Abstract
Numerous studies from different international groups have demonstrated that sensations can be propagated along acupuncture channel pathways (referred to as propagated signals along channel pathways; PSCP). The PSCP can be elicited by electroacupuncture (EA), transcutaneous electrical nerve stimulation (TENS), manual acupuncture (MA), and heat applied to distal acupuncture points (acupoints). Previous experiments with EA in rats reported that nitric oxide (NO) levels were elevated in the gracile nucleus and skin regions near to the EA sites, with higher levels at acupoints associated with an enhanced expression of NO synthase and transient receptor potential vanilloid type 1 (TRPV1). Recent human studies demonstrated that TENS, MA, and electrical heat stimulated an increase in NO release over skin regions and greatest NO concentrations at acupoints. These stimuli, EA, MA, TENS, and heat, have been used to elicit axonal reflexes, which increase local release of NO and neuropeptides such as calcitonin gene related peptide. Furthermore, the sensation of PSCP along the body surface occurs only ipsilateral to the stimulated acupoints in various human studies, which does not support the involvement of the spinal-thalamic pathway, which would involve cross over transmission of the signals. The gracile nucleus receives ascending input from the sciatic nerve projecting from the hindlimb and responds to somatosensory stimulation mainly on the ipsilateral side via the dorsal column pathway. EA at ST36 increases NO release and expression of NO synthase mainly in the ipsilateral side of the gracile nucleus, while the cardiovascular effects and analgesic responses to EA at ST36 are changed by influences of L-arginine-derived NO synthesis in the ipsilateral gracile nucleus in rats. The stimuli-induced release of NOergic molecules (NO synthases, NO, and cGMP) and neuropeptides exist high levels in the acupoints, which contain rich neuronal components and blood vessels. Enhanced NOergic molecules at acupoints cause axon reflexes during the stimuli, which elevate cutaneous blood flow. Elevated NOergic molecules and local blood flow may spread over acupoints one after another along the meridian lines differing from nerve pathways following the stimuli to induce PSCP. The same types of stimulation also elicit NO release in the gracile nucleus, which contributes to the somatosensory signal transduction of PSCP through the dorsal medulla-thalamic pathways. Other substances such as serotonin, catecholamines, and glutamate, are proposed to mediate responses and certain effects of acupuncture-like stimulation but their mechanisms are poorly-understood. In this review we summarize the current understanding of the neurobiological processes of PSCP research with an emphasis on recent developments of NO mediating stimulation-evoked axon reflexes and somatosensory signal transduction for PSCP perceptions through the dorsal medulla-thalamic pathways.
Keywords: Axon reflex, nitric oxide, calcitonin gene related peptide, biophysical approaches, meridian system, dorsal funiculus tract
1. Introduction
The meridian system (Jing Luo) is as an important component of the theories that deal with physiological regulation in traditional Chinese medicine (TCM), which can indicate pathological changes of the human [1]. Acupuncture points, also called acupoints, located along the meridian pathways over the arm, leg and trunk, are fundamental to the theory in acupuncture, electrical acupuncture (EA), transcutaneous electrical nerve stimulation (TENS), moxibustion, qigong, and meditation [1,2]. According to TCM, the meridian systems flow vital energy and blood circulate, and by which exchange, movement, and the internal organs are connected with the arms, legs, and trunk of the body and superficial organs as well as communication with the universe and environment as an organic whole [1,3]. Thus, meridian systems serve as a guiding principle for diagnosis and treatment of diseases and as the central theory of TCM practices and therapies [1–3]. However, the biomolecules, structure, and functions of the meridian system, and mechanistic effects following stimulation of acupoints and meridians are unclear. It is still unknown in modern sciences as to what is the meridian transmission from their somatic pathways to influence the functions of related internal organs.
Over the last few decades there are various studies using biophysical approaches to investigate the properties of acupoints and meridians [3,4]. It appears that propagated sensation along the meridians [5,6], also called propagated sensation along the channel pathways (PSCP) [5,7,8], is a commonly observed phenomenon; it may manifest in certain aspects of the subject’s sensory and affective perceptions, including numbness, pressure, heaviness, warmth, and radiating paranesthesia [3,4,9,10]. Early scientific reports of PSCP were published in the 1950s, which have been summarized in the review and book [3,4], however, it was described in the Chinese medical work, the Yellow Emperor’s Inner Canon (Neijing), that “When hitting a point, a needle seems to move along the street (channels)” [1]. The phenomenon occurs most frequently following stimulation of distal acupoints (the Jing or the Well points), located at the end of the fingers or toes, using manual acupuncture (MA) [4,7–10], EA, or TENS [4,7,8], acupressure with qigong or meditation [4], and moxibustion including heat and laser [4,9,10]. The PSCP has been described internationally by human subjects, and the location and progression of these sensations follow the map of acupoints and meridians, which are different from the sensory neural pathways [3,4,6]. A systematic review found that among 63,228 subjects receiving pulse electrical stimulation of the acupoints, PSCP was reported in 12%–24% [3]. It is reported that low electrical resistance exists with PSCP, which are present not only in the acupoints, but also over the entire lines of the meridians, which are described in traditional charts [3,4].
Although PSCP purports to be genuine evidence for the functional existence of meridians [5,8,9], there is a lack of objective and systematic experimental evidence to support biochemical and morphological mechanisms behind PSCP. There has been increasing experiment reporting that PSCP occurs in the acupoints and medians in humans, however, the direct evidence to identify biomolecules and structures localized to acupoints and meridians related to development of PSCP are still unavailable, especially limited by technological difficulties to explore the gap in human study in vivo.
2. Nitric oxide (NO) and cyclic guanosine 3’,5’-monophosphate (cGMP) levels are higher in acupoints and are increased by EA, TENS, and heat stimuli
Early anatomical studies of acupoints in humans have shown that the tissue surrounding most acupoints contain more neuronal components, hair follicles, sweat glands, and blood vessels than are present in non-acupoint areas, as shown in Fig. 2, bottom panel [3,4,11,12]. Histological experiments have demonstrated that the epidermis and the outer root sheath in the skin tissues possess immune activities of NO synthases, including neuronal NOS (nNOS) and endothelial NOS (eNOS), and nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase reactivity [13]. Previous studies have demonstrated that NO synthase protein levels and NO are consistently higher in skin regions containing acupoints/meridians than in those without meridians in rats, and the meridian levels of NO synthase and NO are further increased following acupuncture stimulation [12,14]. The results are consistent with other studies that found that NADPH diaphorase positive neurons are present in Zusanli (ST36) and some of the neurons are projected from the lamina IX of L4 to S1 in spinal cord [15]. Our previous studies have demonstrated that concentrations of total nitrite and nitrate (NOx-) and cGMP can be quantified over the ventral forearm and leg by using a painless and non-invasive biocapture method, and that NO contents are higher over acupoints compared to the skin over regions containing meridian lines without acupoints and regions without meridians or acupoints [16–19]. As shown in Fig. 1, the region over Chengshan (BL57) is defined as a meridian with an acupoint, the space between Heyang (BL55) and Chengjin (BL56) represents a meridian line without an acupoint (MWOP). Control samples were obtained in a non-meridian control region (NMCR) close to BL. A biocapture device (0.3 cm × 5.0 cm) was adhered to the skin surface over the skin regions covering acupoints, MWOP, and NMCR (Fig. 1). The 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, a NO-scavenging compound) solution (100 μmol/L) was injected into the tubing and kept in contact with the skin surface for 20 min in order to absorb molecules [16–18]. The liquid was drained from the tube and concentrations of NOx- were quantified by using chemiluminescence in a blinded fashion [16–19]. The concentrations of nitrotyrosine and cGMP in the samples were quantified by using an enzyme-linked immunosorbent assay [18,20].
Fig. 2.
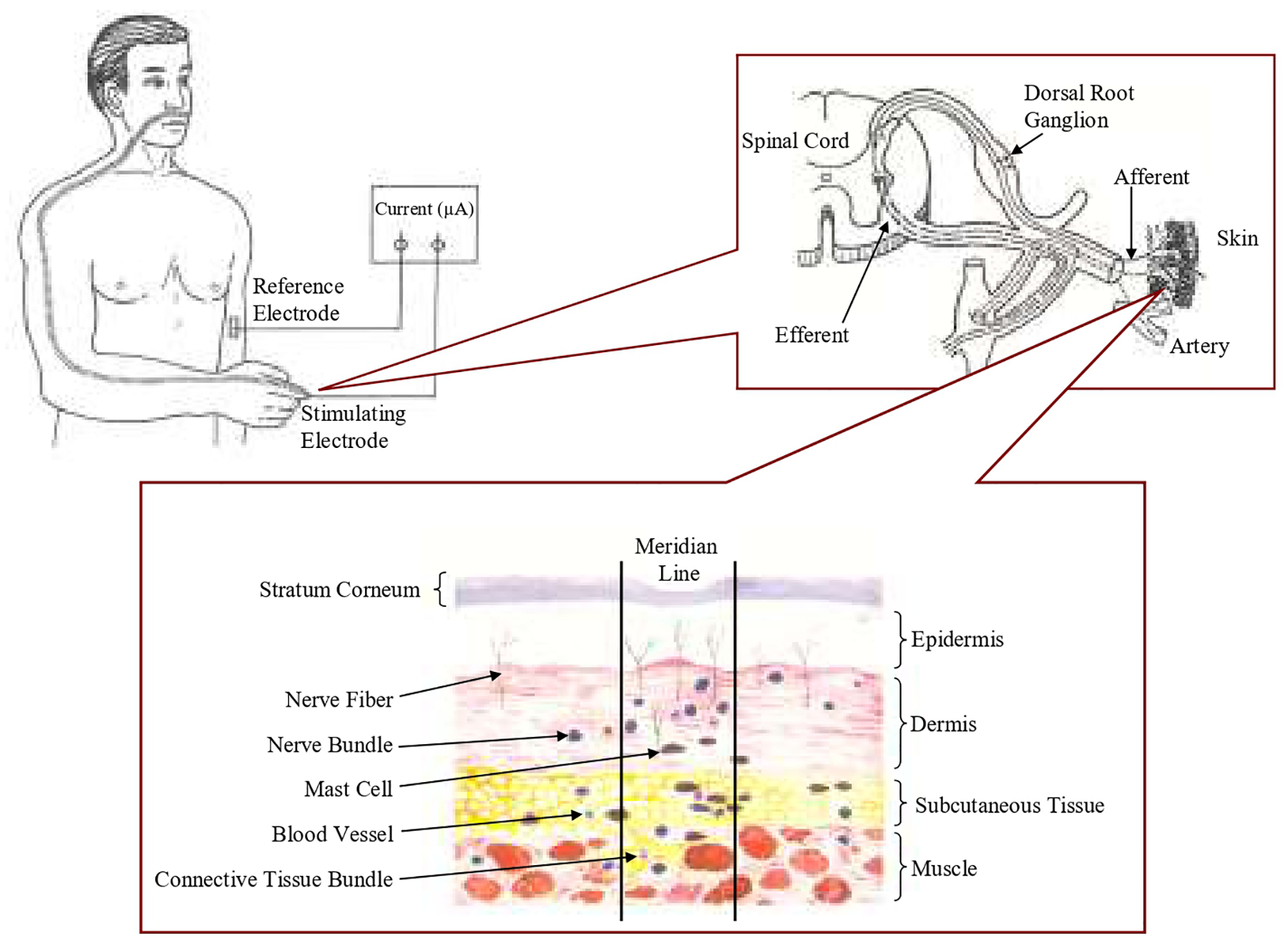
NOergic molecules and neuropeptides mediated stimuli-evoked peripheral axon reflexes involved in sensory signal transduction of propagated signals along channel pathways (PSCP) and morphological studies of acupoints along the skin meridian. The top panels show a somatosensory line path along the acupoints and meridian after a stimulus applied on a distal acupoint. The bottom panel shows anatomical studies of acupoints/meridians which exist higher number of nerve fibers/trunks, blood vessels, hair follicles, and sweat glands compared to their control areas without meridian. The higher number of small nerve fibers, blood vessels with rich endothelia and neuronal NO synthase, TRPV1 and neuropeptides such as calcitonin gene related peptide, and sweat glands in the cutaneous tissues serve as structures for NO signaling molecules and neuropeptides mediated axon reflexes with signal functions.
Fig. 1.
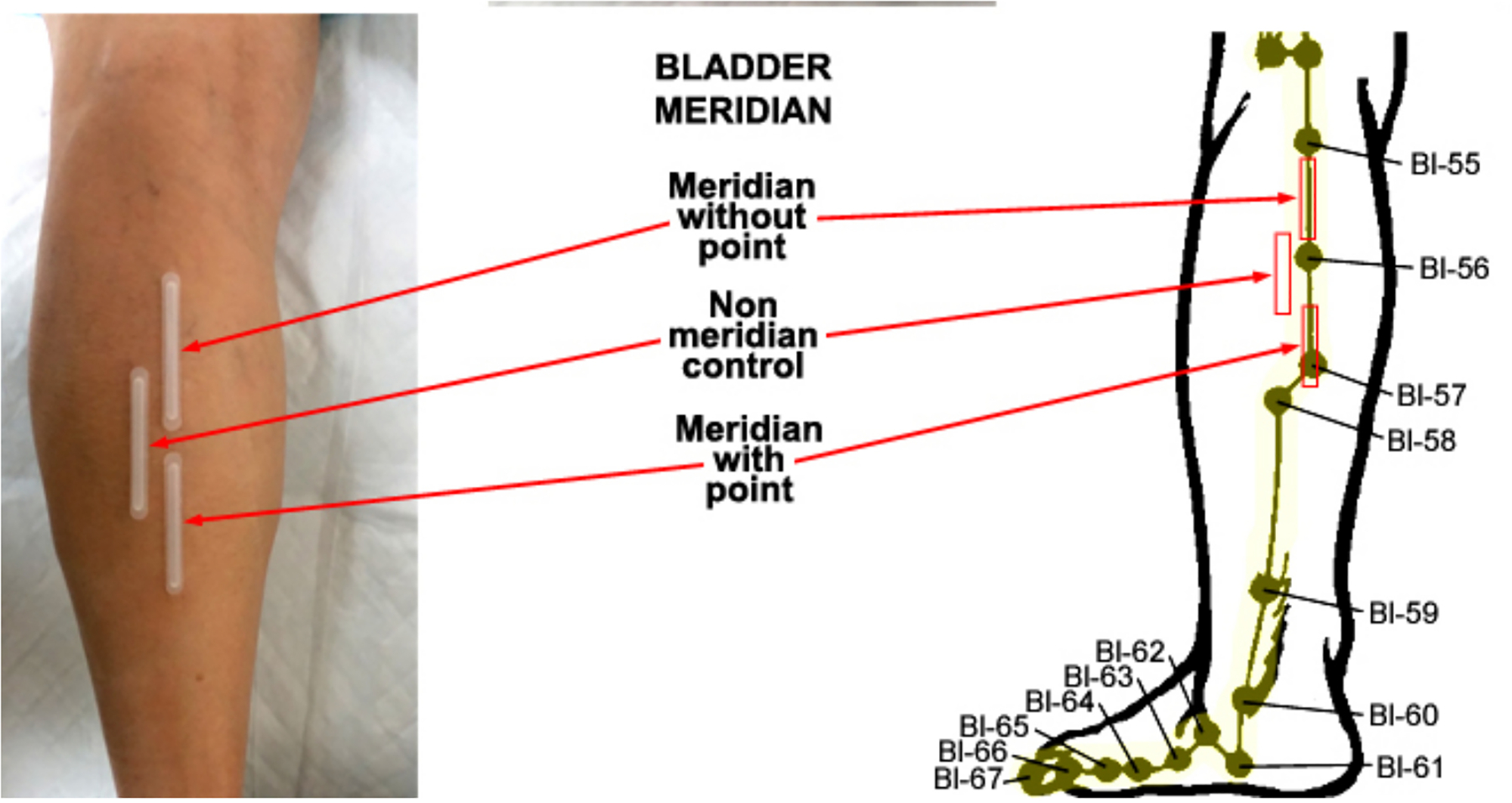
Biocapture of biomolecules from the skin surface over the Bladder meridian (BL) of the leg. The BL meridian and related acupuncture points are illustrated right panel. The region over Chengshan (BL57) is defined as meridian with an acupoint. the space between Heyang (BL55) and Chengjin (BL56) is part of the BL meridian without an acupoint, and a non-meridian control was established over region adjacent to the BL line.
The results agree with data from dermal microdialysis, which found that the amount of NO-cGMP released in the subcutaneous tissue of an acupoint is greater than in the non-meridian control region and can be increased by acupuncture stimulation in healthy humans [20]. The investigation of NO bioavailability over the skin acupoints has also been confirmed by different method in other studies [21], which serves as an important indicator for physiological distribution and therapeutic manipulation of the skin microvasculature. Several studies have demonstrated that NO release is triggered by various stimuli, such as acupuncture, electrical impulses, moxibustion, electrical heat, and laser. The stimuli are similar with the biophysical approaches to induce the PSCP and the latent PSCP (The PSCP is induced by combining stimuli with mechanical tapping but no prominent sensation of propagation could be felt following routine stimuli) over the body surface. The release of NO-cGMP in the subcutaneous tissue of the forearm skin along the pericardium channel (PC) acupoints, as measured by microdialysis, is increased by low-frequency EA [20]. These results are consistent with the report that low-frequency transcutaneous electrical nerve stimulation induces an increase in the release of NO and cGMP over PC acupoints in humans [18]. Consistently, manual acupuncture with low force/frequency and electrical heat stimulates the release of NO over the forearm regions along the PC acupoints [19]. Acupuncture induced cutaneous vasodilatation can be attenuated by the application of an NO synthesis inhibitor to the forearms of humans [22]. Acupuncture stimulation increases blood flow to the skin and muscle, which improves local circulation which helps to clear away algesic or sensitizing substances, leading to pain relief [23,24].
3. NO-cGMP and neuropeptides mediate axon reflexes and sensory signal transduction
Similarly, axonal reflexes have been defined as a response to mechanical, electrical, thermal, or pharmacological stimuli, as shown in Fig.2. [25–29]. The response to local skin stimulus is characterized by its biphasic nature—the first peak is due to C-fiber nociceptor-mediated axon reflex [25,29], which results in vasodilatation through the local release of calcitonin gene related peptide (CGRP) [25,26] and neuropeptide Y (NPY) [27,28]. The second increase in skin blood flow, characterized by the plateau, has been demonstrated to be largely dependent (about 70%) on NO [28,29]. Local NO release is consistently induced by low force/rate of MA, EA and TENS, as well as electrical heat [18–20]. These stimuli have been demonstrated to evoke axon reflexes in various studies [25–28]. Several studies have demonstrated that peripheral axon reflexes play an important role in the regulation of skin blood flow, which is involved in sweating, inflammation, pain, itch, allergic rhinitis, neuropathy, and pathological changes [30–32].
A recent study reported that CGRP, via axon reflex, participates in increasing local muscle blood flow following manual acupuncture in rats [33]. Another study showed that cutaneous vasodilation in response to acupuncture stimulation in humans was significantly inhibited by local treatment with N(ω)-nitro-l-arginine methyl ester, an inhibitor of NO synthesis, but not altered by local application of anesthetics cream, which suggest that NO mechanisms contribute to cutaneous vasodilation in response to acupuncture stimulation in humans but may not occur through an axon reflex [22]. The precise mechanisms of the elevation of NO release affected by the stimulations are still unclear. The results from both anatomical and biochemical studies consistently suggest that these stimuli are linked to the NO signaling molecules that are involved in axon reflexes, and the same NO-mediated axon reflex may also participate in the perception of PSCP. However, experiments are needed to evaluate whether axon reflexes mediated the NO release contribute to the PSCP induced by the same stimuli or other mechanistic link between NO-release and PSCP, which both can be induced by the stimuli. The axon reflex can be evoked by a wide range of physiologic stimuli such as heat, cold, mechanical distension, and ultraviolet light [34,35]. The topical stimulation depolarizes unmyelinated C-fibers in the skin and results in afferent action potentials that are conducted orthodromically toward the spinal cord. The transient receptor potential vanilloid type 1 (TRPV1) is a non-selective cation channel that binds vanilloids and was originally described to be sensitive to a host of stimuli, including mechanical distension and temperature [36–38]. TRPV1 mediates sensory signal transduction [38–40]. In addition, the expression of TRPV1 in cutaneous sensory nerves, mast cells, and epithelial cells suggests a major role for these receptors in the transmission of sensory information [38].
Experimental evidence using double immunostaining of TRPV1 receptor and nNOS revealed co-localization of TRPV1 and nNOS in both subepidermal nerve fibers and in dermal connective tissue cells in rats [41]. A high expression of co-localization of TRPV1 and nNOS in subepidermal nerve fibers is present in acupoints and the expression is increased by EA, which suggests that the increased expression of TRPV1 may play a role in mediating the transduction of EA signals to the CNS [41]. It has been demonstrated that EA can induce expression of TRPV1 and nNOS in the acupoint ST36 and the gracile nucleus and nucleus tractus solitaries, which are involved in the signal transduction of EA stimuli via somatosensory afferents-medulla pathways [14]. Other studies also showed that sensitization of TRPV1 via peripheral metabotropic glutamate receptor 5 signaling contributes to thermal and mechanical hypersensitivity [42], and that the TRPV1 channel contributes to cutaneous thermal hyperemia in humans [43]. These findings are also consistent with results which show that TRPV1 and nNOS levels are enhanced in the acupoints, and suggest that NO may enhance TRPV1 mediated communication which is involved in sensory signal transduction and meridian functions [44,45].
4. NO in the dorsal medulla-thalamic pathways contributes to sensory signal transduction and functions: Stimuli-induced NO release and axon reflex
NADPH phosphate reactivity and nNOS immunoreactivity are increased in the gracile nucleus in response to either unilateral electrical stimulation of the sural nerve or by sciatic nerve injury [46,47]. Experiments have demonstrated that EA stimulation of the foot acupoints BL64, BL65, or the leg acupoint ST36 can increase nNOS immunoreactivity and NADPH diaphorase reactivity in the gracile nucleus [48,49]. NO is synthesized by NO synthase, and nNOS, eNOS, and iNOS exist in many cells and neurons in the brain and peripheral acupoints [50,51]. These results are consistent with the other reports that found somatosensory afferent inputs from the hindlimb project to the gracile nucleus [52–54], and showed that nNOS expression induced by the activation of afferent somatic nerves occurs mainly in the ipsilateral side of gracile nucleus neurons [14,41,46].
Electrophysiological mapping studies and anterograde axon tracing techniques have demonstrated somatotopic organization of the gracile nucleus receiving peripheral somatosensory afferents from the hindlimb in various mammals including the cat, rat, raccoon, sheep, and opossum [52–54]. The gracile nucleus receives ascending input from primary afferent fibers of the sciatic nerve, and the afferent sensory fibers in the sciatic nerves originate from the skin or muscle of the hindlimb, and the synapse is directed on dorsal horn neurons in the spinal cord, which ascend to the gracile nucleus [52–55]. Most responses in the gracile nucleus were to stimulation of skin and hairs of the tail, hind foot and leg, and trunk, and a similar distribution of responses was found in the cuneate nucleus to stimulation of forelimb, neck, and pinna [55,56]. Cutaneous primary afferents projecting from the hindlimb to the medulla oblongata are distributed mainly in the gracile nucleus, and the cuneate nucleus receives somatosensory input chiefly from the upper limb [52,54]. The synapses from these two major dorsal column nuclei receiving somatosensory afferent inputs project to the thalamus [53,57]. It has been suggested that the gracile nucleus contributes to somatic and visceral pain and sensory processing, which serve as an integration center for such information flowing into the thalamus [58,59]. The somatosensory afferent inputs ascend in the paraventricular thalamic nucleus (PVT) and adjacent thalamic nuclei have been identified by electrophysiological studies [60,61]. Central autonomic control of cardiovascular and other integrative functions has been shown in the PVT as the mediodorsal thalamic nucleus [61,62]. These results suggest that a nociceptive/somatosensory pathway in the dorsal funiculus tract is involved in the mechanistic effects of EA stimulation of acupoints in the leg, which produce NO and TRPV1, resulting in somatosensory and cardiovascular and pain regulation.
Experimental evidence further revealed co-expression of TRPV1 with nNOS in subepidermal nerve fibers is greater in acupoints than the surrounding tissue, and the expression is further increased by EA stimulation [41]. Consistently, our other studies have shown that expression of mRNA for TRPV1 is enhanced in the acupoint and modified in the brainstem region of rats following EA at ST36 [63]. NO release is increased in the gracile nucleus following EA stimulation and TRPV3 expression in the dorsal medulla is also enhanced by infrared heat treatment in rats [63–65]. NOergic molecules and TRPV1 play important roles in signal transduction of not only acupuncture/EA but also TENS, and heat induced PSC/LPSC sensory information in peripheral and central sites through the acupoints–dorsal medulla–thalamus–cortex pathways (the dorsal funiculus tract).
The effects of l-arginine-derived NO synthesis in the gracile nucleus on the cardiovascular responses to EA stimulation of ST36 have been studied in rats [52]. EA stimulation of ST36 produces depressor and bradycardic responses in rats, but the same stimulation of non-acupoints caused slight cardiovascular responses [66,67]. The hypotensive and bradycardic responses to EA at ST36 were facilitated by microinjection of l-arginine and blocked by microinjections of lidocaine into the gracile nucleus [66]. Microinjection of nNOS antisense oligonucleotides into the gracile nucleus inhibited the cardiovascular responses to EA at ST36 in rats [67]. The results suggest that the cardiovascular responses to EA at ST36 are mediated by NO in the gracile nucleus. These results are consistent with previous studies showing that the thalamic neurons receive neural inputs from the gracile nucleus and further demonstrate that NO plays a role in the gracile nucleus for mediating the cardiovascular responses to EA at ST36. NO in the gracile nucleus contributes to neuronal activities and therapeutics elicited by EA at ST36, and dorsal medulla-thalamic pathways are responsible for EA signal transduction [66,67]. It is proposed that substances including serotonin, catecholamines, inorganic chemicals, transient receptor potential channels, mas-related G protein-coupled receptor D, mas-related G protein-coupled receptor member A3, and amino acids such as glutamate and γ-aminobutyric acid may mediate the responses and certain cardiovascular and analgesic effects of acupuncture-like stimulations, but their mechanisms are poorly understood.
5. EA triggers release of NO and neuropeptides in the ipsilateral gracile nucleus and is consistent with the phenomenon of PSCP
The perception of PSCP are similar with the “de qi” sensation that arises during acupuncture treatment. Traditionally, sensory perception is believed to be generally transduced through the spinothalamic pathway which is a sensory tract that carries signal of nociception, temperature, crude touch, and pressure from our skin to the somatosensory area of the thalamus and sensory cortex. In the spinothalamic pathway, the axons of the second-order neurons cross over the spinal cord to the opposite side, two segments above the level of entry via the anterior white commissure, therefore, pain and temperature sensations are mainly over the entire contralateral side of the body. However, it is reported that PSCP is induced by ipsilateral stimulation but not contralateral stimulation of acupoints in various studies involving human subjects [3–5]. An analysis of 57 cases showed that following stimulation, 85% of PSCP was on the ipsilateral side of limb, and 14% was on the contralateral side [68]. These results do not support that the transduction of main PSCP sensations over the somatic-body to the sensory cortex are through the spinothalamic pathway.
Earlier investigators have demonstrated that transection injury of the sciatic nerve causes dystrophic central terminals in the ipsilateral gracile nucleus [69]. Unilateral transection injury of the sciatic nerve upregulated NPY also in the ipsilateral gracile nucleus in rats [70]. Studies have demonstrated that sciatic axotomy induces nNOS immunoreactivity and NADPH phosphate reactivity predominately in the ipsilateral gracile nucleus in rats, which suggests that sciatic injury induces transganglionic or trans-synaptic nNOS expression and NO release mainly in the ipsilateral site of the gracile nucleus [46,65]. Consistently, our recent studies show that 2,5-hexanedione intoxication increases nNOS positive neurons in the ipsilateral gracile nucleus of Zucker diabetic fatty rats and control rats [71]. The results support that lesion of the peripheral nerves upregulated NPY in the ipsilateral gracile nucleus [69]. The data also agree with studies reporting that EA-induced NO release and synthesis in the gracile nucleus in addition to the local stimulated acupoints [14,41,63], and EA-induced expression of NOS in the gracile nucleus is similar to lesion-induced transganglionic and/or trans-synaptic expression of an endogenous substance or pathological changes [46,70], occurs predominately in the ipsilateral gracile nucleus. These experimental results agree with the description in the neuroscience textbook that somatosensory afferent inputs from the hindlimb projecting to the gracile nucleus are mainly in the ipsilateral side of thalamus [55]. The results are consistent with the human studies reporting that PSCP sensations over the somatic-body occur mainly along the ipsilateral side of a limb following the stimulation [68] and further suggest that local stimulation of acupoints causes up-regulation of NOergic molecules and neuropeptides mainly in the ipsilateral gracile nucleus, which contribute to signal transduction of PSCP sensory information from the peripheral to central sites through the dorsal funiculus tract.
6. Future perspective
There has been a widespread and increasing interest in scientific examinations of the meridian system and the use of acupuncture for treating disorders all over the world. However, the direct experiment and technology to identify a specific anatomic structure and pathway of acupoints-meridian system are still lack [2–4,45,72]. The chemicals, structure, functions and mechanisms of the meridians are unclear. One of the 125 major exploration and discovery questions is that “Is there a scientific basis to the meridian system in traditional Chinese medicine?” as the international frontier, global common needs and gathering foresight released in 2021 by Shanghai Jiao Tong University and Science magazine [72]. During the last few decades, various studies have taken biophysical approaches to their investigation of phenomenon of meridians [3–10]. The PSCPs follow routes over the body surface that are consistent with the classical meridians and have been internationally elicited by various stimuli applied on acupoints in many human subjects. Although the phenomena of PSCP has been confirmed and published about 20 years ago and PSCP purports to be genuine evidence for the functional existence of meridians [5,8,9], there is a lack of objective and systematic experimental studies to support biochemical and morphological evidence and mechanisms of PSCP generation in humans. Physiological, histological, and clinical observations suggest that the human body’s fascia network resembles the theoretical meridian system, which may be the physical substrate represented by the meridians of TCM [73,74]. However, few studies exploring the structure, bomolecules and mechanisms behind the meridian phenomenon have been published over the past 20 years, perhaps because existing methods were insufficient to study the meridian system [2–4,45,72].
The increased interest in TCM and acupuncture has led to an increasing number of studies investigating its mechanisms of action, from the sensations of “de qi” and PSCP to the transduction of needling stimulation signals through the peripheral and central nervous system. The understanding of the neurobiological processes for signal transduction of acupuncture and PSCP sensory perceptions were initially believed to be involved in the spinothalamic pathway. However, unilateral stimuli cause mainly ipsilateral PSCP but no contralateral response, which does not support the role of the spinal-thalamic pathway in the transmission of the signal. In addition, the afferent fibers for these PSCP-induced stimuli are mainly from mechanoreceptors. Although the results from animal and human studies consistently suggest that axon reflexed mediated by NO signaling molecules and neuropeptides including CGRP, NPY, and substance P, can be triggered by MA, EA, electrical stimulation, and heat, which also induce PSCP and elevate NO production at acupoints and gracile nucleus, more sophisticated approaches are needed to understand how NOergic molecules and neuropeptides may be involved in propagation of the axon reflex over acupoints and meridian lines in PSCP in humans. In addition, the somatic-organ interactions are also unexplored areas, and whether the PSCP pathways along the meridian lines also spread into related organs needs to be systemically examined.
It has been shown that NOergic signaling molecules and neuropeptides play important roles in mediating the skin conductance responses to electrical stimulation and contribute to the low electrical resistance and high electric conductance that are characteristic of acupoints and meridians [75]. Norepinephrine synthesis and release was enhanced in acupoints and facilitated by the presence of an exogenous NO donor and inhibited by an inhibitor of NO synthesis [76]. A recent review summarized that NO concentrations are enhanced in skin acupoints and meridians, and l-arginine-derived NO synthesis and noradrenergic transmission modify skin electric conductance, which contributes to the low resistance characteristics of acupoints and meridians [45]. In addition to local release of NO, EA-induced NO synthase expression and NO release exist mainly in the ipsilateral gracile nucleus [49], which contribute to cardiovascular effects and analgesic responses to EA ST36 [67,68]. The gracile nucleus receives peripheral somatosensory nociceptive/sensory afferents projecting from the hindlimb, and neurons in the nucleus respond to innocuous and somatosensory stimulation mainly on the ipsilateral side via the dorsal column pathway [52–57]. A number of studies have established that the gracile nucleus is an integration center for cutaneous and visceral information flowing into the thalamus, which plays an important role in somatic sensory and pain processing [58,59]. Our results are consistent with previous studies showing that the thalamic neurons receive neural inputs from the gracile nucleus and further demonstrate that NO in the nucleus contributes to neuronal activities and therapeutics elicited by EA at ST36 through dorsal medulla-thalamic pathways, which are responsible for EA signal transduction, as concluded in a review article published in 2004 [44]. Moreover, animal studies have consistently found that NO levels are elevated by EA in the acupoints and meridians of rats and are associated with an enhanced expression of NO synthase and TRPV1 [14,41]. TRPV3 expression in the dorsal medulla is also enhanced by infrared heat treatment in rats [64]. Dermal microdialysis in humans showed that NO and cGMP release in the subcutaneous tissue of acupoints is increased by EA [20]. NO and cGMP released over skin acupoints are consistently increased by MA, EA, and TENS with low stimulation frequency/force, but not by treatments with high stimulation frequency/force [18,19]. The results show that NO level is higher over acupoints at physiological levels, and stimulus-evoked NO release is higher at acupoints [16–20]. A review article [65] summarizing these results suggested that higher levels of NO signaling molecules in acupoints may be involved in its functional specificity. Further, higher expression of NO synthase and TRPV1 in the gracile nucleus and in the subepidermal nerve fibers of acupoints and their subsequent upregulation after EA stimulation may play a key role in mediating the transduction of EA signals to the CNS supporting the postulated role of the dorsal funiculus tract in sensory and nociceptive regulation as well as mediating mechanisms of acupuncture. Consistently, the stimuli have been used to elicit axonal reflex, which are characterized by local release of NO and neuropeptides such as calcitonin related polypeptide (CGRP), TRPV1, and NPY. Interestedly, the same stimuli can be used to evoke both axon reflex and PSCP. Further, the stimuli-induced NO signaling molecules and neuropeptides contribute to the biochemical and –physiological processes of the axon reflex, leading to the suggestion that a similar driver induces NO signaling molecules and neuropeptide-mediated axon reflexes, spreading over acupoints one after another along the median lines, which may participate in the somatosensory signal transduction of PSCP.
The biomolecular changes in the acupoints and gracile nucleus play important roles in transductions of the sensory signals-induced by PSCP through the acupoints-dorsal medulla-thalamic pathways. The PSCP perceptions are similar to the de qi during acupuncture treatment. That is the reason why stronger de qi may indicate better therapeutic effects following MA, EA, electrical stimulation, and heat therapies. NO-mediated axon reflex in acupoints and the sensory signals mediated by NO release in the gracile nucleus through the acupoints-dorsal medulla-thalamic pathways may also participate in the processes of de qi as well as produce therapeutic effects through central regulation induced by the stimuli. Moreover, whether the PSCP pathways along the meridian lines spread into related organs and influence organ functions, therefore, contribute to the therapeutic effects of the stimuli are important areas to be further studied.
7. Conclusion
Previous anatomical studies have demonstrated that acupoints and meridians contain higher numbers of nerve fibers and trunks, blood vessels, hair follicles, and sweat glands [3,4,11,12]. Several studies demonstrated that low force/frequency of TENS, MA, EA and electrical and heat produce an elevation of NO release in all skin areas including non-acupoints and non-meridian skin regions, but with higher levels at acupoints [12,14,19,20]. Recent results have also showed that expression of TRPV1 endowed with NO synthase in subepidermal nerve fibers exist a higher level in the acupoints which is further increased by EA stimulation. These stimuli are often used to be applied on the distal acupuncture points (the Jing or the Well points) for eliciting PSCP. The same stimuli also elicit NO release in the gracile nucleus which contributes to the transduction of the sensory signals (including both stimuli-evoked signals and sensory signals from elevated local blood flow) that are induced by PSCP through the acupoints-dorsal medulla-thalamic-cortex pathways. The stimuli-evoked axon reflex and NOergic biomolecules/neuropeptides over acupoints one after another along the median lines, which increases local blood flow and somatosensory signals in the skin and subcutaneous tissue under a linear path resembling acupoints and meridians. The increased interest in the acupoints and meridians has led to an open-minded attitude towards understanding this system and related therapies, which will bring groundbreaking results not only into the TCM but also neuro-physiological regulation and the pathophysiology of clinical disorders in biomedical sciences.
Funding
This project was made possible by National Institutes of Health Grant (AT002478, AT004620, and AT004504) from the National Center for Complementary and Integrative Medicine.
Footnotes
CRediT authorship contribution statement
Contributed to manuscript preparation: SXM
Declaration of competing interests
The author has stated explicitly that there are no competing financial or personal interest in connection with this article.
References
- [1].The Acupuncture Institute of the Academy of Traditional Chinese Medicine. Essentials of Chinese acupuncture. Beijing: Foreign Languages Press; 1980. p. 76–88. https://search.yahoo.com/yhs/search/?hspart=pty&hsimp=yhs-browser_wavebrowser&grd=1¶m2=6e93e1e6-ece6-4286-a195-bd2cf4a98705¶m3=wav~US~appfocus1~¶m4=d-cp12919082543-lp5-hh6-obem-wav-vuentp:on-igiuzAryEeSWquRGFYzQ-ab36-w64-brwsr-df-sl-ntp~Chrome~essentials%20of%20chinese%20acupuncture%20book~B2D7D7656EB4E5153688637C8FBF7B49~Win10¶m1=20220331&p=essentials%20of%20chinese%20acupuncture%20book&type=A1-brwsr-~2022-14~&guccounter=1 [Google Scholar]
- [2].Chan SH. What is being stimulated in acupuncture: evaluation of the existence of a specific substrate? Neurosci Biobehav Rev 1984; 8(1): 25–33. https://pubmed.ncbi.nlm.nih.gov/6328387 [DOI] [PubMed] [Google Scholar]
- [3].Wang GJ, Ayati MH, Zhang WB. Meridian studies in China: a systematic review. J Acupunct Meridian Stud 2010; 3(1): 1–9. https://pubmed.ncbi.nlm.nih.gov/20633509 [DOI] [PubMed] [Google Scholar]
- [4].Zhu ZX, Hao JK. Acupuncture meridian biophysics—scientific verification of the first great invention of China. Beijing: Beijing Press; 1989. p.17–126, 146–420.[Chinese]. [Google Scholar]
- [5].Xu JS, Zheng SX, Pan XH, Zhu XX, Hu XL. The existence of propagated sensation along the meridian proved by neuroelectrophysiology. Neural Regen Res 2013; 8(28): 2633–40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4146028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang WB, Zhao Y, Kjell F. Understanding propagated sensation along meridians by volume transmission in peripheral tissue. Chin J Integr Med 2013; 19(5):330–9. https://pubmed.ncbi.nlm.nih.gov/23674110/ [DOI] [PubMed] [Google Scholar]
- [7].Beissner F, Marzolff I. Investigation of acupuncture sensation patterns under sensory deprivation using a geographic information system. Evid Based Complement Alternat Med 2012; 2012: 591304. https://pubmed.ncbi.nlm.nih.gov/23243458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang WB, Tian YY, Li H, Tian JH, Luo MF, Xu FL, et al. A discovery of low hydraulic resistance channel along meridians. J Acupunct Meridian Stud 2008; 1(1): 20–8. https://pubmed.ncbi.nlm.nih.gov/20633451 [DOI] [PubMed] [Google Scholar]
- [9].Chen XZ, Yang YK, Yang J, Yang MX, Feng SW, Hu XJ, et al. Acupuncture deqi intensity and propagated sensation along channels may, respectively, differ due to different body positions of subjects. Evid Based Complement Alternat Med 2013; 2013: 897048. https://www.hindawi.com/journals/ecam/2013/897048/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Razavy S, Gadau M, Zhang SP, Wang FC, Bangrazi S, Berle C, et al. Investigation of the phenomenon of propagated sensation along the channels in the upper limb following administration of acupuncture and mock laser. J Acupunct Meridian Stud 2017; 10(5): 307–16. https://pubmed.ncbi.nlm.nih.gov/29078965 [DOI] [PubMed] [Google Scholar]
- [11].Monteiro-Riviere NA, Hwang YC, Stromberg MW. Light microscopic morphology of low resistance skin points in the guinea pig. Am J Chin Med 1981; 9(2): 155–63. https://pubmed.ncbi.nlm.nih.gov/7345920 [DOI] [PubMed] [Google Scholar]
- [12].Ma SX. Enhanced nitric oxide concentrations and expression of nitric oxide synthase in acupuncture points/meridians. J Altern Complement Med 2003; 9(2): 207–15. https://pubmed.ncbi.nlm.nih.gov/12804074 [DOI] [PubMed] [Google Scholar]
- [13].Dippel E, Mayer B, Schönfelder G, Czarnetzki BM, Paus R. Distribution of constitutive nitric oxide synthase immunoreactivity and NADPH-diaphorase activity in murine telogen and anagen skin. J Invest Dermatology 1994; 103(1): 112–5. https://pubmed.ncbi.nlm.nih.gov/7517979 [DOI] [PubMed] [Google Scholar]
- [14].Ji B, Hu J, Ma SX. Effects of electroacupuncture Zusanli (ST36) on food intake and expression of POMC and TRPV1 through afferents-medulla. pathway in Obese Prone Rats. Peptides 2013; 40: 188–94. https://pubmed.ncbi.nlm.nih.gov/23116614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiong KR, Li HB, Wang T. Origin of nitric oxide synthase positive nerve fibers at Zusanli area in rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998; 18: 230–2 [Chinese with abstract in English]. [CNKI] https://en.cnki.com.cn/Article_en/CJFDTOTAL-ZZXJ199804016.htm [PubMed] [Google Scholar]
- [16].Ma SX, Li XY, Sakurai T, Pandjaitan M. Evidence of enhanced non-enzymatic nitric oxide generation on the skin surface of acupuncture points: An innovative approach in humans. Nitric Oxide 2007; 17(2): 60–8. https://pubmed.ncbi.nlm.nih.gov/17613264 [DOI] [PubMed] [Google Scholar]
- [17].Ma SX, Lee PC, Jiang I, Ma E, Hu JS, Li XY. Influence of age, gender, and race on nitric oxide release over acupuncture points-meridians. Sci Rep 2015; 5: 17547. https://pubmed.ncbi.nlm.nih.gov/26621821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ma SX, Mayer E, Lee P, Li XY, Gao EZ. Transcutaneous electrical nerve stimulation increased nitric oxide-cyclic cGMP release biocaptured over skin surface of the pericardium meridian and acupuncture points in humans. Acup Electrother 2015; 40(2):73–86. https://pubmed.ncbi.nlm.nih.gov/26369251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ma SX, Lee PC, Anderson TL, Li XY, Jiang IZ. Response of local nitric oxide release to manual acupuncture and electrical heat in humans: Effects of reinforcement methods. Evid Based Complement Alternat Med 2017; 2017: 4694238. https://pubmed.ncbi.nlm.nih.gov/28717380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lim NT, Ma SX. Responses of nitric oxide-cGMP releases in acupuncture point to electroacupuncture in human skin in vivo using dermal microdialysis. Microcirculation 2009; 16(5): 434–43. https://pubmed.ncbi.nlm.nih.gov/19468961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ha Y, Kim M, Nah J, Suh M, Lee Y. Measurements of location-dependent nitric oxide levels on skin surface in relation to acupuncture point. Evid Based Complement Alternat Med 2012; 2012: 781460. https://pubmed.ncbi.nlm.nih.gov/23049611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kimura K, Takeuchi H, Yuri K, Wakayama I. Effects of nitric oxide synthase inhibition on cutaneous vasodilation in response to acupuncture stimulation in humans. Acupunct Med 2013; 31(1): 74–80. https://pubmed.ncbi.nlm.nih.gov/23076431 [DOI] [PubMed] [Google Scholar]
- [23].Sandberg M, Lundeberg T, Lindberg LG, Gerdle B. Effects of acupuncture on skin and muscle blood flow in healthy subjects. Eur J Appl Physiol 2003; 90(1–2): 114–9. https://pubmed.ncbi.nlm.nih.gov/12827364 [DOI] [PubMed] [Google Scholar]
- [24].Sandberg M, Larsson B, Lindberg LG, Gerdle B. Different patterns of blood flow response in the trapezius muscle following needle stimulation (acupuncture) between healthy subjects and patients with fibromyalgia and work-related trapezius myalgia. Eur J Pain 2005; 9(5): 497–510. https://pubmed.ncbi.nlm.nih.gov/16139178 [DOI] [PubMed] [Google Scholar]
- [25].Schmelz M, Michael K, Weidner C, Schmidt R, Torebjörk HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 2000; 11(3): 645–8. https://pubmed.ncbi.nlm.nih.gov/10718329 [DOI] [PubMed] [Google Scholar]
- [26].Wallengren J, Håkanson R: Effects of substance P, neurokinin A and calcitonin gene-related peptide in human skin and their involvement in sensory nerve-mediated responses. Eur J Pharmacol 1987; 143(2): 267–73. https://pubmed.ncbi.nlm.nih.gov/2446892/ [DOI] [PubMed] [Google Scholar]
- [27].Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol (1985) 2008; 105(1): 233–40. https://pubmed.ncbi.nlm.nih.gov/18483164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kellogg DL Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 1999; 86: 1185–90. https://pubmed.ncbi.nlm.nih.gov/10194201 [DOI] [PubMed] [Google Scholar]
- [29].Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol 1996; 497(Pt 3): 837–48. https://pubmed.ncbi.nlm.nih.gov/9003568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maselli RA, Jaspan JB, Soliven BC, Green AJ, Spire JP, Arnason BG. Comparison of sympathetic skin response with quantitative sudomotor axon reflex test in diabetic neuropathy. Muscle Nerve 1989; 12(5): 420–3. https://pubmed.ncbi.nlm.nih.gov/2725569 [DOI] [PubMed] [Google Scholar]
- [31].Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 2006; 572(Pt 3): 811–20. https://pubmed.ncbi.nlm.nih.gov/16497714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hijazi MM, Buchmann SJ, Sedghi A, Illigens BM, Reichmann H, Schackert G, et al. Assessment of cutaneous axon-reflex responses to evaluate functional integrity of autonomic small nerve fibers. Neurol Sci 2020; 41(7): 1685–96. https://pubmed.ncbi.nlm.nih.gov/32125538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shinbara H, Okubo M, Kimura K, Mizunuma K, Sumiya E, Shinbara H, et al. Participation of calcitonin gene related peptide released via axon reflex in the local increase in muscle blood flow following manual acupuncture. Acupunct Med 2013; 31(1): 81–7. https://pubmed.ncbi.nlm.nih.gov/23305727 [DOI] [PubMed] [Google Scholar]
- [34].Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol 2003; 139(11): 1479–88. https://pubmed.ncbi.nlm.nih.gov/14623709 [DOI] [PubMed] [Google Scholar]
- [35].Hassan AA, Rayman G, Tooke JE. Effect of indirect heating on the postural control of skin blood flow in the human foot. Clin Sci 1986; 70(6): 577–82. https://pubmed.ncbi.nlm.nih.gov/3519056 [DOI] [PubMed] [Google Scholar]
- [36].Hong SS, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem 2005; 280(1): 618–27. https://pubmed.ncbi.nlm.nih.gov/15513920 [DOI] [PubMed] [Google Scholar]
- [37].Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol 2004; 500(1–3): 351–69. https://pubmed.ncbi.nlm.nih.gov/15464045 [DOI] [PubMed] [Google Scholar]
- [38].Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells and epithelial cells of appendage structures. Exp Dermatol 2004; 13(3): 129–39. https://pubmed.ncbi.nlm.nih.gov/14987252 [DOI] [PubMed] [Google Scholar]
- [39].Cioffi DL. The Skinny on TRPV1. Circ Res 2007; 100(7): 934–6. https://pubmed.ncbi.nlm.nih.gov/17431193 [DOI] [PubMed] [Google Scholar]
- [40].Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1997; 400(6743): 452–7. https://pubmed.ncbi.nlm.nih.gov/10440374 [DOI] [PubMed] [Google Scholar]
- [41].Ibrahim TS, Chen ML, Ma SX. TRPV1 expression in acupuncture points: response to electroacupuncture stimulation. J Chem Neuroanat 2011; 41(3): 129–36. https://pubmed.ncbi.nlm.nih.gov/21256210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Honda K, Shinoda M, Kondo M, Shimizu K, Yonemoto H, Otsuki K, et al. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain 2017; 158(9): 1754–64. https://pubmed.ncbi.nlm.nih.gov/28621704 [DOI] [PubMed] [Google Scholar]
- [43].Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 2010; 588(Pt 21): 4317–26. https://pubmed.ncbi.nlm.nih.gov/20807792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ma SX: Neurobiology of acupuncture: Toward CAM. Evid Based Complement Alternat Med 2004; 1(1): 41–7. https://pubmed.ncbi.nlm.nih.gov/15257325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ma SX. Low electrical resistance properties of acupoints: roles of NOergic signaling molecules and neuropeptides in skin electrical conductance. Chin J Integr Med 2021; 27(8): 563–9. https://pubmed.ncbi.nlm.nih.gov/34319572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ma SX, Cornford ME, Vahabnezhad I, Wei SM, Li XY. Responses of nitric oxide synthase expression in the gracile nucleus to sciatic nerve injury in young and aged rats. Brain Res 2000; 855(1): 124–31. https://pubmed.ncbi.nlm.nih.gov/10650138 [DOI] [PubMed] [Google Scholar]
- [47].Ma SX. Nitric oxide in the dorsal medulla modulates excitatory somatosympathetic reflexes. Curr Cardiol Reviews 2007; 3: 35–42. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=5de689a0fbf62da8d6d00b603f8b64e62d40587f [Google Scholar]
- [48].Ma SX, Li XY. Increased neuronal nitric oxide synthase expression in the gracile nucleus of brainstem following electroacupuncture given between cutaneous hindlimb acupuncture points BL 64 & BL 65 in rats. Acup Electrother Res 2000; 27(3–4): 157–69. https://pubmed.ncbi.nlm.nih.gov/12638736 [DOI] [PubMed] [Google Scholar]
- [49].Ma SX, Ma J, Moise G, Li XY. Responses of neuronal nitric oxide synthase expression in the brainstem to electroacupuncture Zusanli (ST36) in rats. Brain Res 2005; 1037(1–2): 70–7. https://pubmed.ncbi.nlm.nih.gov/15777754 [DOI] [PubMed] [Google Scholar]
- [50].Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest 1991; 21(4): 361–74. https://pubmed.ncbi.nlm.nih.gov/1718757 [DOI] [PubMed] [Google Scholar]
- [51].Bredt DS, Snyde SH. Nitric oxide, a novel neuronal messenger. Neuron 1992; 8(1): 3–11. https://pubmed.ncbi.nlm.nih.gov/1370373 [DOI] [PubMed] [Google Scholar]
- [52].Gulley RL. Golgi studies of the nucleus gracilis in the rat. Anat Rec 1973; 177(3): 325–42. https://pubmed.ncbi.nlm.nih.gov/4754155 [DOI] [PubMed] [Google Scholar]
- [53].Leem JW, Lee BH, Willis WD, Chung JM. Grouping of somatosensory neurons in the spinal cord and the gracile nucleus of the rat by cluster analysis. J Neuropysiol 1994; 72(6): 2590–7. https://pubmed.ncbi.nlm.nih.gov/7897476 [DOI] [PubMed] [Google Scholar]
- [54].Ueyama T, Houtani T, Ikeda M, Sato K, Sugimato T, Mizuno N. Distribution of primary afferent fibers projecting from hindlimb cutaneous nerves to the medulla oblongata in the cat and rat. J Comp Neurol 1994; 341(2): 145–58. https://pubmed.ncbi.nlm.nih.gov/7512998 [DOI] [PubMed] [Google Scholar]
- [55].Martin JH. Anatomical substrates for somatic sensation. In: Kandel ER, Schwartz JH (eds.). Principles of neural science, second edition. New York: Elsevier; 1985. p. 301–315. [Google Scholar]
- [56].Ostapoff EM, Johnson JI, Albright BC. Mechanosensory projections to cuneate, gracile, and external cuneate nuclei in a tree squirrel (fox squirrel, Sciurus niger). Neuroscience 1983; 9(1): 107–27. https://pubmed.ncbi.nlm.nih.gov/6308501 [DOI] [PubMed] [Google Scholar]
- [57].Cliffer KD, Hasegawa T, Willis WD. Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli: basic characterization and antidromic activation from the thalamus. J Neurophysiol 1992; 68(3): 818–32. https://pubmed.ncbi.nlm.nih.gov/1432050 [DOI] [PubMed] [Google Scholar]
- [58].Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 1996; 76(4): 2661–74. https://pubmed.ncbi.nlm.nih.gov/8899636 [DOI] [PubMed] [Google Scholar]
- [59].Al-Chaer ED, Westlund KN, Willis WD. Nucleus gracilis: an integrator for visceral and somatic information. J Neurophysiol 1997; 78(1): 521–7. https://pubmed.ncbi.nlm.nih.gov/9242300 [DOI] [PubMed] [Google Scholar]
- [60].Shin HC, Chapin JK. Mapping the effects of motor cortex stimulation on somatosensory relay neurons in the rat thalamus: direct responses and afferent modulation. Brain Res Bull 1990; 24(2): 257–65. https://pubmed.ncbi.nlm.nih.gov/2322860 [DOI] [PubMed] [Google Scholar]
- [61].Yen CT, Honda CN, Jones EG. Electrophysiological study of spinothalamic inputs to ventrolateral and adjacent thalamic nuclei of the cat. J Neurophys 1991; 66(3): 1033–47. https://pubmed.ncbi.nlm.nih.gov/1753274 [DOI] [PubMed] [Google Scholar]
- [62].Angyán L Somatomotor and cardiorespiratory response to basal ganglia stimulation in cats. Physiol Behav 1994; 56(1): 167–73. https://pubmed.ncbi.nlm.nih.gov/8084896 [DOI] [PubMed] [Google Scholar]
- [63].Rong PJ, Ma SX: Electroacupuncture Zusanli (ST36) on release of nitric oxide in the gracile nucleus and improvement of sensory neuropathies in Zucker diabetic fatty rats. Evid Based Complement Alternat Med 2011; 2011: 134545. https://pubmed.ncbi.nlm.nih.gov/19679645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu J, Choo HJ, Ma SX: Infrared heat treatment reduces food intake and modifies expressions of TRPV3-POMC in the dorsal medulla of obese prone rats. Int J Hyperthermia 2011; 27(7): 708–16. https://pubmed.ncbi.nlm.nih.gov/21967110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ma SX. Nitric oxide signaling molecules in acupoints: toward mechanisms of acupuncture. Chin J Integr Med 2017; 23(11): 812–5. https://pubmed.ncbi.nlm.nih.gov/29080196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen S, Ma SX. Effects of l-arginine-derived nitric oxide synthesis on cardiovascular responses to stimulus-evoked somatosympathetic reflexes in the gracile nucleus. Brain Res 2002; 958: 330–7. https://pubmed.ncbi.nlm.nih.gov/12470869 [DOI] [PubMed] [Google Scholar]
- [67].Chen S, Ma SX. Nitric oxide on acupuncture (ST36)-induced depressor response in the gracile nucleus. J Neurophysiol 2003; 90(2): 780–5. https://pubmed.ncbi.nlm.nih.gov/12672780 [DOI] [PubMed] [Google Scholar]
- [68].Liu CZ. Clinical meridian phenomenology. Dalian: Dalian Publishing House; 1994. p. 311–31 [Chinese]. [Google Scholar]
- [69].Wessels WJT, Feirabend HKP, Marani E. Development of projections of primary afferent fibers from the hindlimb to the gracile nucleus: a WGA-HRP study in the rat. Dev Brain Res 1991; 63(1–2): 265–79. https://pubmed.ncbi.nlm.nih.gov/1724211 [DOI] [PubMed] [Google Scholar]
- [70].Zhang X, Meister B, Elde R, Verge VMK, Hökfelt T. Large calibre primary afferent neurons projecting of the gracile nucleus express neuropeptide Y after sciatic nerve lesions: an immunohistochemical and in situ hybridization study in rats. Eur J Neurosci 1993; 5(11): 1510–9. https://pubmed.ncbi.nlm.nih.gov/7506974 [DOI] [PubMed] [Google Scholar]
- [71].Ma SX, Peterson RG, Magee EM, Lee P, Lee WP, Li XY. Impaired expression of neuronal nitric oxide synthase in the gracile nucleus is involved in neuropathic changes in Zucker diabetic fatty rats with and without 2,5-hexanedione intoxication. Neuro Res 2016; 106: 47–54. https://pubmed.ncbi.nlm.nih.gov/26519861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Levine AG. 125 questions: exploration and discovery. In: Sanders S (Eds.). Washington, DC: Science/AAAS Custom Publishing; 2021. p. 10. https://www.science.org/do/10.1126/resource.2499249/full/sjtu-booklet-1714066892333.pdf [Google Scholar]
- [73].Bai Y, Wang J, Wu JP, Dai JX, Ou S, Yew DT, et al. Review of evidence suggesting that the fascia network could be the anatomical basis for acupoints and meridians in the human body. Evid Based Complement Alternat Med 2011; 2011: 260510. https://pubmed.ncbi.nlm.nih.gov/21584283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Maurer N, Nissel H, Egerbacher M, Gornik E, Schuller P, Traxler H. Anatomical evidence of acupuncture meridians in the human extracellular matrix: results from a macroscopic and microscopic interdisciplinary multicentre study on human corpses. Evid Based Complement Alternat Med 2019; 2019: 6976892. https://pubmed.ncbi.nlm.nih.gov/31015853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen JX, Ma SX. Effects of nitric oxide and noradrenergic function on skin electric resistance of acupoints and meridians. J Altern Complement Med 2005; 11(3): 423–31. https://pubmed.ncbi.nlm.nih.gov/15992225 [DOI] [PubMed] [Google Scholar]
- [76].Chen JX, Ibe BO, Ma SX. Nitric oxide on modulation of norepinephrine production in acupuncture points. Life Sci 2006; 79(23): 2157–64. https://pubmed.ncbi.nlm.nih.gov/16890244 [DOI] [PubMed] [Google Scholar]


