Abstract
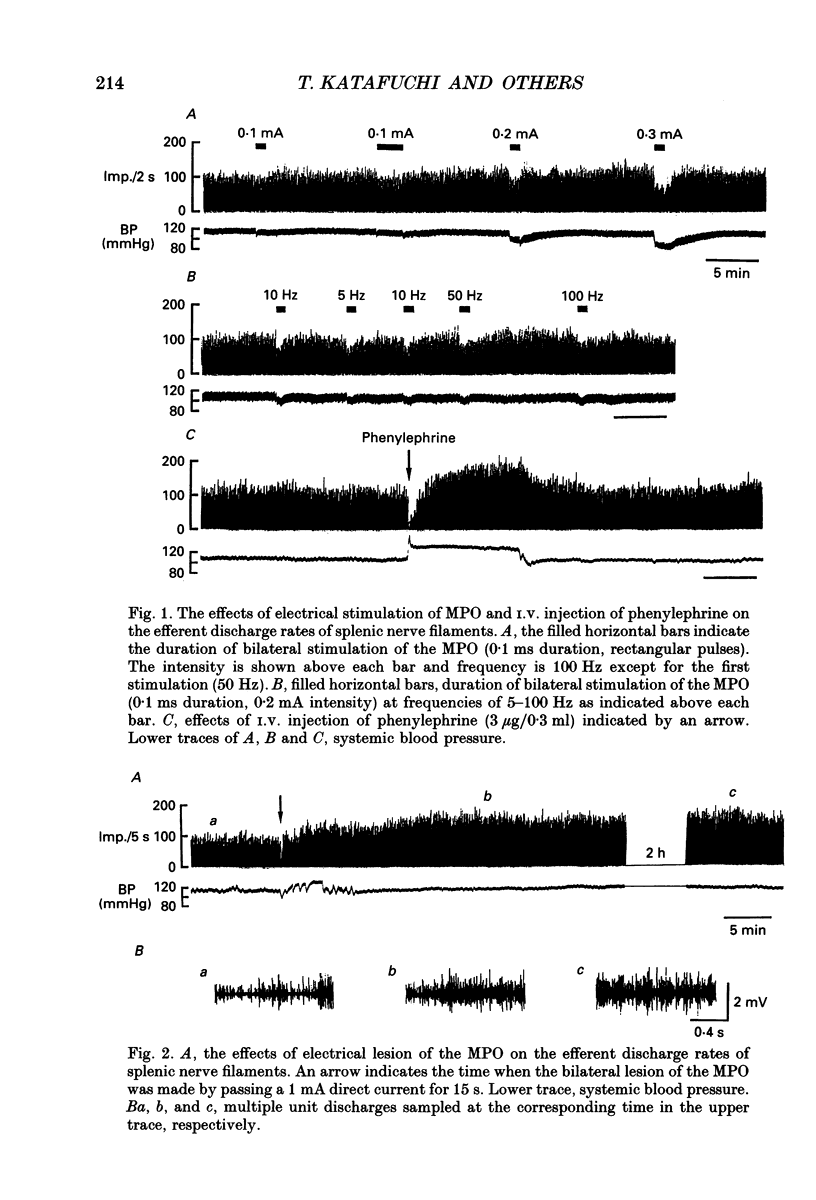
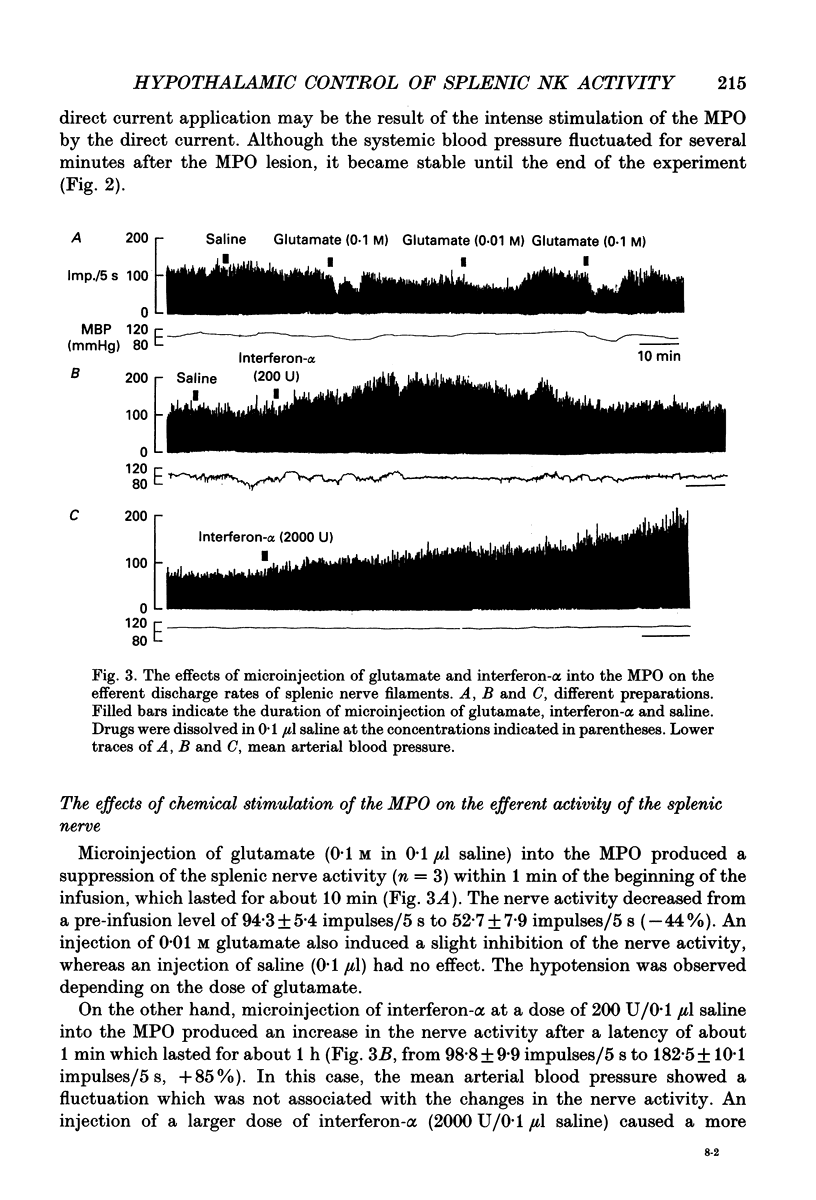
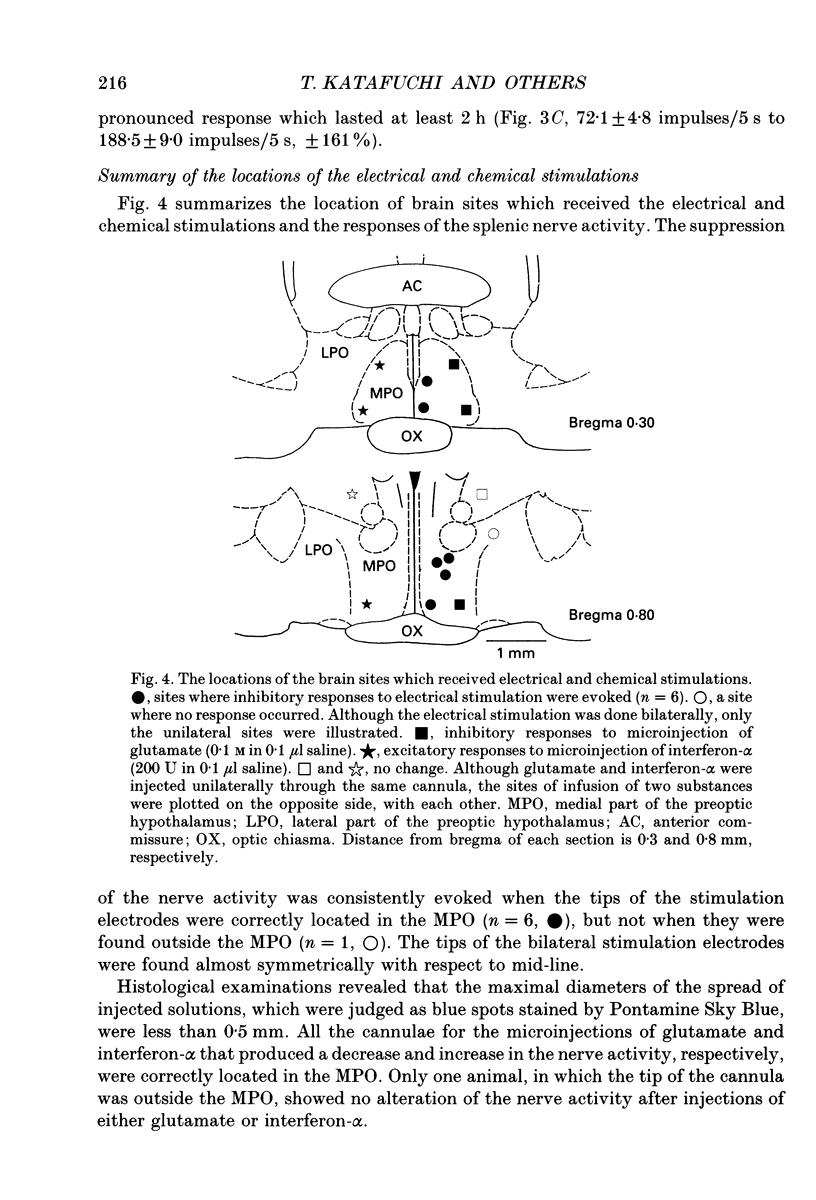
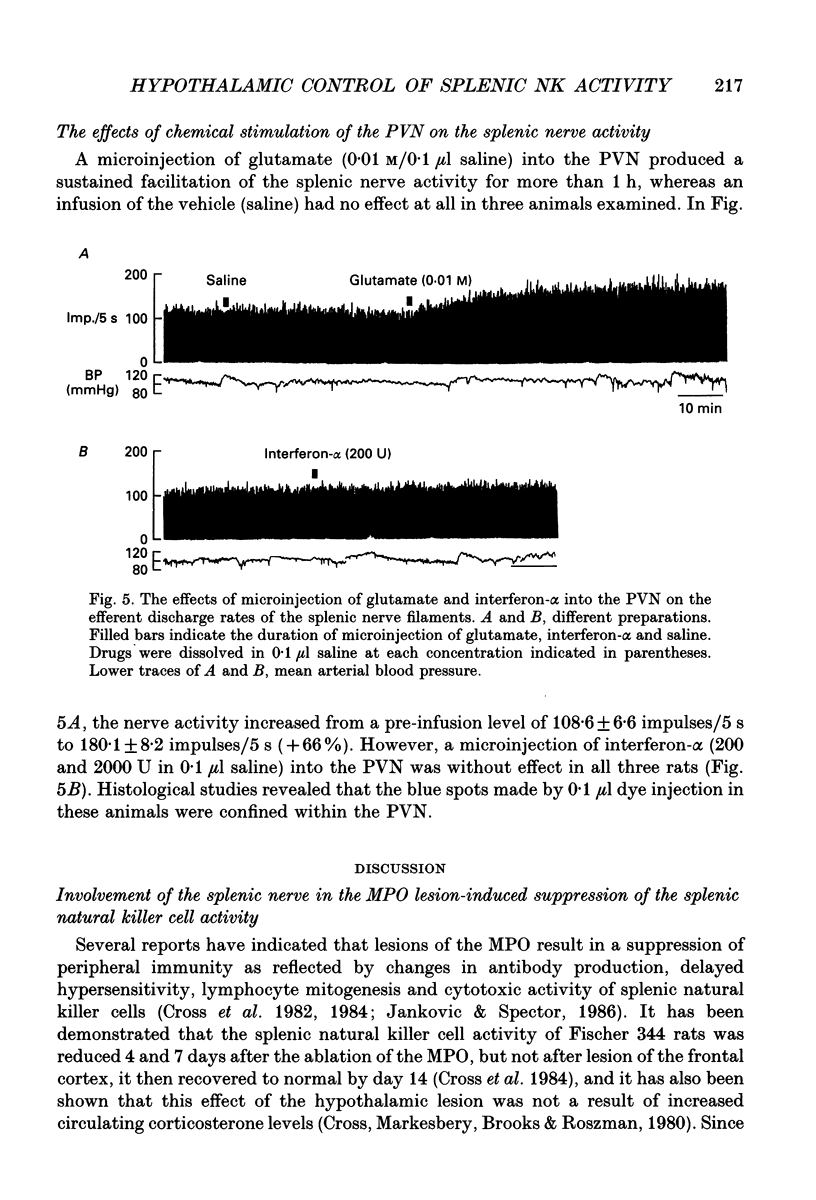
1. The cytotoxic activity of splenic natural killer cells measured by a standard chromium release assay in urethane and alpha-chloralose-anaesthetized rats was significantly suppressed 20 min after bilateral ablation of the medial part of the preoptic hypothalamus (MPO). The suppression was completely blocked by prior splenic denervation. The splenic natural killer cell activity of MPO sham-lesioned rats or thalamus-lesioned rats, both having an intact splenic innervation, were not different from that of a non-treated control group. 2. Electrical stimulation of the bilateral MPO (0.1 ms, 0.1-0.3 mA, 5-100 Hz) suppressed the efferent activity of the splenic nerve in all six rats examined. The reduction of the nerve activity was accompanied by a transient fall in blood pressure. An I.V. injection of phenylephrine (3 micrograms/0.3 ml) also evoked a suppression of the nerve activity, which was accompanied by transient hypertension, suggesting that the suppressive effect of the MPO stimulation was independent of changes in blood pressure. On the other hand, a bilateral lesion of the MPO resulted in a sustained increase in the electrical activity of the splenic sympathetic nerve filaments which lasted for more than 2 h. 3. Microinjection of monosodium-L-glutamate (0.1 and 0.01 M in 0.1 microliters saline) unilaterally into the MPO evoked a transient suppression of the efferent discharge rate of the splenic nerve activity within 1 min, which was also accompanied by a decrease in blood pressure. The injection of saline (0.1 microliter) into the MPO had no effect. The microinjection of recombinant human interferon-alpha (200 and 2000 U in 0.1 microliter saline) into the MPO dose dependently increased the splenic nerve activity without any change in blood pressure. 4. In contrast, microinjection of interferon-alpha into the paraventricular nucleus of the hypothalamus (PVN) had no effect on splenic nerve activity, although an injection of glutamate increased the nerve activity. 5. The present results, taken together with previous reports, suggest that the neuronal networks between the MPO and the splenic sympathetic nerve, which may be activated by centrally administered interferon-alpha, are important in the suppression of the splenic cellular immunity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellinger D. L., Felten S. Y., Lorton D., Felten D. L. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989 Dec;3(4):291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Pfaff D. W. Axonal projections of medial preoptic and anterior hypothalamic neurons. Science. 1975 Dec 12;190(4219):1112–1114. doi: 10.1126/science.1188390. [DOI] [PubMed] [Google Scholar]
- Cross R. J., Brooks W. H., Roszman T. L., Markesbery W. R. Hypothalamic-immune interactions. Effect of hypophysectomy on neuroimmunomodulation. J Neurol Sci. 1982 Mar;53(3):557–566. doi: 10.1016/0022-510x(82)90250-7. [DOI] [PubMed] [Google Scholar]
- Cross R. J., Markesbery W. R., Brooks W. H., Roszman T. L. Hypothalamic-immune interactions. I. The acute effect of anterior hypothalamic lesions on the immune response. Brain Res. 1980 Aug 25;196(1):79–87. doi: 10.1016/0006-8993(80)90717-9. [DOI] [PubMed] [Google Scholar]
- Cross R. J., Markesbery W. R., Brooks W. H., Roszman T. L. Hypothalamic-immune interactions: neuromodulation of natural killer activity by lesioning of the anterior hypothalamus. Immunology. 1984 Feb;51(2):399–405. [PMC free article] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Hellstrand K., Hermodsson S., Strannegård O. Evidence for a beta-adrenoceptor-mediated regulation of human natural killer cells. J Immunol. 1985 Jun;134(6):4095–4099. [PubMed] [Google Scholar]
- Hori T., Nakashima T., Take S., Kaizuka Y., Mori T., Katafuchi T. Immune cytokines and regulation of body temperature, food intake and cellular immunity. Brain Res Bull. 1991 Sep-Oct;27(3-4):309–313. doi: 10.1016/0361-9230(91)90117-3. [DOI] [PubMed] [Google Scholar]
- Irwin M., Hauger R. L., Brown M., Britton K. T. CRF activates autonomic nervous system and reduces natural killer cytotoxicity. Am J Physiol. 1988 Nov;255(5 Pt 2):R744–R747. doi: 10.1152/ajpregu.1988.255.5.R744. [DOI] [PubMed] [Google Scholar]
- Katafuchi T., Hori T., Take S. Central administration of interferon-alpha enhances rat sympathetic nerve activity to the spleen. Neurosci Lett. 1991 Apr 15;125(1):37–40. doi: 10.1016/0304-3940(91)90125-d. [DOI] [PubMed] [Google Scholar]
- Katafuchi T., Take S., Hori T. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol. 1993 Jun;465:343–357. doi: 10.1113/jphysiol.1993.sp019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeur M., Nahmod V. E., Finkielman S., Arzt E. Lesions of the medial septal nucleus produce a long-lasting inhibition of T lymphocyte proliferation. Neurosci Lett. 1991 Apr 29;125(2):129–132. doi: 10.1016/0304-3940(91)90008-h. [DOI] [PubMed] [Google Scholar]
- Nakashima T., Hori T., Kuriyama K., Matsuda T. Effects of interferon-alpha on the activity of preoptic thermosensitive neurons in tissue slices. Brain Res. 1988 Jun 28;454(1-2):361–367. doi: 10.1016/0006-8993(88)90838-4. [DOI] [PubMed] [Google Scholar]
- Nance D. M., Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989 Dec;3(4):281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Ninomiya I., Nisimaru N., Irisawa H. Sympathetic nerve activity to the spleen, kidney, and heart in response to baroceptor input. Am J Physiol. 1971 Nov;221(5):1346–1351. doi: 10.1152/ajplegacy.1971.221.5.1346. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Yoshimatsu H. Neural network of glucose monitoring system. J Auton Nerv Syst. 1984 May-Jun;10(3-4):359–372. doi: 10.1016/0165-1838(84)90033-x. [DOI] [PubMed] [Google Scholar]
- Reder A., Checinski M., Chelmicka-Schorr E. The effect of chemical sympathectomy on natural killer cells in mice. Brain Behav Immun. 1989 Jun;3(2):110–118. doi: 10.1016/0889-1591(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. Direct hypothalamo-autonomic connections. Brain Res. 1976 Nov 26;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Saphier D., Feldman S. Effects of stimulation of the preoptic area on hypothalamic paraventricular nucleus unit activity and corticosterone secretion in freely moving rats. Neuroendocrinology. 1986;42(2):167–173. doi: 10.1159/000124269. [DOI] [PubMed] [Google Scholar]
- Sundar S. K., Cierpial M. A., Kilts C., Ritchie J. C., Weiss J. M. Brain IL-1-induced immunosuppression occurs through activation of both pituitary-adrenal axis and sympathetic nervous system by corticotropin-releasing factor. J Neurosci. 1990 Nov;10(11):3701–3706. doi: 10.1523/JNEUROSCI.10-11-03701.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol. 1976 May 15;167(2):227–256. doi: 10.1002/cne.901670207. [DOI] [PubMed] [Google Scholar]
- Take S., Mori T., Kaizuka Y., Katafuchi T., Hori T. Central interferon alpha suppresses the cytotoxic activity of natural killer cells in the mouse spleen. Ann N Y Acad Sci. 1992 Apr 15;650:46–50. doi: 10.1111/j.1749-6632.1992.tb49093.x. [DOI] [PubMed] [Google Scholar]


