Abstract
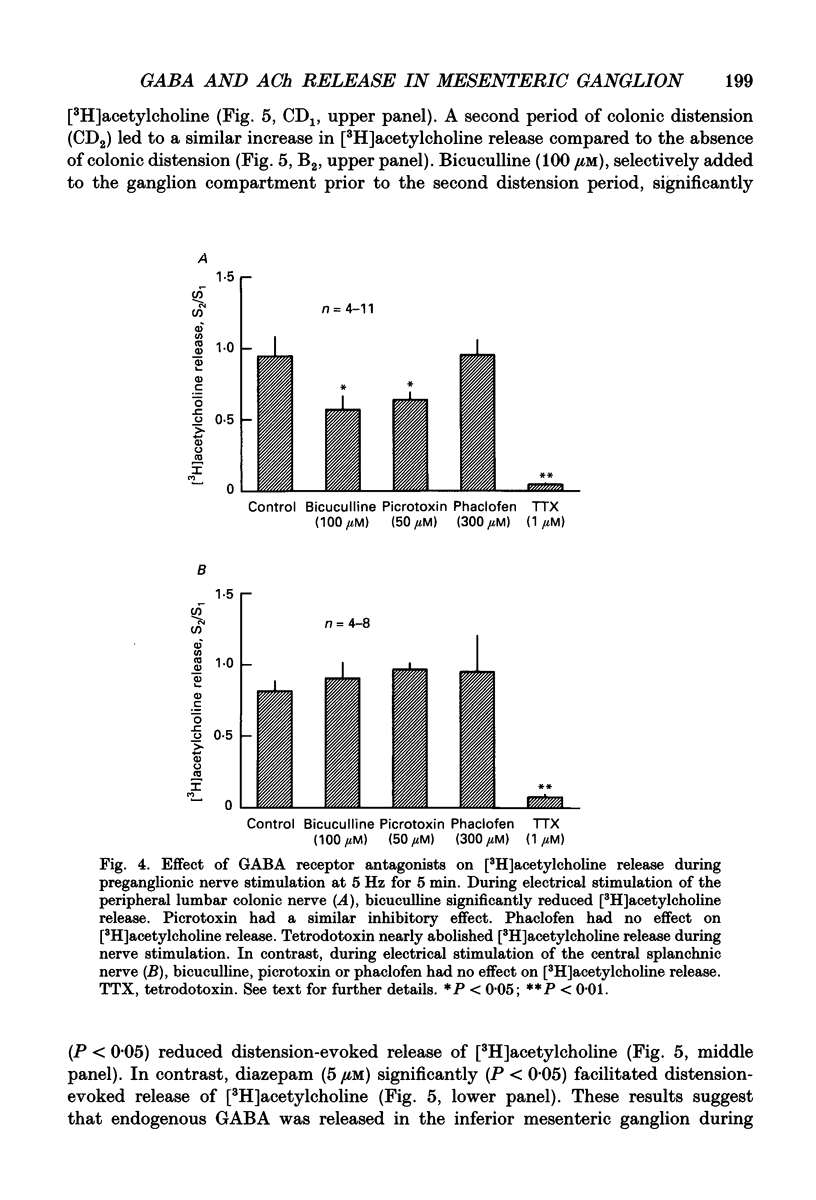
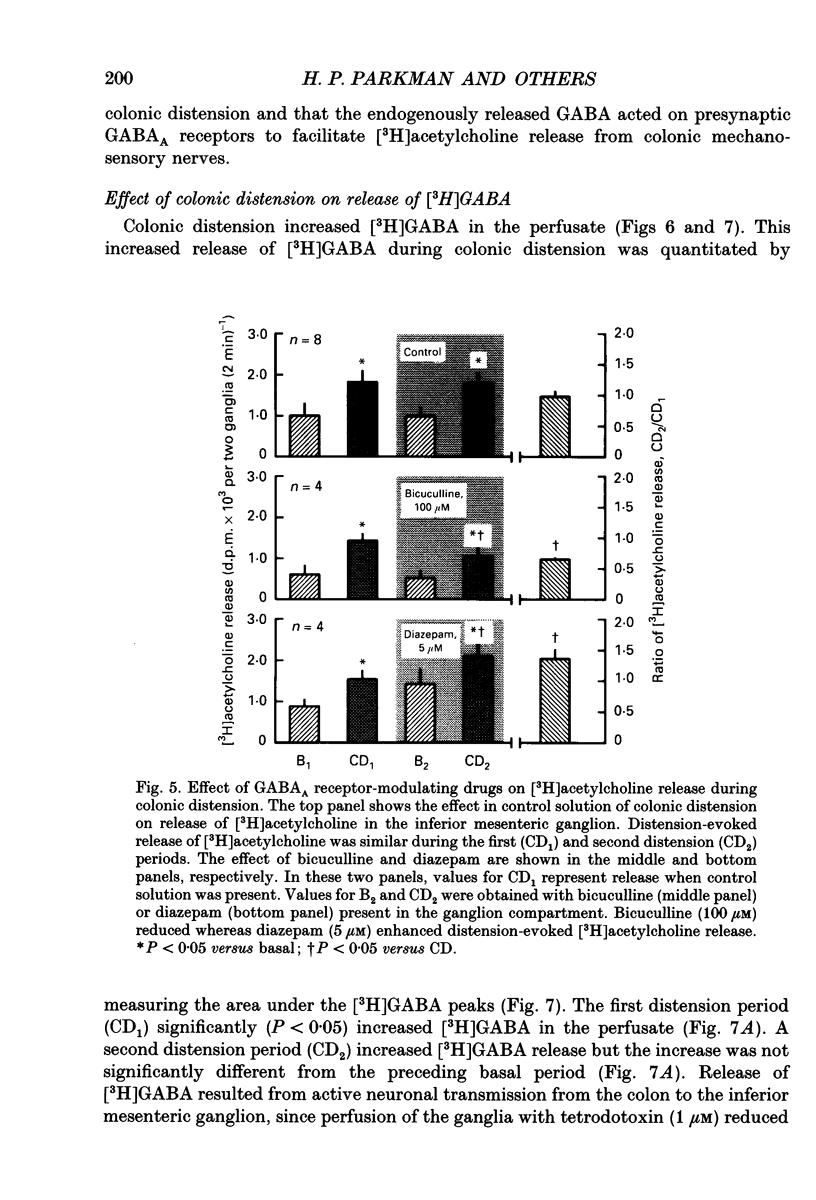
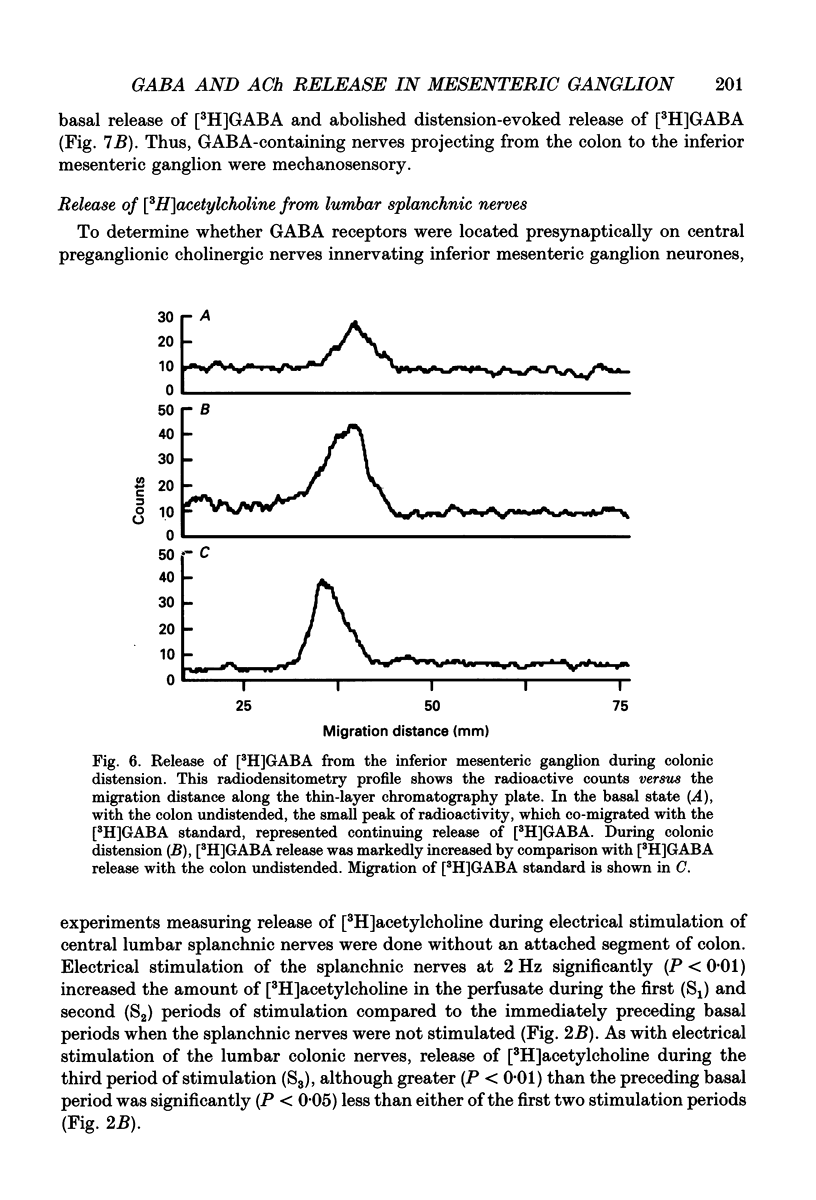
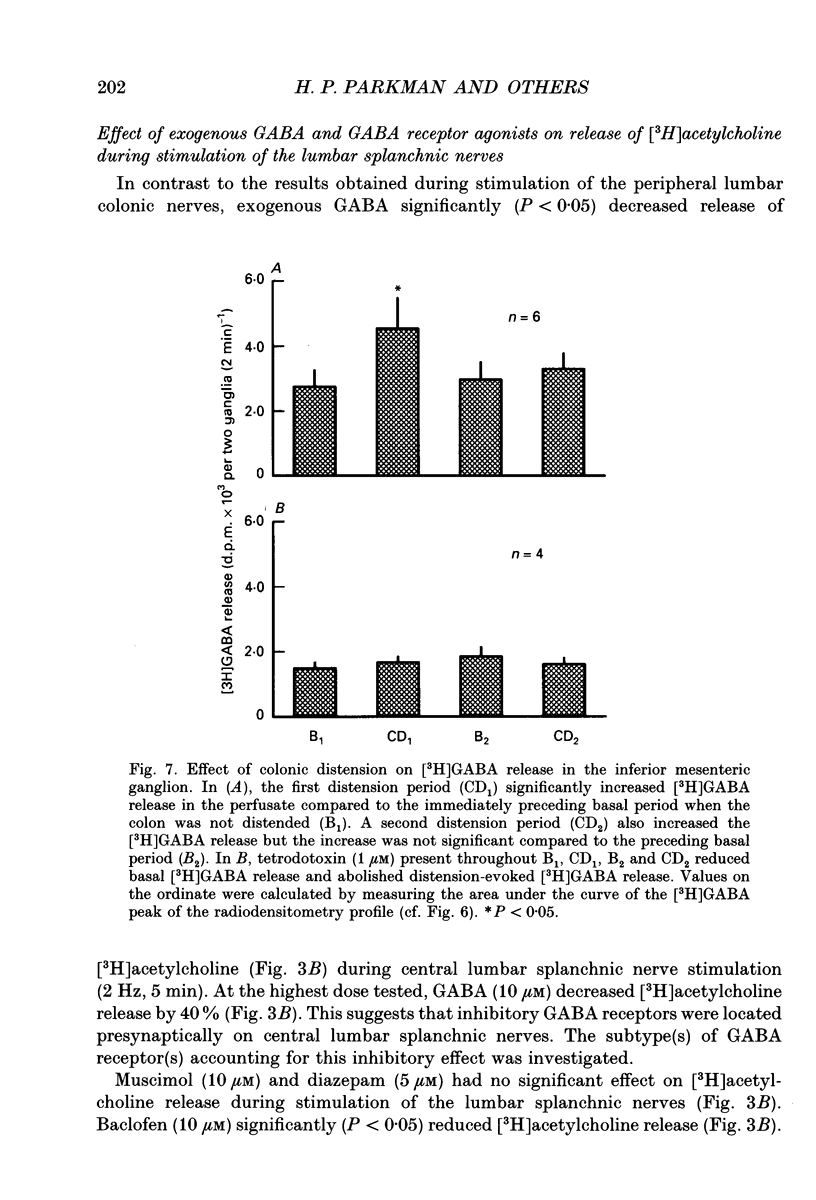
1. The effect of GABA and GABA receptor-modulating drugs on release of [3H]acetylcholine was studied in the guinea-pig inferior mesenteric ganglion. 2. GABA caused a dose-dependent increase in [3H]acetylcholine release during stimulation of the lumbar colonic nerves. Muscimol (10 microM) and diazepam (5 microM) also increased [3H]acetylcholine release during stimulation of the lumbar colonic nerves whereas baclofen (10 microM) had no effect. 3. Bicuculline (20-100 microM) and picrotoxin (50 microM) alone reduced [3H]acetylcholine release during electrical stimulation of the lumbar colonic nerves whereas phaclofen (300 microM) had no effect. 4. Bicuculline (100 microM) significantly decreased whereas diazepam (5 microM) significantly increased distension-induced [3H]acetylcholine release. 5. Colonic distension significantly increased [3H]GABA release in the inferior mesenteric ganglion compared to basal periods when the colon was not distended. Distension-induced release of [3H]GABA resulted from active neuronal transmission from the colon to the inferior mesenteric ganglion, since perfusion of the inferior mesenteric ganglion with tetrodotoxin (1 microM) reduced basal release of [3H]GABA and abolished distension-evoked increases in the release of [3H]GABA. 6. In contrast to its excitatory effects on peripheral colonic afferent cholinergic nerves, exogenous GABA caused a dose-dependent decrease in [3H]acetylcholine release during electrical stimulation of the central lumbar splanchnic nerves. Baclofen (10 microM) also inhibited [3H]acetylcholine release whereas muscimol (10 microM) or diazepam (5 microM) had no effect. Phaclofen (300 microM) antagonized the inhibitory effects of exogenous GABA (10 microM) and of baclofen (10 microM). Bicuculline (100 microM), picrotoxin (50 microM) and phaclofen (300 microM) alone had no effect on [3H]acetylcholine release during splanchnic nerve stimulation. 7. Phaclofen (300 microM) increased [3H]acetylcholine release during simultaneous electrical stimulation of the lumbar colonic nerves and splanchnic nerves and when GABAA receptors were blocked by bicuculline (20 microM). 8. The data suggest that GABAA receptors facilitate release of acetylcholine from peripheral cholinergic mechanosensory nerves projecting from the colon to the inferior mesenteric ganglion and that GABAB receptors inhibit release of acetylcholine from central cholinergic nerves. Enteric GABA-containing nerves projecting to the inferior mesenteric ganglion are mechanosensory. Endogenous release of GABA may act on GABAA receptors to facilitate peripheral cholinergic mechanosensory transmission and/or on GABAB receptors to inhibit central cholinergic transmission.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. M., Barnes N. M., Costall B., Naylor R. J., Tyers M. B. 5-HT3 receptors mediate inhibition of acetylcholine release in cortical tissue. Nature. 1989 Apr 27;338(6218):762–763. doi: 10.1038/338762a0. [DOI] [PubMed] [Google Scholar]
- Beani L., Bianchi C., Siniscalchi A., Sivilotti L., Tanganelli S., Veratti E. Different approaches to study acetylcholine release: endogenous ACh versus tritium efflux. Naunyn Schmiedebergs Arch Pharmacol. 1984 Dec;328(2):119–126. doi: 10.1007/BF00512060. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Tanganelli S., Marzola G., Beani L. GABA induced changes in acetylcholine release from slices of guinea-pig brain. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):253–258. doi: 10.1007/BF00501162. [DOI] [PubMed] [Google Scholar]
- Birnbaum D., Ben-Menachem J., Schwartz A. The influence of oral diazepam on gastrointestinal motility. A preliminary report. Am J Proctol. 1970 Aug;21(4):263–266. [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., Marsh S. gamma-Aminobutyric acid efflux from sympathetic glial cells: effect of 'depolarizing' agents. J Physiol. 1979 Aug;293:75–101. doi: 10.1113/jphysiol.1979.sp012879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., White R. D., Yamini G. [3H]gamma-Aminobutyric acid uptake into neuroglial cells of rat superior cervical sympathetic ganglia. J Physiol. 1979 Aug;293:51–74. doi: 10.1113/jphysiol.1979.sp012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobó E., Kása P., Wenthold R. J., Joó F., Wolff J. R. Evidence for GABAergic fibers entering the superior cervical ganglion of rat from the preganglionic nerve trunk. Histochemistry. 1989;92(2):133–136. doi: 10.1007/BF00490232. [DOI] [PubMed] [Google Scholar]
- Friesen A. J., Khatter J. C. The effect of preganglionic stimulation on the acetylcholine and choline content of a sympathetic ganglion. Can J Physiol Pharmacol. 1971 May;49(5):375–381. doi: 10.1139/y71-043. [DOI] [PubMed] [Google Scholar]
- Galvan M., Grafe P., ten Bruggencate G. Presynaptic actions of 4-aminopyridine and gamma-aminobutyric acid on rat sympathetic ganglia in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1980 Nov;314(2):141–147. doi: 10.1007/BF00504530. [DOI] [PubMed] [Google Scholar]
- Giotti A., Luzzi S., Spagnesi S., Zilletti L. GABAA and GABAB receptor-mediated effects in guinea-pig ileum. Br J Pharmacol. 1983 Mar;78(3):469–478. doi: 10.1111/j.1476-5381.1983.tb08807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider J. R., Makhlouf G. M. Enteric GABA: mode of action and role in the regulation of the peristaltic reflex. Am J Physiol. 1992 Apr;262(4 Pt 1):G690–G694. doi: 10.1152/ajpgi.1992.262.4.G690. [DOI] [PubMed] [Google Scholar]
- Hills J. M., King B. F., Mirsky R., Jessen K. R. Immunohistochemical localisation and electrophysiological actions of GABA in prevertebral ganglia in guinea-pig. J Auton Nerv Syst. 1988 Mar;22(2):129–140. doi: 10.1016/0165-1838(88)90086-0. [DOI] [PubMed] [Google Scholar]
- Kato E., Kuba K. Inhibition of transmitter release in bullfrog sympathetic ganglia induced by gamma-aminobutyric acid. J Physiol. 1980 Jan;298:271–283. doi: 10.1113/jphysiol.1980.sp013080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Krantis A., Kerr D. I. Gaba induced excitatory responses in the guinea-pig small intestine are antagonized by bicuculline, picrotoxinin and chloride ion blockers. Naunyn Schmiedebergs Arch Pharmacol. 1981 Nov;317(3):257–261. doi: 10.1007/BF00503827. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Starr M. S. Effect of inhibitors of -aminobutyrate aminotransferase on the accumulation of 3H- -aminobutyric acid by the retina. Br J Pharmacol. 1973 Mar;47(3):543–555. [PMC free article] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Bonanno G., Fedele E. Release of gamma-[3H]aminobutyric acid (GABA) from electrically stimulated rat cortical slices and its modulation by GABAB autoreceptors. J Pharmacol Exp Ther. 1989 Aug;250(2):648–653. [PubMed] [Google Scholar]
- Ritchie J. A., Truelove S. C. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J. 1979 Feb 10;1(6160):376–378. doi: 10.1136/bmj.1.6160.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon F., Kelly J. S. Selective uptake of (3H)beta-alanine by glia: association with glial uptake system for GABA. Brain Res. 1975 Mar 21;86(2):243–257. doi: 10.1016/0006-8993(75)90700-3. [DOI] [PubMed] [Google Scholar]
- Stapelfeldt W. H., Parkman H. P., Szurszewski J. H. The electrophysiological effects of endogenous GABA in the guinea-pig inferior mesenteric ganglion. J Physiol. 1993 Nov;471:175–189. doi: 10.1113/jphysiol.1993.sp019896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapelfeldt W. H., Szurszewski J. H. Neurotensin facilitates release of substance P in the guinea-pig inferior mesenteric ganglion. J Physiol. 1989 Apr;411:325–345. doi: 10.1113/jphysiol.1989.sp017576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989 Mar;25(3):213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Release of 3H-acetylcholine from isolated guinea pig ileum. A radiochemical method for studying the release on the cholinergic neurotransmitter in the intestine. Acta Physiol Scand. 1977 Nov;101(3):302–317. doi: 10.1111/j.1748-1716.1977.tb06012.x. [DOI] [PubMed] [Google Scholar]
- Young J. A., Brown D. A., Kelly J. S., Schon F. Autoradiographic localization of sites of (3H)gamma-aminobutyric acid accumulation in peripheral autonomic ganglia. Brain Res. 1973 Dec 7;63:479–486. doi: 10.1016/0006-8993(73)90128-5. [DOI] [PubMed] [Google Scholar]
- Yunger L. M., Fowler P. J., Zarevics P., Setler P. E. Novel inhibitors of gamma-aminobutyric acid (GABA) uptake: anticonvulsant actions in rats and mice. J Pharmacol Exp Ther. 1984 Jan;228(1):109–115. [PubMed] [Google Scholar]