Prog Brain Res. 1986;68:193–203. doi: 10.1016/s0079-6123(08)60239-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990 Mar-Apr;304:7–33. [PubMed] [Google Scholar]
- Costa M., Furness J. B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984 Nov;13(3):911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudl R. J., Anderson D. S., Forsythe A. B., Ziegler M. G., O'Dorisio T. M. Treatment of diabetic diarrhea and orthostatic hypotension with somatostatin analogue SMS 201-995. Am J Med. 1987 Sep;83(3):584–588. doi: 10.1016/0002-9343(87)90777-7. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Distribution of intrinsic nerve cell bodies and axons which take up aromatic amines and their precursors in the small intestine of the guinea-pig. Cell Tissue Res. 1978 Apr 28;188(3):527–543. doi: 10.1007/BF00219790. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry. 1975;41(4):335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Wilson A. J. Water-stable fluorophores, produced by reaction with aldehyde solutions, for the histochemical localization of catechol- and indolethylamines. Histochemistry. 1977 May 20;52(2):159–170. doi: 10.1007/BF00492292. [DOI] [PubMed] [Google Scholar]
- Gaginella T. S., O'Dorisio T. M., Fassler J. E., Mekhjian H. S. Treatment of endocrine and nonendocrine secretory diarrheal states with Sandostatin. Metabolism. 1990 Sep;39(9 Suppl 2):172–175. doi: 10.1016/0026-0495(90)90239-9. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Costa M., Furness J. B. Changes in surviving nerve fibers associated with submucosal arteries following extrinsic denervation of the small intestine. Cell Tissue Res. 1988 Sep;253(3):647–656. doi: 10.1007/BF00219756. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Iversen S. D., Bloom F., Douglas C., Brown M., Vale W. Calcium-dependent release of somatostatin and neurotensin from rat brain in vitro. Nature. 1978 May 11;273(5658):161–163. doi: 10.1038/273161a0. [DOI] [PubMed] [Google Scholar]
- Jiang M. M., Surprenant A. Re-innervation of submucosal arterioles by myenteric neurones following extrinsic denervation. J Auton Nerv Syst. 1992 Feb;37(2):145–154. doi: 10.1016/0165-1838(92)90243-a. [DOI] [PubMed] [Google Scholar]
- Keast J. R. Mucosal innervation and control of water and ion transport in the intestine. Rev Physiol Biochem Pharmacol. 1987;109:1–59. doi: 10.1007/BFb0031024. [DOI] [PubMed] [Google Scholar]
- Kluxen F. W., Bruns C., Lübbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Molecular and regulatory properties of adrenergic receptors. Recent Prog Horm Res. 1987;43:469–497. doi: 10.1016/b978-0-12-571143-2.50018-6. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Clapham D. E. Somatostatin activates an inwardly rectifying K+ channel in neonatal rat atrial cells. Pflugers Arch. 1989 Aug;414(4):492–494. doi: 10.1007/BF00585062. [DOI] [PubMed] [Google Scholar]
- Li X. J., Forte M., North R. A., Ross C. A., Snyder S. H. Cloning and expression of a rat somatostatin receptor enriched in brain. J Biol Chem. 1992 Oct 25;267(30):21307–21312. [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Mihara S., Nishi S. Muscarinic excitation and inhibition of neurons in the submucous plexus of the guinea-pig caecum. Neuroscience. 1989;31(1):247–257. doi: 10.1016/0306-4522(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Mihara S., Nishi S., North R. A., Surprenant A. A non-adrenergic, non-cholinergic slow inhibitory post-synaptic potential in neurones of the guinea-pig submucous plexus. J Physiol. 1987 Sep;390:357–365. doi: 10.1113/jphysiol.1987.sp016705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya K. I., Arem R. Diabetic diarrhea. Pathophysiology, diagnosis, and management. Arch Intern Med. 1990 Feb;150(2):262–267. doi: 10.1001/archinte.150.2.262. [DOI] [PubMed] [Google Scholar]
- Raynor K., Reisine T. Analogs of somatostatin selectively label distinct subtypes of somatostatin receptors in rat brain. J Pharmacol Exp Ther. 1989 Nov;251(2):510–517. [PubMed] [Google Scholar]
- Raynor K., Wang H. L., Dichter M., Reisine T. Subtypes of brain somatostatin receptors couple to multiple cellular effector systems. Mol Pharmacol. 1991 Aug;40(2):248–253. [PubMed] [Google Scholar]
- Reisine T. D., Takahashi J. S. Somatostatin pretreatment desensitizes somatostatin receptors linked to adenylate cyclase and facilitates the stimulation of cyclic adenosine 3':5'-monophosphate accumulation in anterior pituitary tumor cells. J Neurosci. 1984 Mar;4(3):812–819. doi: 10.1523/JNEUROSCI.04-03-00812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T. Multiple mechanisms of somatostatin inhibition of adrenocorticotropin release from mouse anterior pituitary tumor cells. Endocrinology. 1985 Jun;116(6):2259–2266. doi: 10.1210/endo-116-6-2259. [DOI] [PubMed] [Google Scholar]
- Shen K. Z., North R. A., Surprenant A. Potassium channels opened by noradrenaline and other transmitters in excised membrane patches of guinea-pig submucosal neurones. J Physiol. 1992 Jan;445:581–599. doi: 10.1113/jphysiol.1992.sp018941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Phosphorylation of cell surface receptors: a mechanism for regulating signal transduction pathways. Endocr Rev. 1988 Feb;9(1):38–56. doi: 10.1210/edrv-9-1-38. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Lussier B. T., Kraicer J. Somatostatin activates an inwardly rectifying K+ conductance in freshly dispersed rat somatotrophs. J Physiol. 1991 Sep;441:615–637. doi: 10.1113/jphysiol.1991.sp018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., North R. A. Mechanism of synaptic inhibition by noradrenaline acting at alpha 2-adrenoceptors. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):85–114. doi: 10.1098/rspb.1988.0039. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M. Regulatory peptides of the hypothalamus. Annu Rev Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]


