Abstract
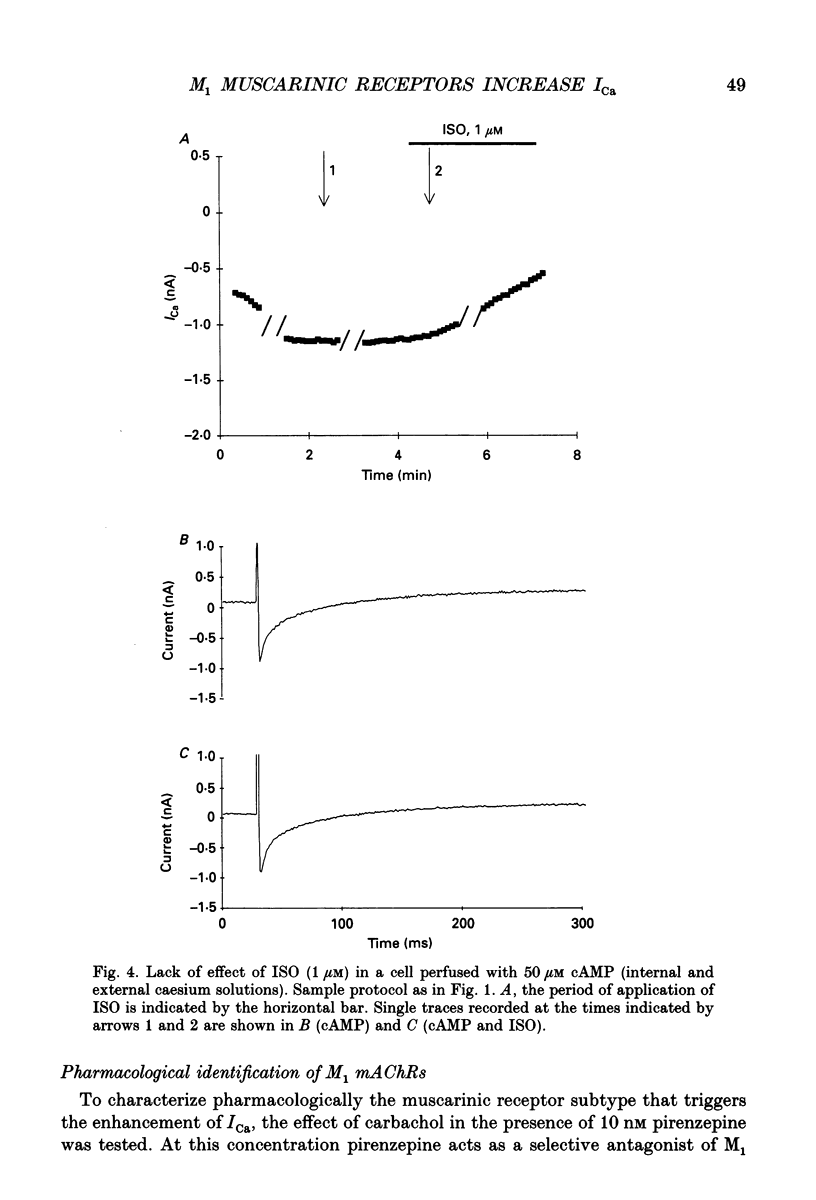
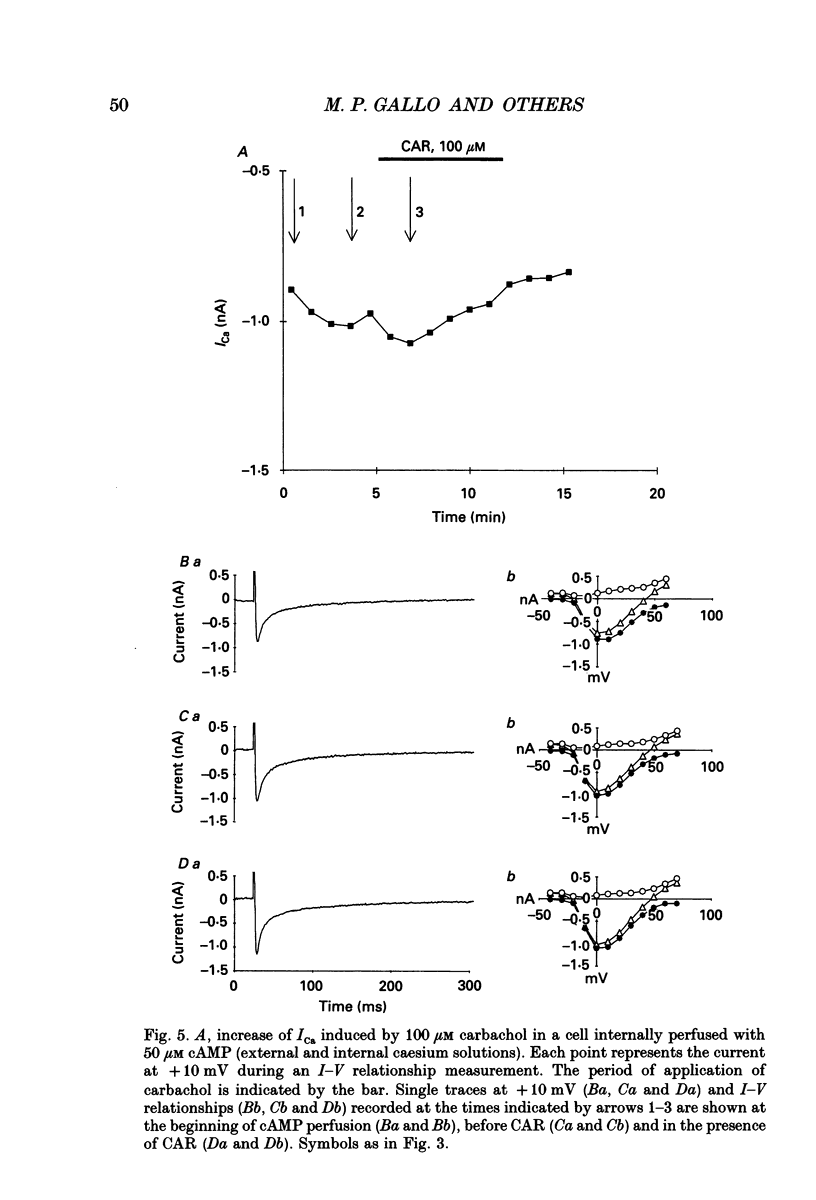
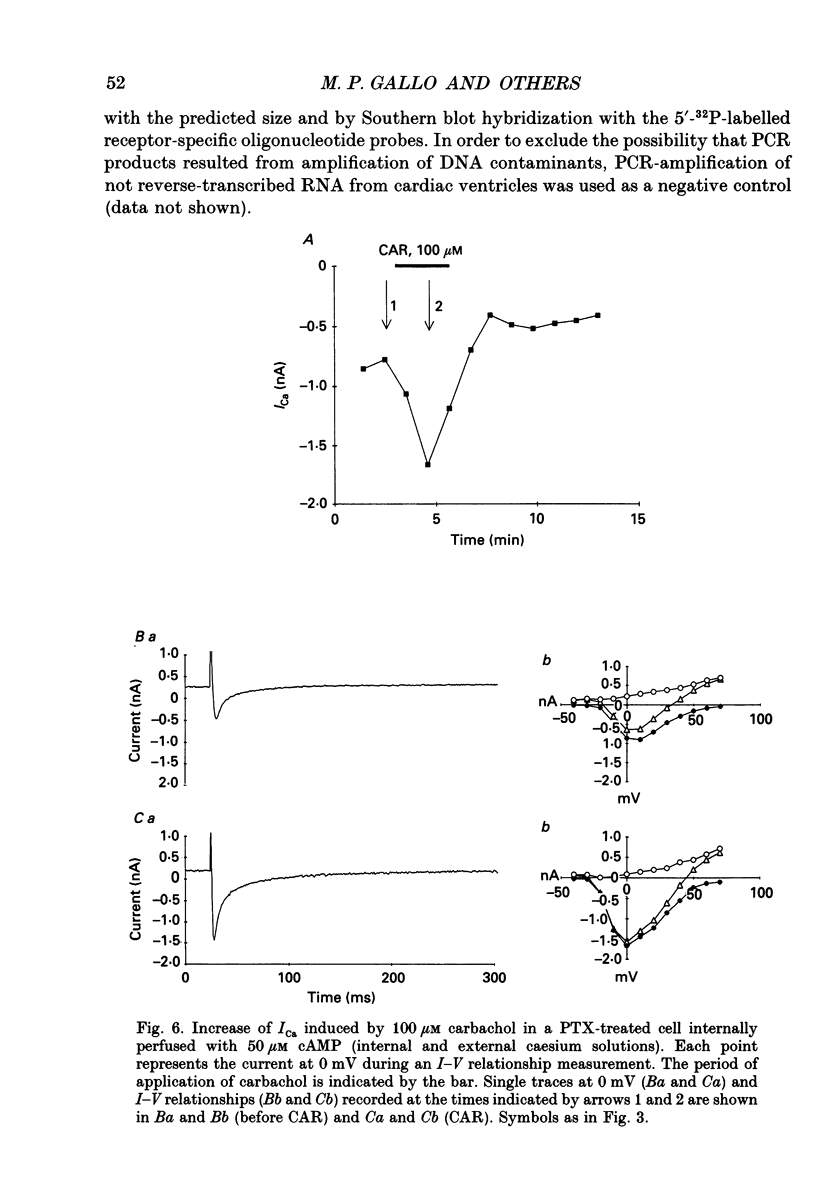
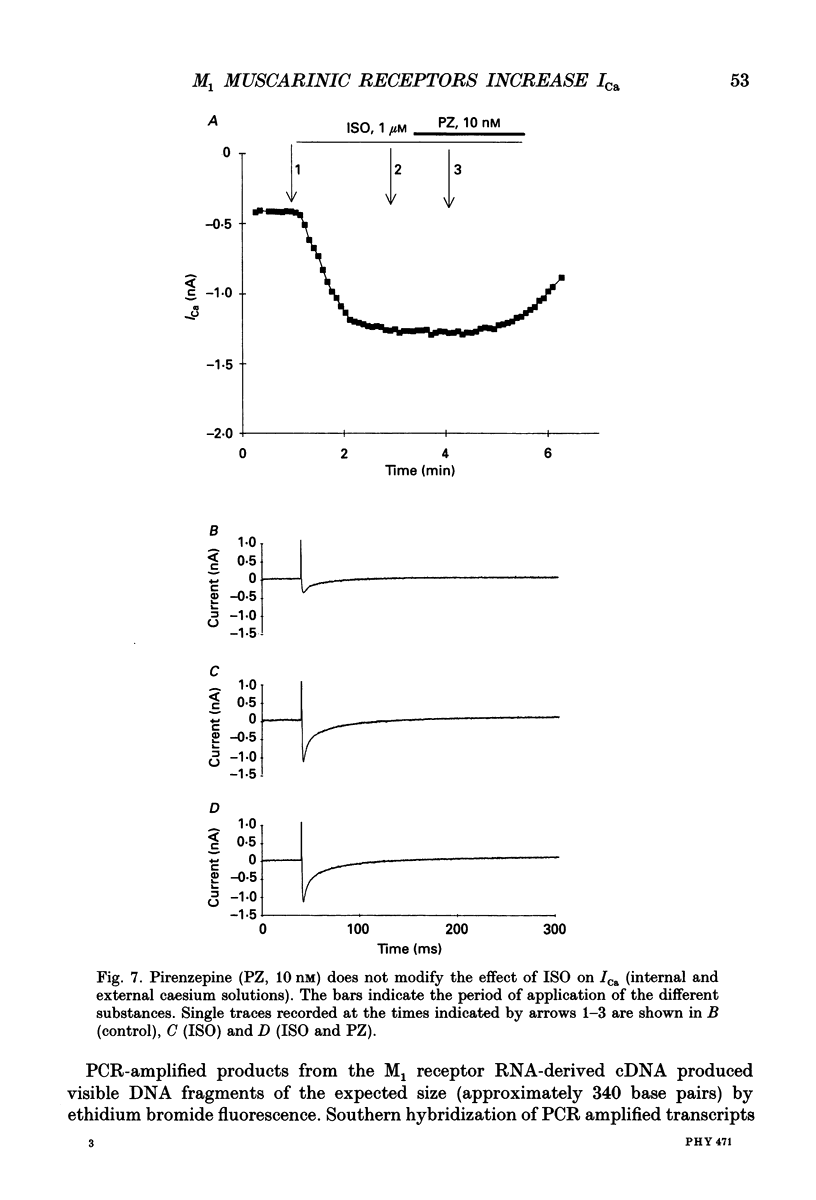
1. Physiological and molecular evidence for the presence and functional role of M1 muscarinic cholinergic receptors (mAChRs) in adult guinea-pig ventricular cells is presented. 2. Whole-cell clamp measurements of the L-type calcium current (ICa) in isolated myocytes were performed. Caesium was used to suppress potassium currents. ICa was increased by the muscarinic agonist carbachol in cells pretreated with pertussis toxin which blocked the M2 mAChR-triggered cascade of intracellular signalling, while it was not changed in untreated cells. 3. If the M2-mediated regulation of ICa was blocked by directly saturating the cell with cyclic adenosine monophosphate (cAMP) through the patch pipette, application of carbachol induced a further small increase of the current above the level reached after cAMP perfusion. This increase was more pronounced in cells pretreated with pertussis toxin. 4. The carbachol-induced increase of ICa was blocked by the selective M1 mAChR antagonist pirenzepine. 5. The application of high concentrations of carbachol increased the accumulation of [3H]inositol monophosphate up to 240% above control levels. This increase was reduced by application of pirenzepine. 6. The expression of M1 receptor mRNA in ventricular cardiocytes was shown by reverse transcriptase-polymerase chain reaction. 7. These results suggest that M1 mAChR regulation of ICa can be a component of the paradoxical positive inotropism induced by high concentrations of muscarinic agonists.
Full text
PDF





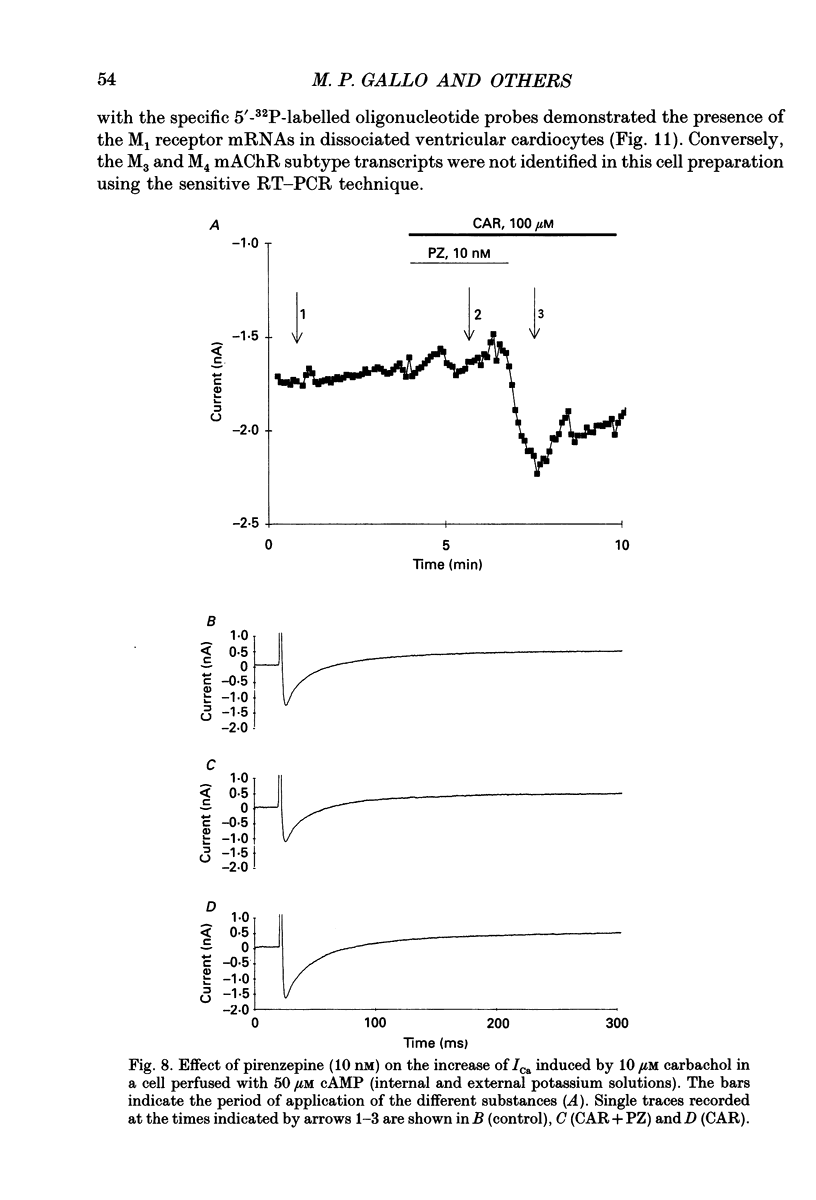
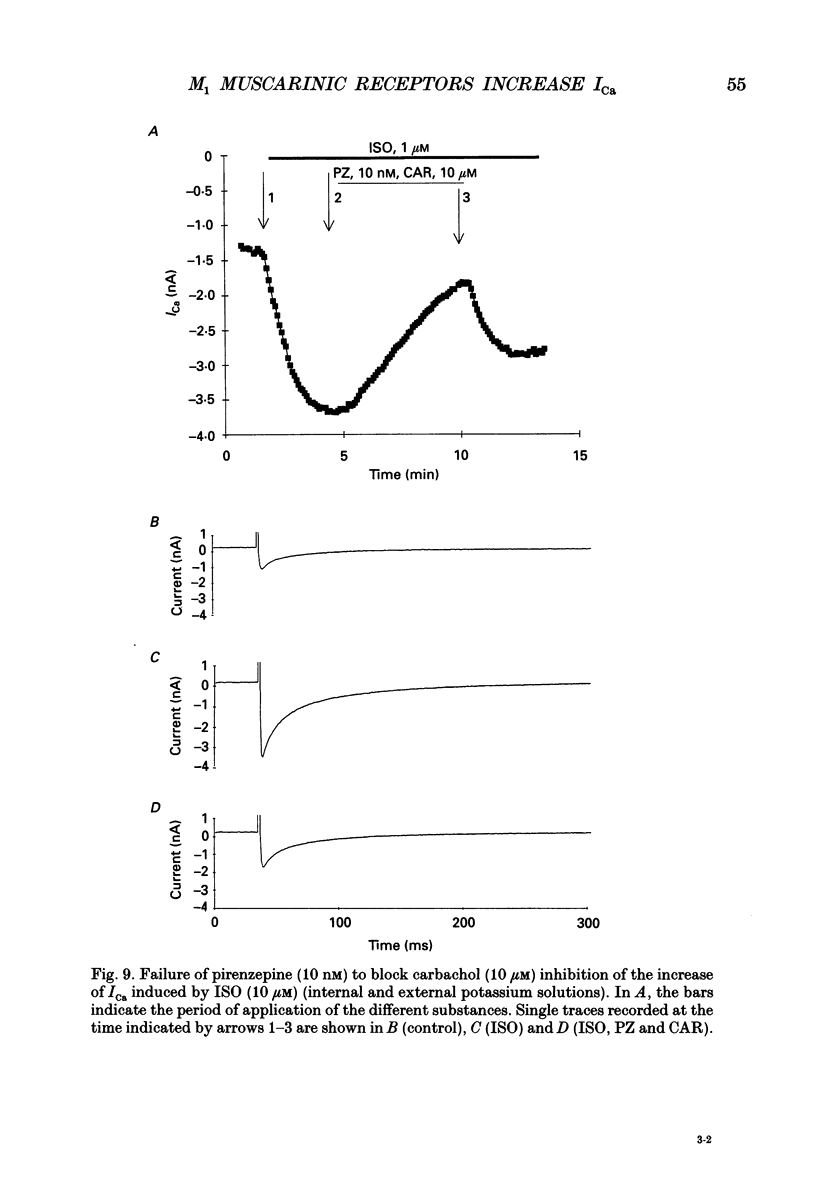
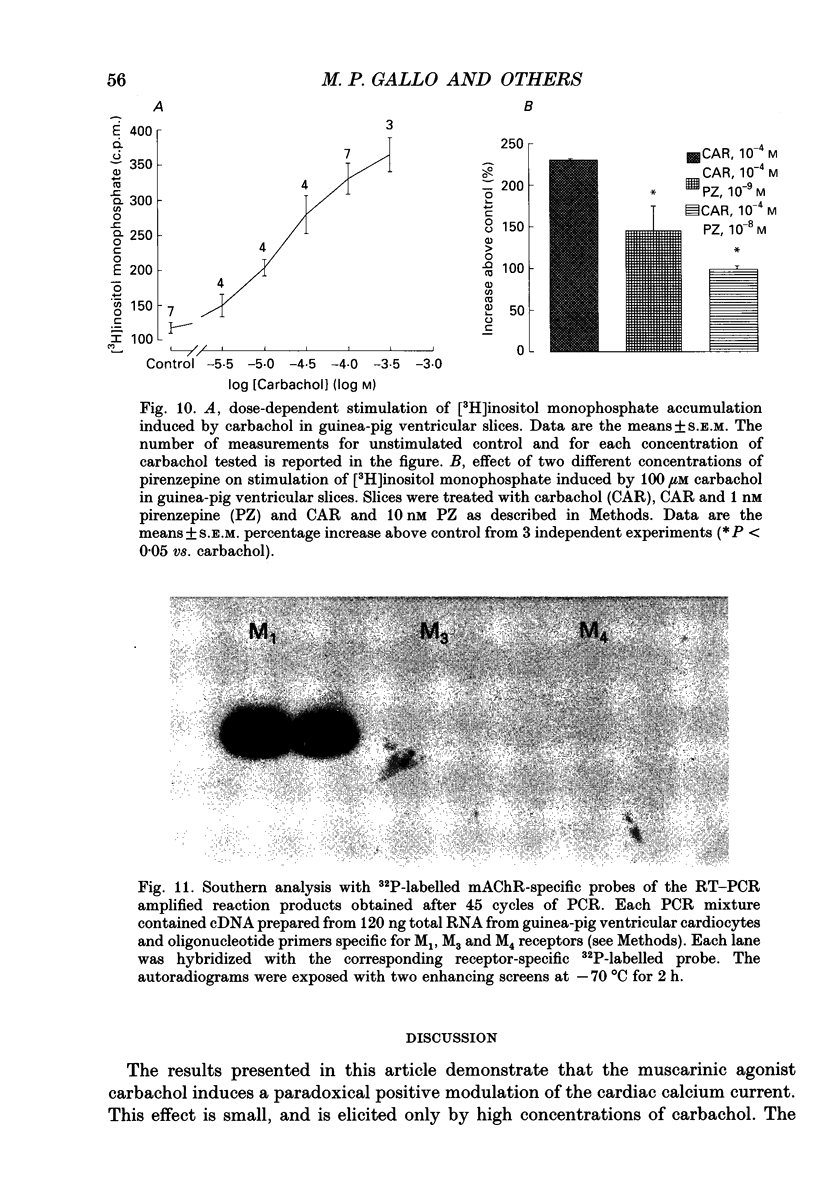




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloatti G., Serazzi L., Levi R. C. Prostaglandin I2 (PGI2) enhances calcium current in guinea-pig ventricular heart cells. J Mol Cell Cardiol. 1991 Jul;23(7):851–860. doi: 10.1016/0022-2828(91)90218-b. [DOI] [PubMed] [Google Scholar]
- Alvarez J. L., Mongo K., Scamps F., Vassort G. Effects of purinergic stimulation on the Ca current in single frog cardiac cells. Pflugers Arch. 1990 Apr;416(1-2):189–195. doi: 10.1007/BF00370241. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I. New subtypes of muscarinic acetylcholine receptors. Trends Pharmacol Sci. 1989 Dec;Suppl:11–15. [PubMed] [Google Scholar]
- Bourinet E., Fournier F., Lory P., Charnet P., Nargeot J. Protein kinase C regulation of cardiac calcium channels expressed in Xenopus oocytes. Pflugers Arch. 1992 Jun;421(2-3):247–255. doi: 10.1007/BF00374834. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Eccles J. C. The action of a single vagal volley on the rhythm of the heart beat. J Physiol. 1934 Sep 19;82(2):211–241. doi: 10.1113/jphysiol.1934.sp003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983 Sep 10;258(17):10233–10239. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christie M. J., North R. A. Control of ion conductances by muscarinic receptors. Trends Pharmacol Sci. 1988 Feb;Suppl:30–34. [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflugers Arch. 1987 Sep;410(1-2):139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- Ehlert F. J., Delen F. M., Yun S. H., Friedman D. J., Self D. W. Coupling of subtypes of the muscarinic receptor to adenylate cyclase in the corpus striatum and heart. J Pharmacol Exp Ther. 1989 Nov;251(2):660–671. [PubMed] [Google Scholar]
- Eva C., Bovolin P., Balzac F., Botta C., Gamalero S. R., Vaccarino F. M. Primary cultures of corticostriatal cells from newborn rats: a model to study muscarinic receptor subtypes regulation and function. J Mol Neurosci. 1990;2(3):143–153. doi: 10.1007/BF02896839. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Ito H., Miller S. C., Akimoto H., Torti S. V., Taylor A., Billingham M. E., Torti F. M. Evaluation of mRNA levels by the polymerase chain reaction in small cardiac tissue samples. J Mol Cell Cardiol. 1991 Oct;23(10):1117–1125. doi: 10.1016/0022-2828(91)90201-v. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- Legssyer A., Poggioli J., Renard D., Vassort G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J Physiol. 1988 Jul;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G. Histamine modulates calcium current in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 1988 Jul;246(1):377–383. [PubMed] [Google Scholar]
- Matsumoto K., Pappano A. J. Sodium-dependent membrane current induced by carbachol in single guinea-pig ventricular myocytes. J Physiol. 1989 Aug;415:487–502. doi: 10.1113/jphysiol.1989.sp017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Matsumoto K., Tajima T., Agnarsson U., Webb W. Pertussis toxin-insensitive mechanism for carbachol-induced depolarization and positive inotropic effect in heart muscle. Trends Pharmacol Sci. 1988 Feb;Suppl:35–39. [PubMed] [Google Scholar]
- Peralta E. G., Winslow J. W., Ashkenazi A., Smith D. H., Ramachandran J., Capon D. J. Structural basis of muscarinic acetylcholine receptor subtype diversity. Trends Pharmacol Sci. 1988 Feb;Suppl:6–11. [PubMed] [Google Scholar]
- Rosen M. R., Steinberg S. F., Danilo P., Jr Developmental changes in the muscarinic stimulation of canine Purkinje fibers. J Pharmacol Exp Ther. 1990 Jul;254(1):356–361. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scamps F., Legssyer A., Mayoux E., Vassort G. The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ Res. 1990 Oct;67(4):1007–1016. doi: 10.1161/01.res.67.4.1007. [DOI] [PubMed] [Google Scholar]
- Schimerlik M. I. Structure and regulation of muscarinic receptors. Annu Rev Physiol. 1989;51:217–227. doi: 10.1146/annurev.ph.51.030189.001245. [DOI] [PubMed] [Google Scholar]