Abstract
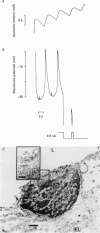
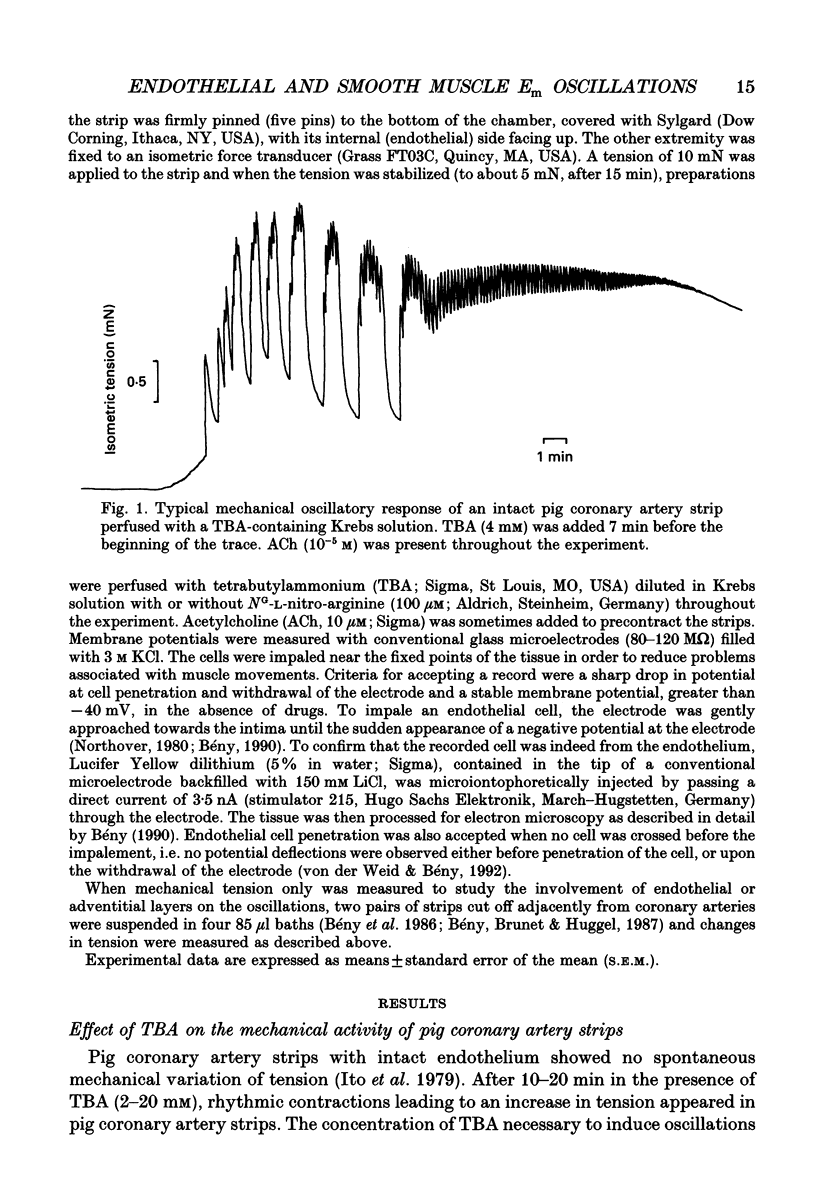
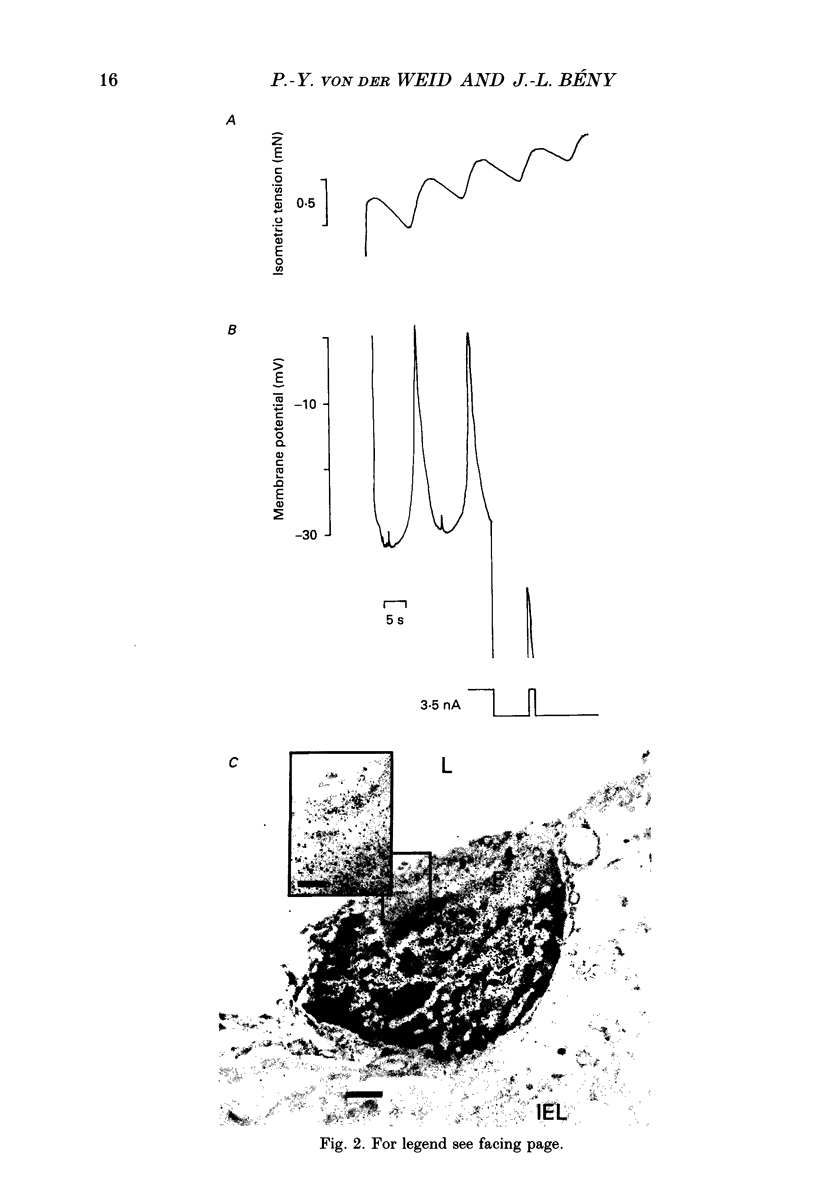
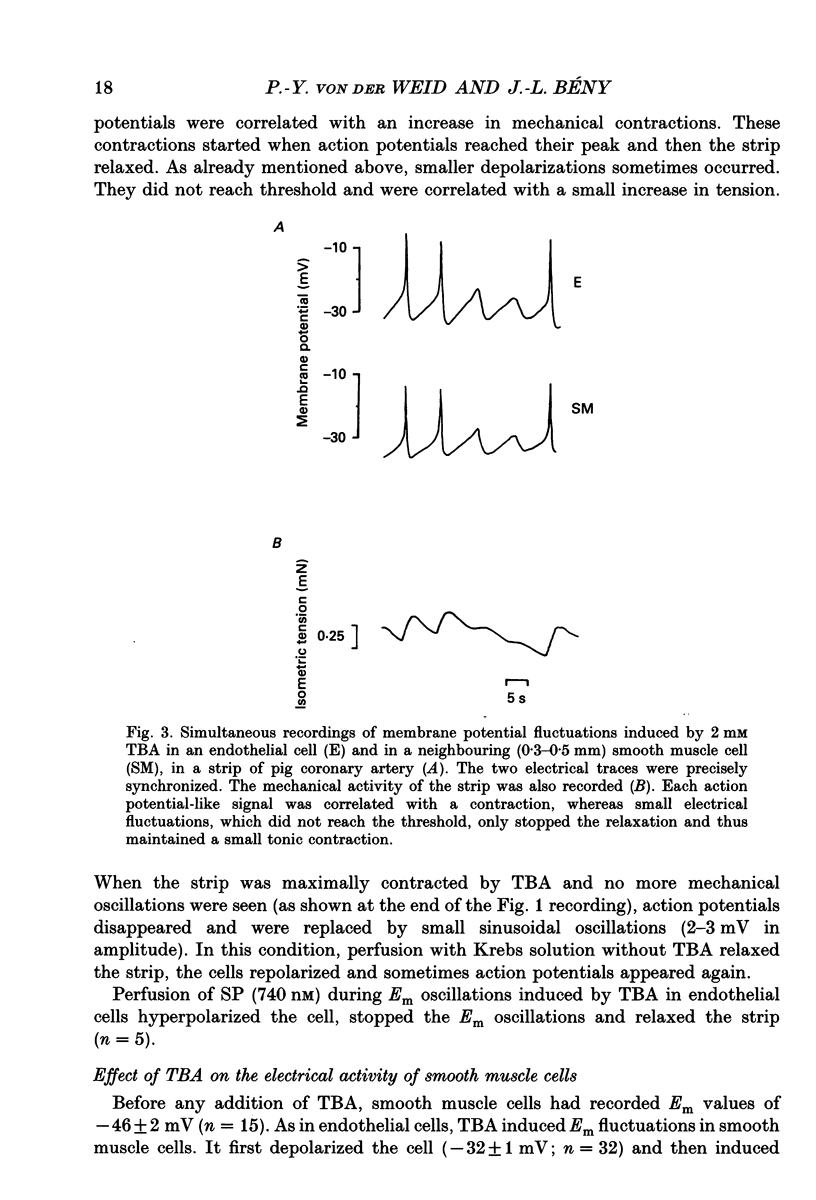
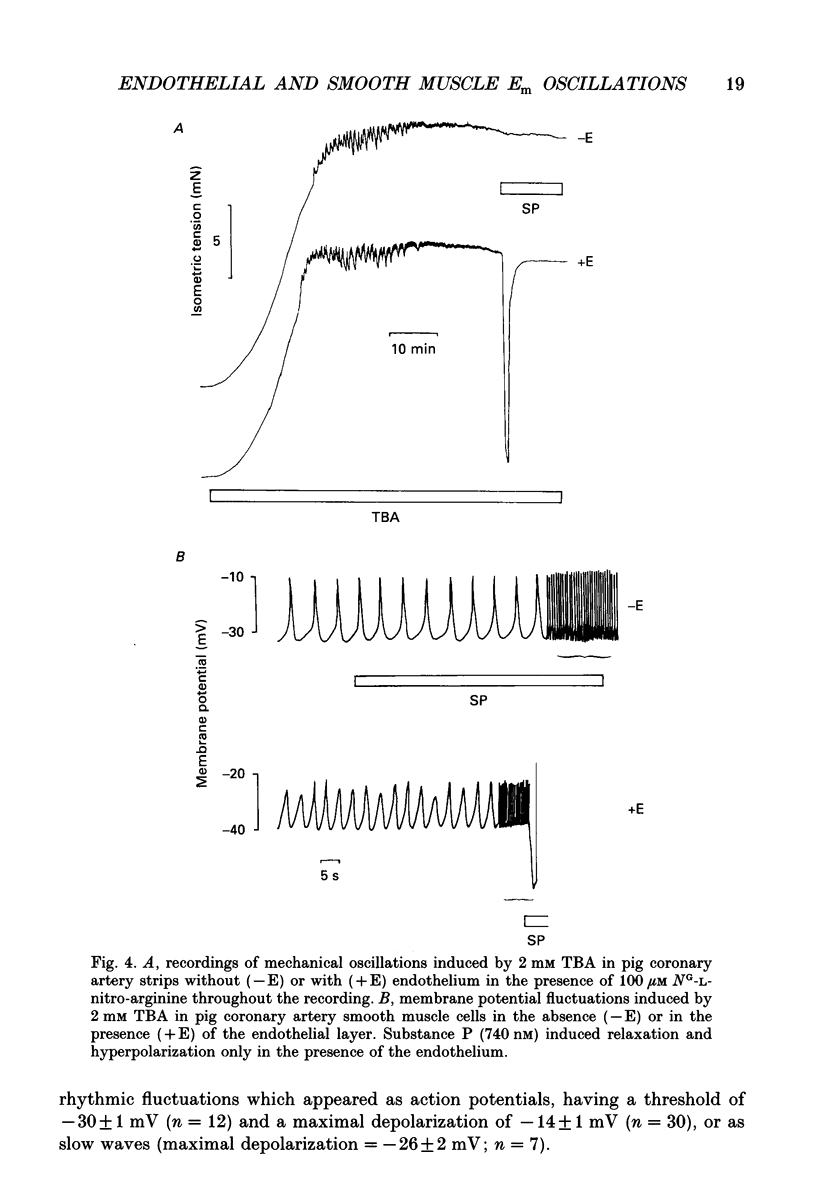
1. The effects of tetrabutylammonium (TBA) on the mechanical tension and on the electrical behaviour of endothelial and smooth muscle cells were studied in intact porcine coronary artery strips. 2. Superfusion of strips with TBA (2-20 mM) induced mechanical oscillations, leading to an increase in tonic isometric tension. 3. TBA-induced mechanical oscillations were correlated with fluctuations of the membrane potential of endothelial cells, which were identified by iontophoretic injection of Lucifer Yellow. 4. The endothelial cell membrane potential fluctuations appeared as action potentials or smaller amplitude slow waves, and were synchronized with electrical membrane potential fluctuations of the underlying coronary smooth muscle cells. 5. Oscillations induced by TBA in smooth muscle cells were not affected by removal of the endothelium, and depended on the presence of calcium in the external medium. 6. To our knowledge, this is the first description of action potential-like fluctuations in the endothelium. It is concluded that the oscillations were generated in the smooth muscle and that they propagate to the endothelium. The question of the mode of propagation of the signal is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beny J. L., Brunet P., Huggel H. Interaction of bradykinin and des-Arg9-bradykinin with isolated pig coronary arteries: mechanical and electrophysiological events. Regul Pept. 1987 Apr;17(4):181–190. doi: 10.1016/0167-0115(87)90061-9. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Elhamdani A., Feltz A. Voltage-dependent calcium entry in confluent bovine capillary endothelial cells. FEBS Lett. 1992 Mar 16;299(3):239–242. doi: 10.1016/0014-5793(92)80123-x. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C., Huggel H. Effects of substance P, calcitonin gene-related peptide and capsaicin on tension and membrane potential of pig coronary artery in vitro. Regul Pept. 1989 Apr;25(1):25–36. doi: 10.1016/0167-0115(89)90245-0. [DOI] [PubMed] [Google Scholar]
- Bény J. L. Endothelial and smooth muscle cells hyperpolarized by bradykinin are not dye coupled. Am J Physiol. 1990 Mar;258(3 Pt 2):H836–H841. doi: 10.1152/ajpheart.1990.258.3.H836. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Gribi F. Dye and electrical coupling of endothelial cells in situ. Tissue Cell. 1989;21(6):797–802. doi: 10.1016/0040-8166(89)90030-x. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Possani L. D., Kunze D. L. Bradykinin-induced potassium current in cultured bovine aortic endothelial cells. J Membr Biol. 1990 Jul;116(3):227–238. doi: 10.1007/BF01868462. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Davies P. F. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis. Lab Invest. 1986 Jul;55(1):5–24. [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Belardinelli L., Sperelakis N., Rubio R., Berne R. M. Differential effects of adenosine and nitroglycerin on the action potentials of large and small coronary arteries. Circ Res. 1979 Feb;44(2):176–182. doi: 10.1161/01.res.44.2.176. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Sperelakis N. Action potentials induced in guinea pig arterial smooth muscle by tetraethylammonium. Am J Physiol. 1979 Jul;237(1):C75–C80. doi: 10.1152/ajpcell.1979.237.1.C75. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Sperelakis N. Membrane electrical properties of vascular smooth muscle from the guinea pig superior mesenteric artery. Pflugers Arch. 1978 Dec 28;378(2):111–119. doi: 10.1007/BF00584443. [DOI] [PubMed] [Google Scholar]
- Herman I. M. Extracellular matrix-cytoskeletal interactions in vascular cells. Tissue Cell. 1987;19(1):1–19. doi: 10.1016/0040-8166(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Hermsmeyer K. Contraction and membrane activation in several mammalian vascular muscles. Life Sci I. 1971 Feb 15;10(4):223–234. doi: 10.1016/0024-3205(71)90251-7. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Suzuki H. The effects of diltiazem (CRD-401) on the membrane and mechanical properties of vascular smooth muscles of the rabbit. Br J Pharmacol. 1978 Dec;64(4):503–510. doi: 10.1111/j.1476-5381.1978.tb17311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. F., Mülsch A., Busse R. Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am J Physiol. 1991 Jan;260(1 Pt 2):H248–H253. doi: 10.1152/ajpheart.1991.260.1.H248. [DOI] [PubMed] [Google Scholar]
- Jackson W. F. Oscillations in active tension in hamster aortas: role of the endothelium. Blood Vessels. 1988;25(3):144–156. doi: 10.1159/000158728. [DOI] [PubMed] [Google Scholar]
- Janssen L. J., Daniel E. E. Depolarizing agents induce oscillations in canine bronchial smooth muscle membrane potential: possible mechanisms. J Pharmacol Exp Ther. 1991 Oct;259(1):110–117. [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Cannell M., van Breemen C. Calcium entry-dependent oscillations of cytoplasmic calcium concentration in cultured endothelial cell monolayers. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1690–1694. doi: 10.1073/pnas.89.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D., Bacal K., Kunze D. L. Bradykinin-activated calcium influx pathway in bovine aortic endothelial cells. Am J Physiol. 1992 Apr;262(4 Pt 2):H942–H948. doi: 10.1152/ajpheart.1992.262.4.H942. [DOI] [PubMed] [Google Scholar]
- Myers J. H., Lamb F. S., Webb R. C. Norepinephrine-induced phasic activity in tail arteries from genetically hypertensive rats. Am J Physiol. 1985 Mar;248(3 Pt 2):H419–H423. doi: 10.1152/ajpheart.1985.248.3.H419. [DOI] [PubMed] [Google Scholar]
- Omote M., Kajimoto N., Mizusawa H. The role of endothelium in the phenylephrine-induced oscillatory responses of rabbit mesenteric arteries. Jpn J Pharmacol. 1992 May;59(1):37–41. doi: 10.1254/jjp.59.37. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. Endothelium-derived relaxing and contracting factors. J Cell Biochem. 1991 May;46(1):27–36. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. S., Bény J. L. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992 Jul;263(1 Pt 2):H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- Spagnoli L. G., Villaschi S., Neri L., Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982 Jan 15;38(1):124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Stein P. G., Driska S. P. Histamine-induced rhythmic contraction of hog carotid artery smooth muscle. Circ Res. 1984 Oct;55(4):480–485. doi: 10.1161/01.res.55.4.480. [DOI] [PubMed] [Google Scholar]
- Takeda K., Schini V., Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pflugers Arch. 1987 Nov;410(4-5):385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Bény J. L. Effect of Ca2+ ionophores on membrane potential of pig coronary artery endothelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):H1823–H1831. doi: 10.1152/ajpheart.1992.262.6.H1823. [DOI] [PubMed] [Google Scholar]