Abstract
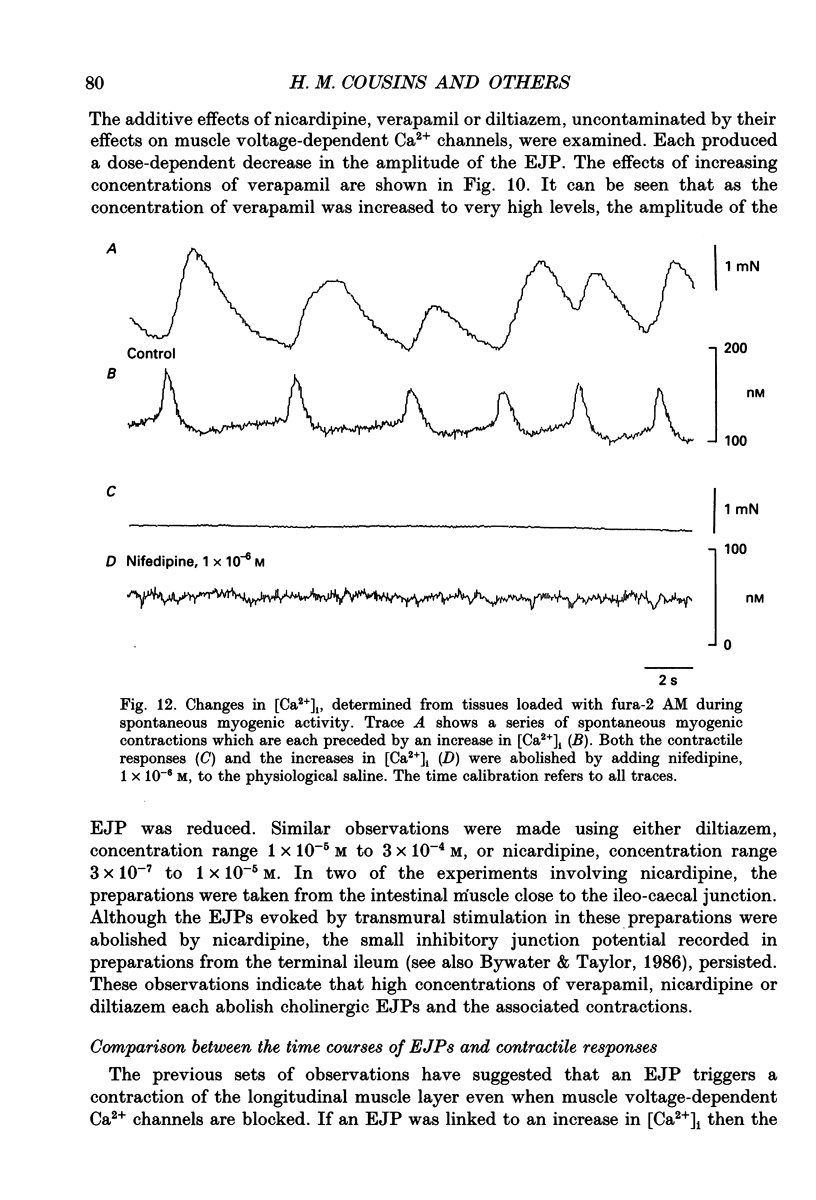
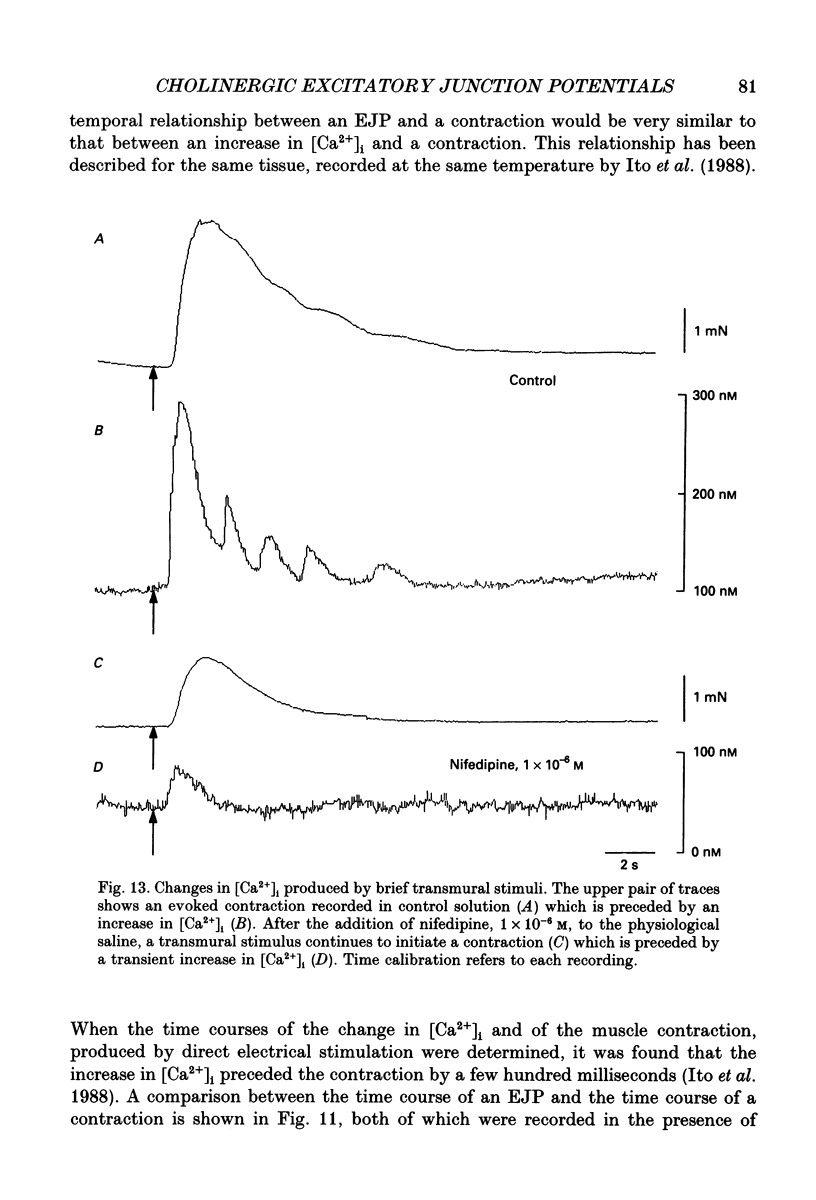
1. Brief transmural stimuli, 0.5-1 ms, initiated contractions of the longitudinal muscle taken from the guinea-pig ileum that were recorded isometrically. In separate preparations similar stimuli were found to initiate excitatory junction potentials which were recorded using intracellular recording electrodes. All of these responses were abolished by either tetrodotoxin, omega-conotoxin or hyoscine. 2. The contractions produced by increasing [K+]o were blocked by nifedipine, 1 x 10(-7) M; nicardipine, 1 x 10(-7) M; verapamil, 1 x 10(-5) M or diltiazem, 1 x 10(-5) M. In these solutions brief stimuli continued to initiate contractions: this indicates that neuronally released acetylcholine continues to trigger a contraction when muscle voltage-dependent calcium channels appear to have been blocked. 3. When membrane potential recordings were made from the smooth muscle layer, brief transmural stimuli initiated excitatory junction potentials that triggered muscle action potentials. Although muscle action potentials were abolished by low concentrations of a range of organic calcium antagonists, excitatory junction potentials persisted and continued to initiate contractions of reduced amplitude. 4. When the internal concentration of calcium ions, [Ca2+]i, was measured using fura-2, brief transmural stimuli caused an increase in [Ca2+]i. Part of this response, which occurred at a time corresponding to the unblocked excitatory junction potential, persisted in the presence of the organic calcium antagonist nifedipine. 5. Two explanations appear possible. Neuronally released acetylcholine may simultaneously activate non-selective cation channels and cause the release of Ca2+ from an internal store. Alternatively, neuronally released acetylcholine may cause an increase in [Ca2+]i which is separate from that which accompanies the activation of voltage-dependent calcium channels. At this stage there is little other anatomical or electrophysiological evidence to support this view.
Full text
PDF



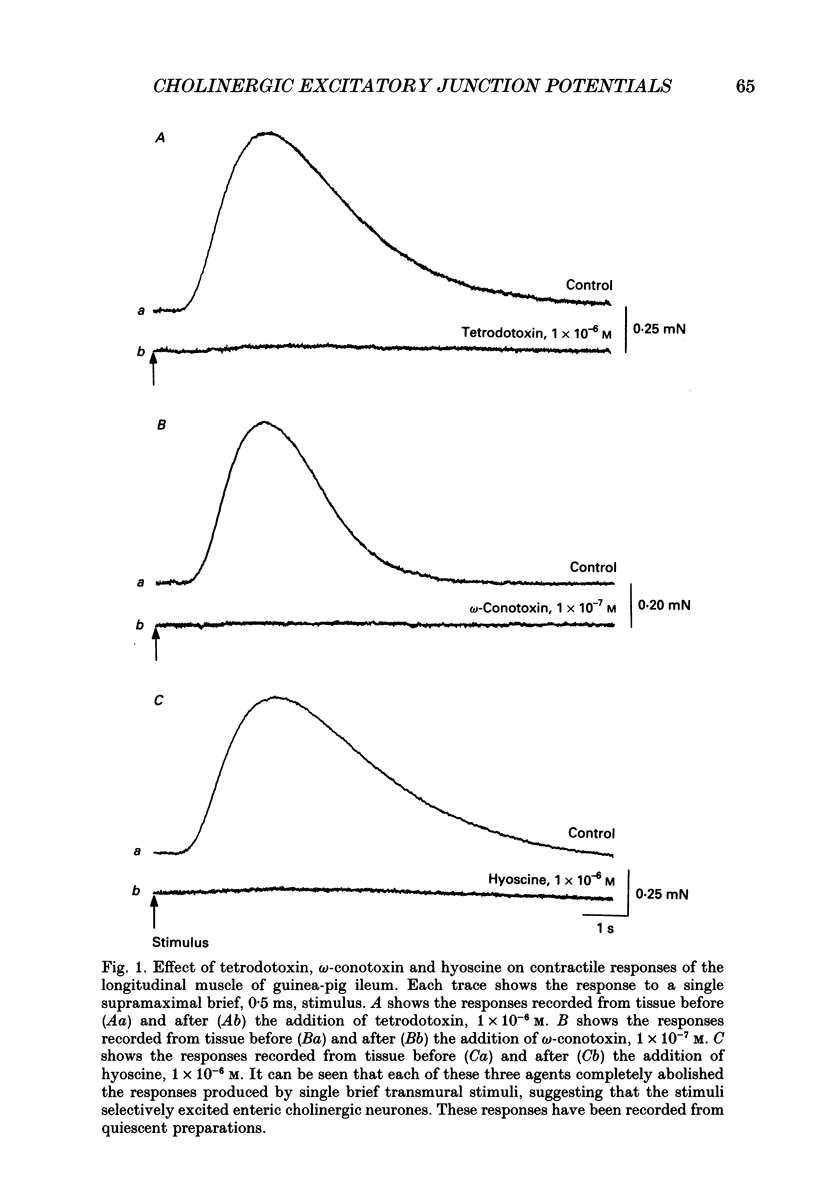
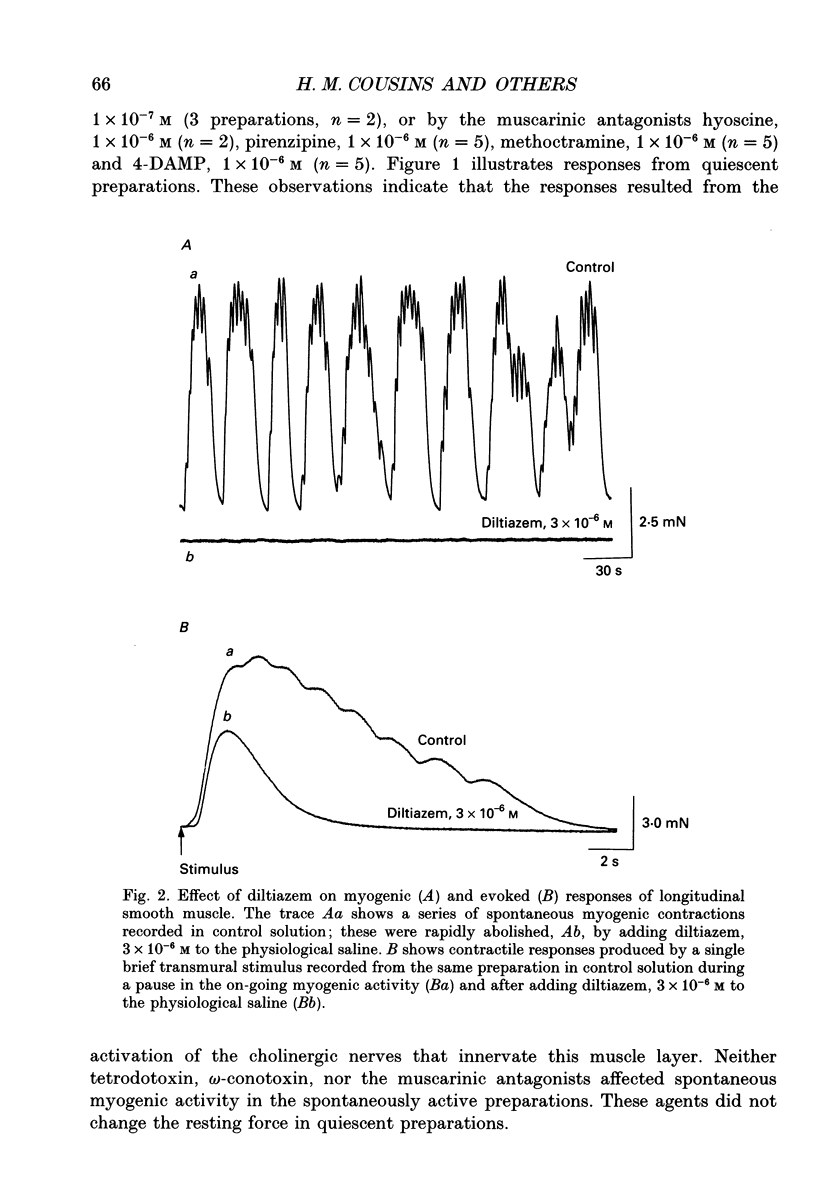
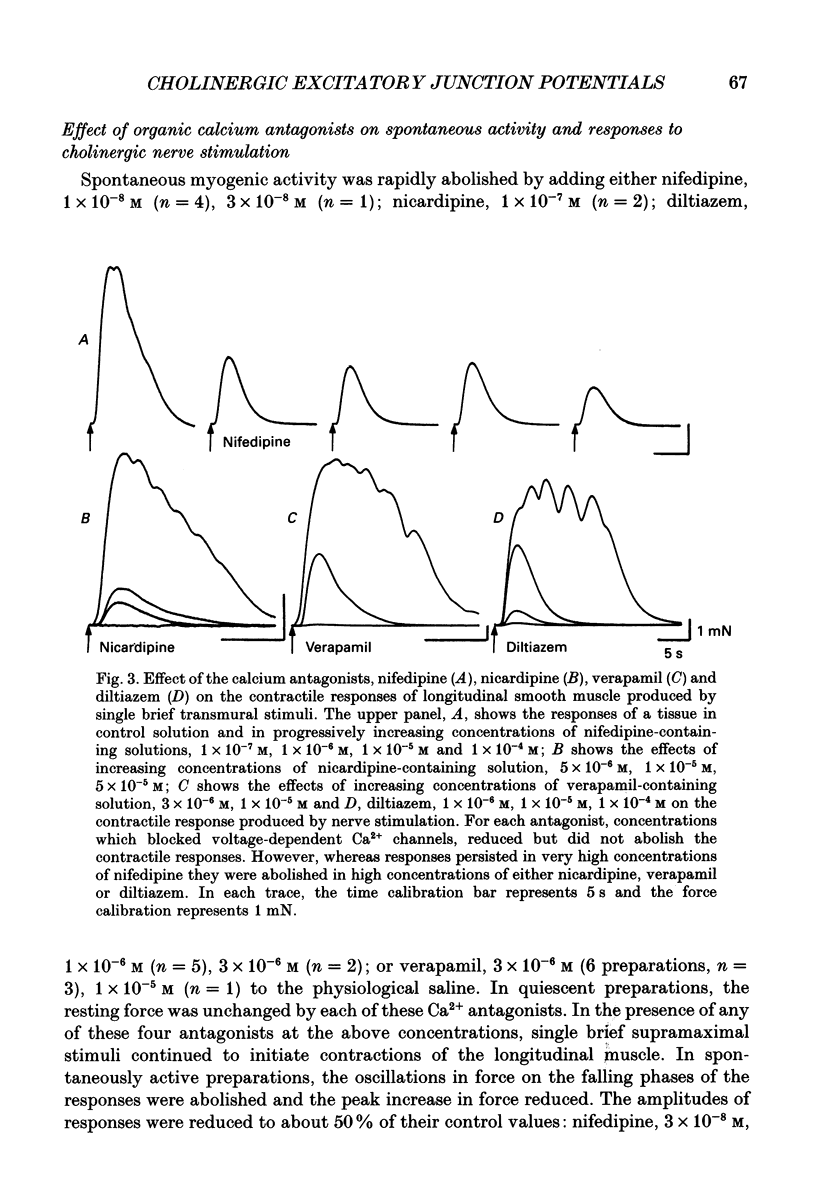

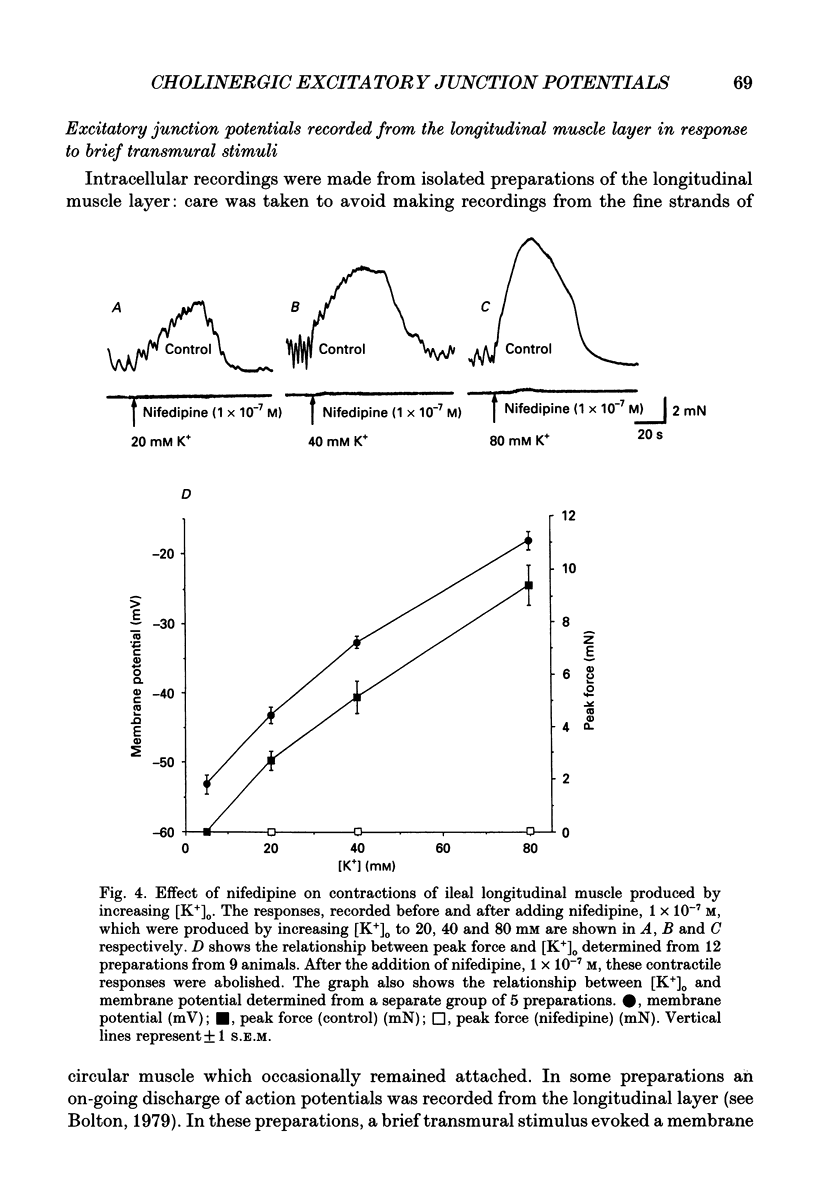

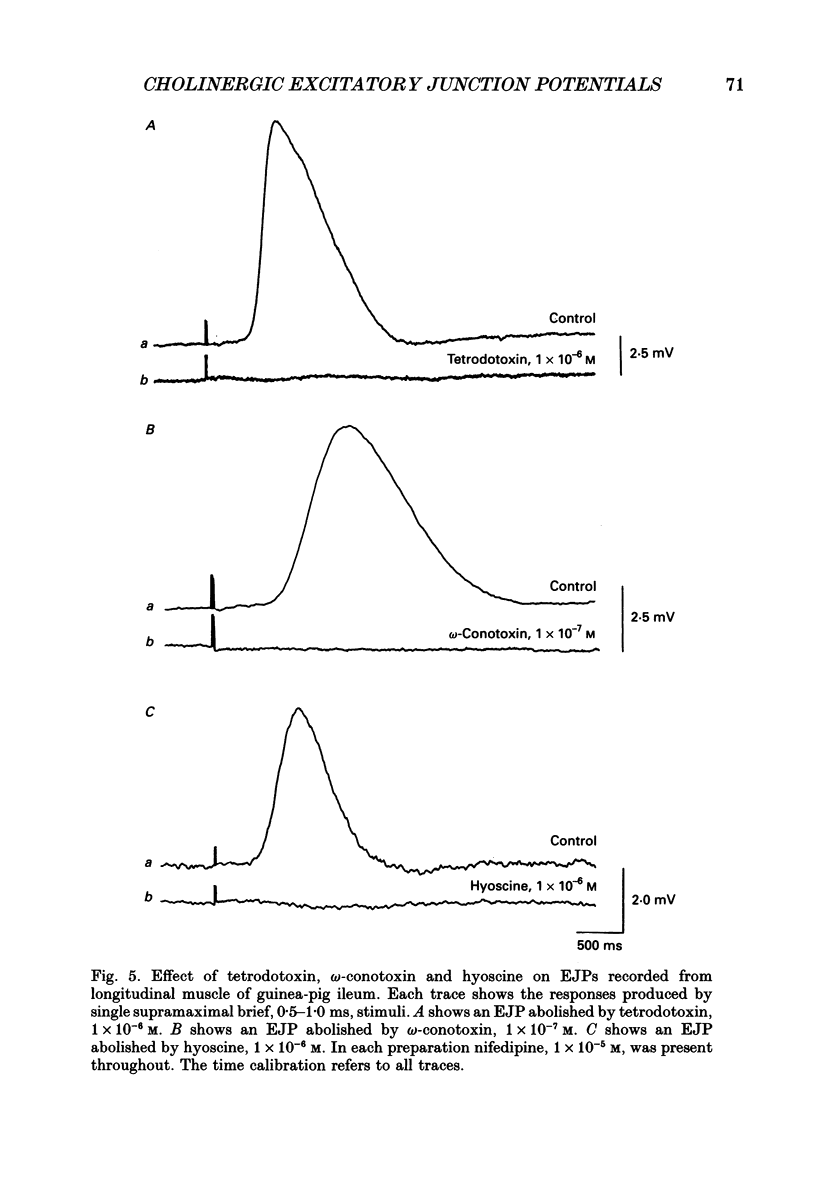
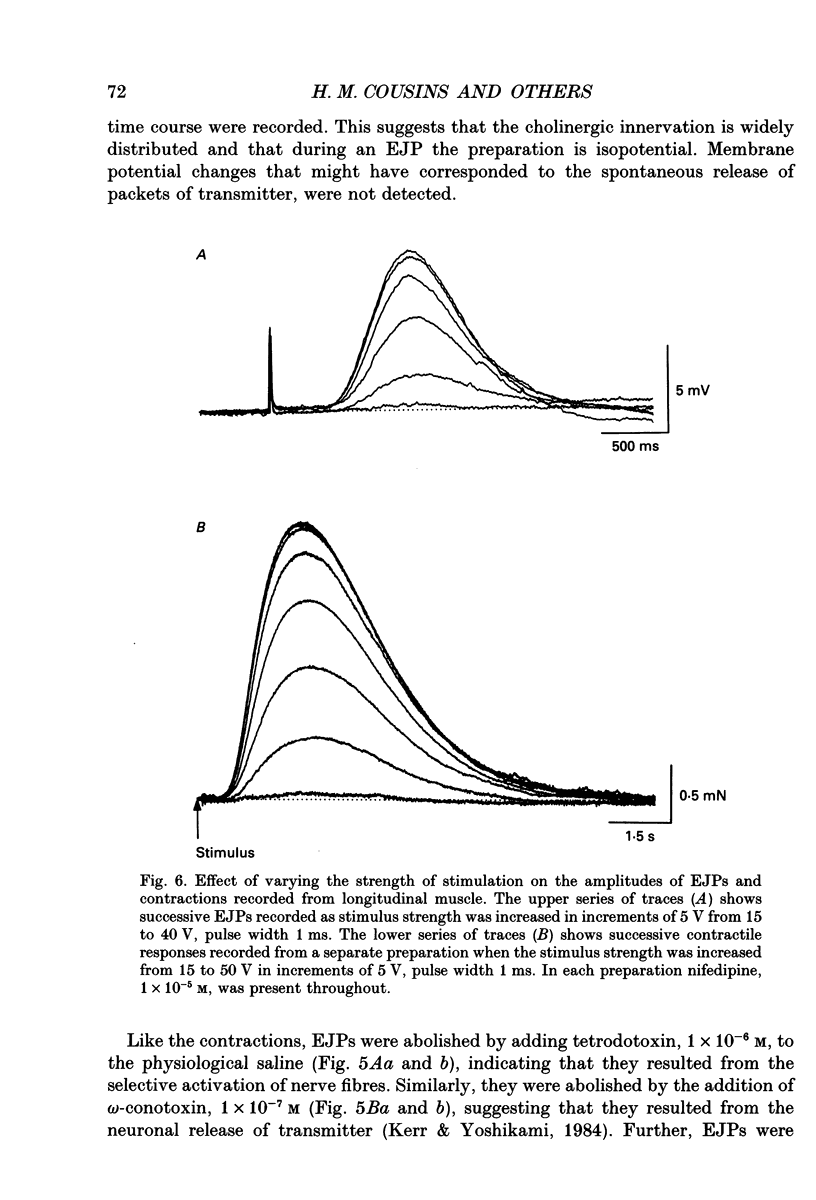

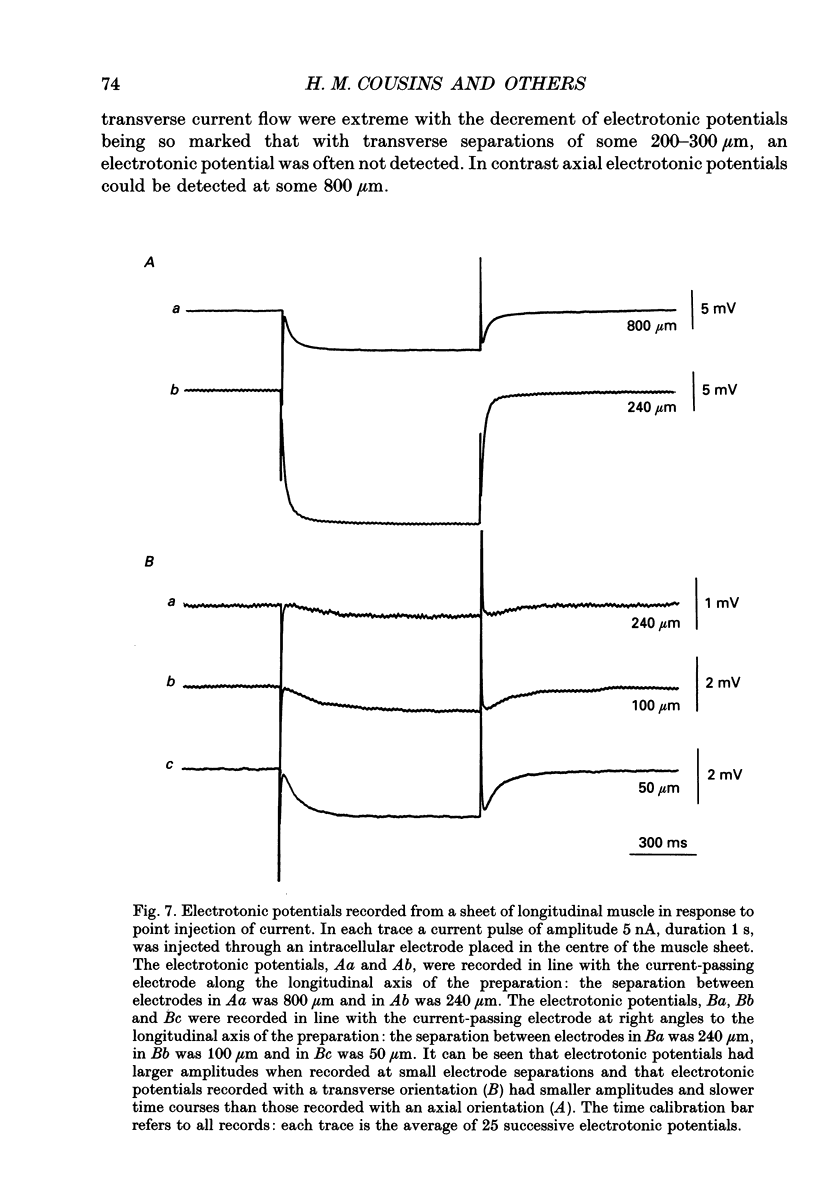
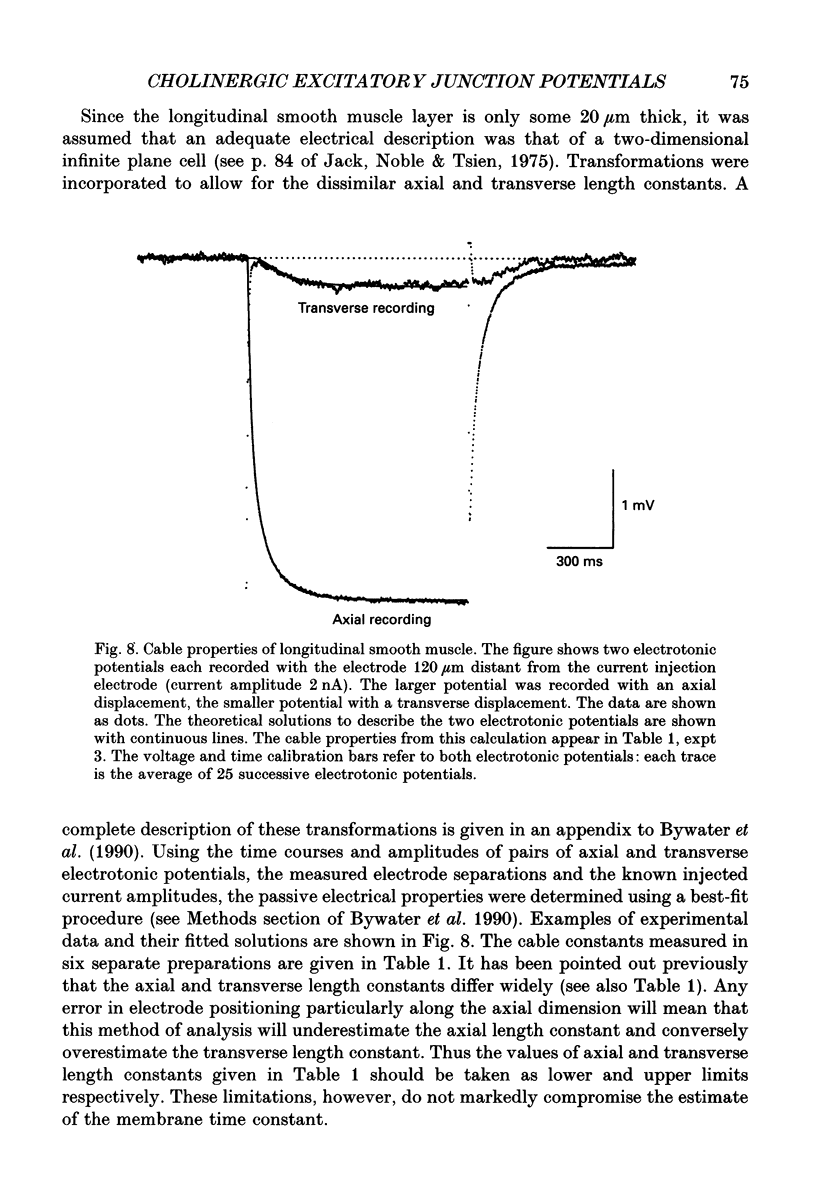
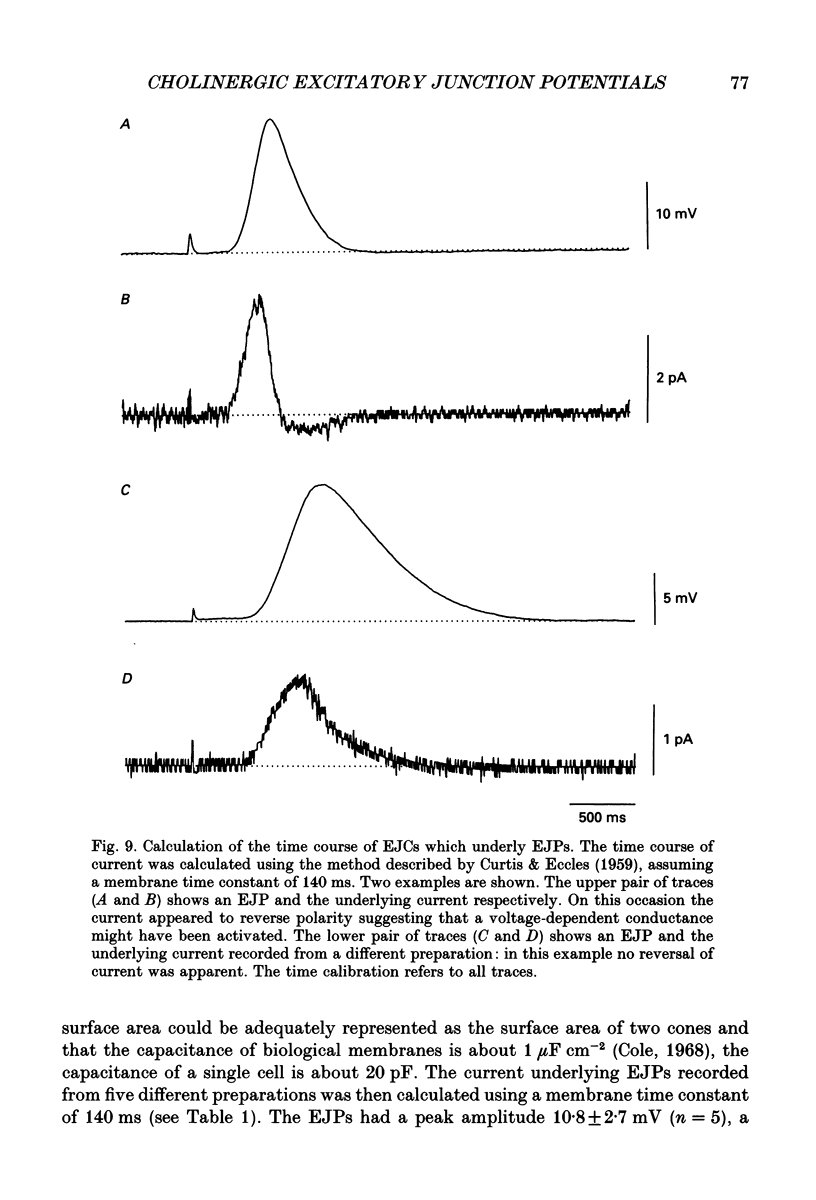
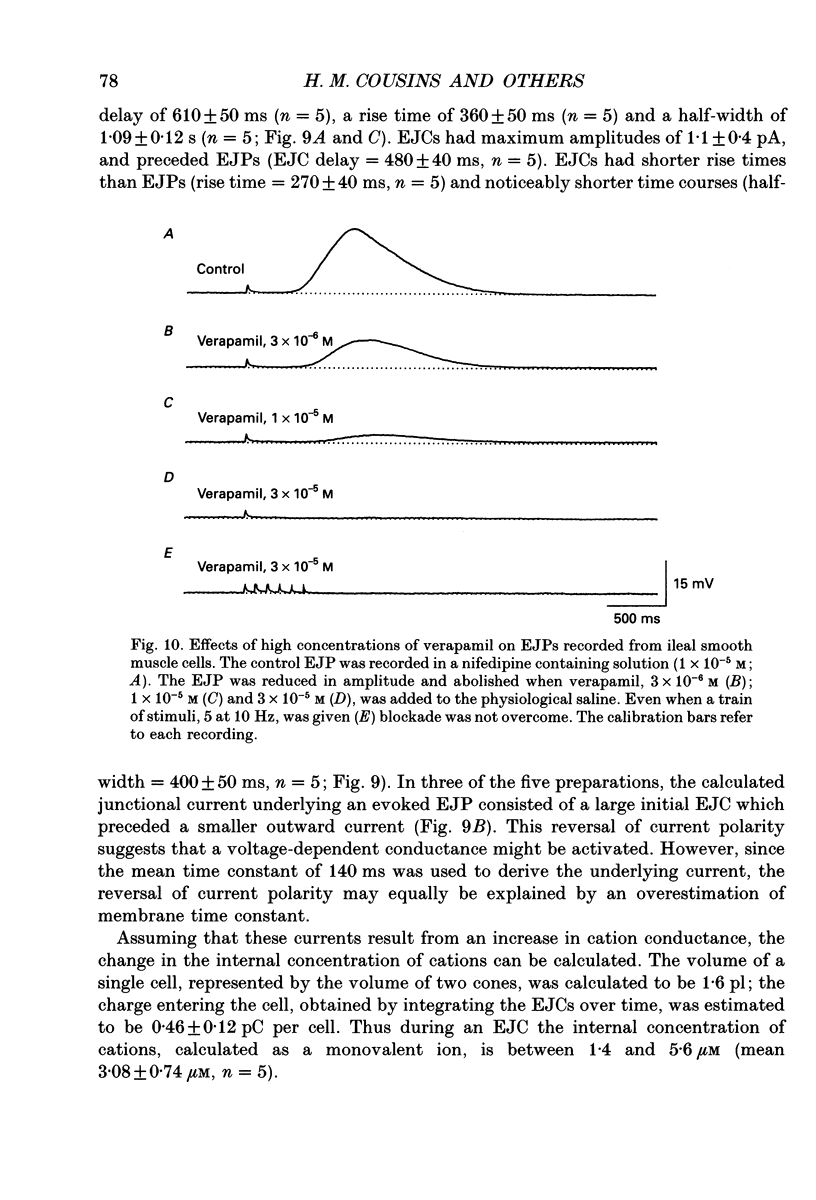
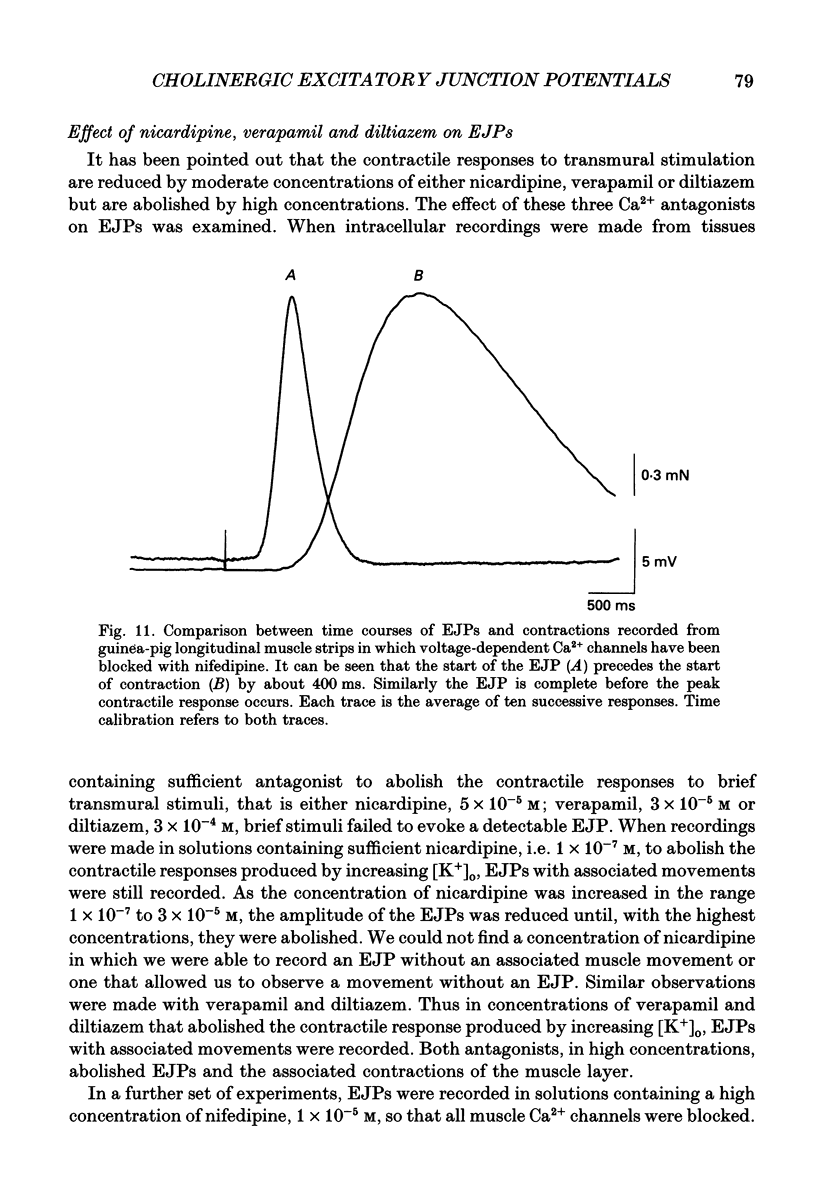





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
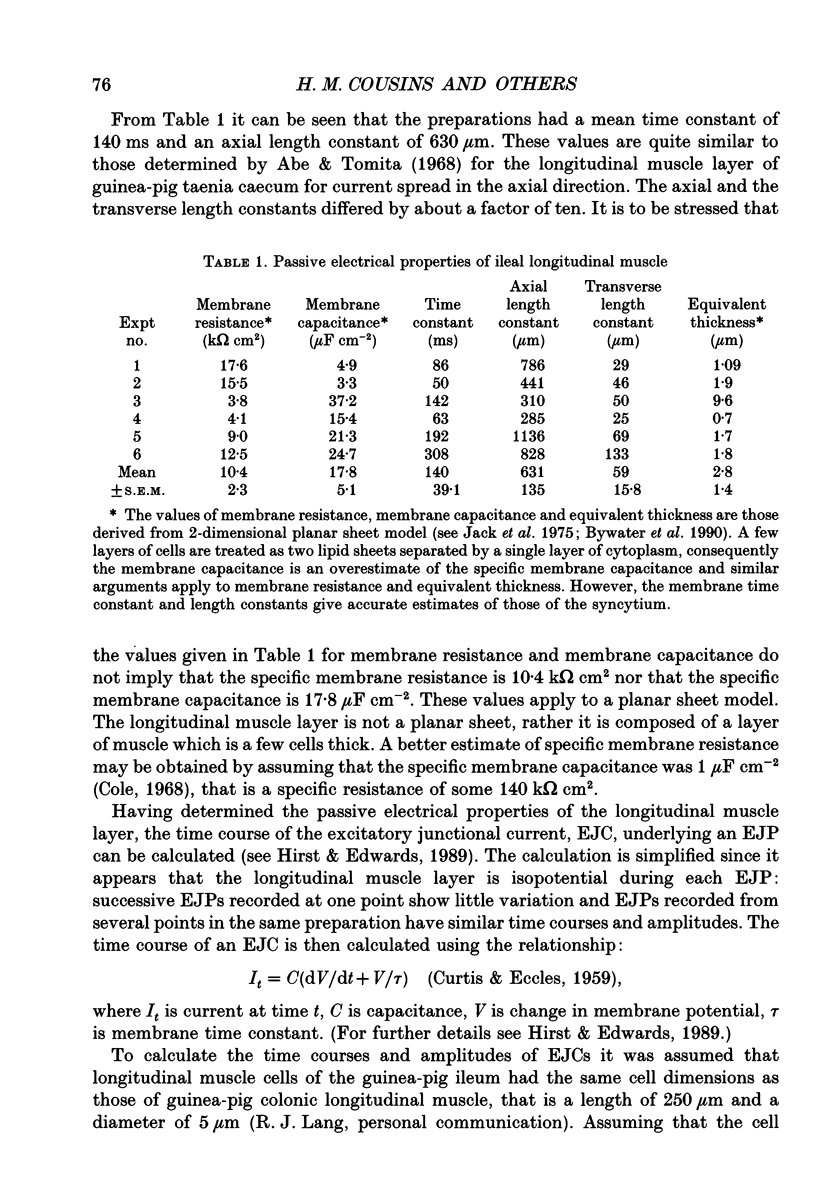
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer V., Holzer P., Ito Y. Role of extra- and intracellular calcium in the contractile action of agonists in the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1991 Jan;343(1):58–64. doi: 10.1007/BF00180677. [DOI] [PubMed] [Google Scholar]
- Bauer V., Kuriyama H. Evidence for non-cholinergic, non-adrenergic transmission in the guinea-pig ileum. J Physiol. 1982 Sep;330:95–110. doi: 10.1113/jphysiol.1982.sp014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Campbell G. D., Edwards F. R., Hirst G. D. Effects of vagal stimulation and applied acetylcholine on the arrested sinus venosus of the toad. J Physiol. 1990 Jun;425:1–27. doi: 10.1113/jphysiol.1990.sp018089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol. 1986 May;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins H., Edwards F., Hirst D., Wendt I. Cholinergic neuroeffector transmission in the longitudinal muscle of guinea-pig ileum. Jpn J Pharmacol. 1992;58 (Suppl 2):301P–301P. [PubMed] [Google Scholar]
- Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat. 1972 Jan;111(Pt 1):69–97. [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Bramich N. J., Edwards F. R., Klemm M. Transmission at autonomic neuroeffector junctions. Trends Neurosci. 1992 Feb;15(2):40–46. doi: 10.1016/0166-2236(92)90024-3. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol. 1990 Jun;258(6 Pt 1):C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Parker I. Calcium transients evoked by electrical stimulation of smooth muscle from guinea-pig ileum recorded by the use of Fura-2. J Physiol. 1988 Dec;407:117–134. doi: 10.1113/jphysiol.1988.sp017406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplita P. V., Triggle D. J. Actions of Ca2+ antagonists on the guinea-pig ileal myenteric plexus preparation. Biochem Pharmacol. 1983 Jan 1;32(1):65–68. doi: 10.1016/0006-2952(83)90653-6. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Klemm M., Hirst G. D., Campbell G. Structure of autonomic neuromuscular junctions in the sinus venosus of the toad. J Auton Nerv Syst. 1992 Jun 15;39(2):139–150. doi: 10.1016/0165-1838(92)90054-k. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Kawai M., Takewaki T., Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992 May;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. J., Paul R. J. Effects of 2,3-butanedione monoxime on whole-cell Ca2+ channel currents in single cells of the guinea-pig taenia caeci. J Physiol. 1991 Feb;433:1–24. doi: 10.1113/jphysiol.1991.sp018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. P., Bolton T. B. A calcium-dependent rather than a G-protein mechanism is involved in the inward current evoked by muscarinic receptor stimulation in dialysed single smooth muscle cells of small intestine. Br J Pharmacol. 1988 Oct;95(2):325–327. doi: 10.1111/j.1476-5381.1988.tb11649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niel J. P., Bywater R. A., Taylor G. S. Effect of substance P on non-cholinergic fast and slow post-stimulus depolarization in the guinea-pig ileum. J Auton Nerv Syst. 1983 Dec;9(4):573–584. doi: 10.1016/0165-1838(83)90114-5. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Sanders K. M. Cholinergic stimulation activates a non-selective cation current in canine pyloric circular muscle cells. J Physiol. 1990 Oct;429:223–236. doi: 10.1113/jphysiol.1990.sp018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar M. A., Gooptu D. Effect of nifedipine on the contractile responses of human colonic muscle. Br J Clin Pharmacol. 1983 Sep;16(3):339–340. doi: 10.1111/j.1365-2125.1983.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


