Abstract
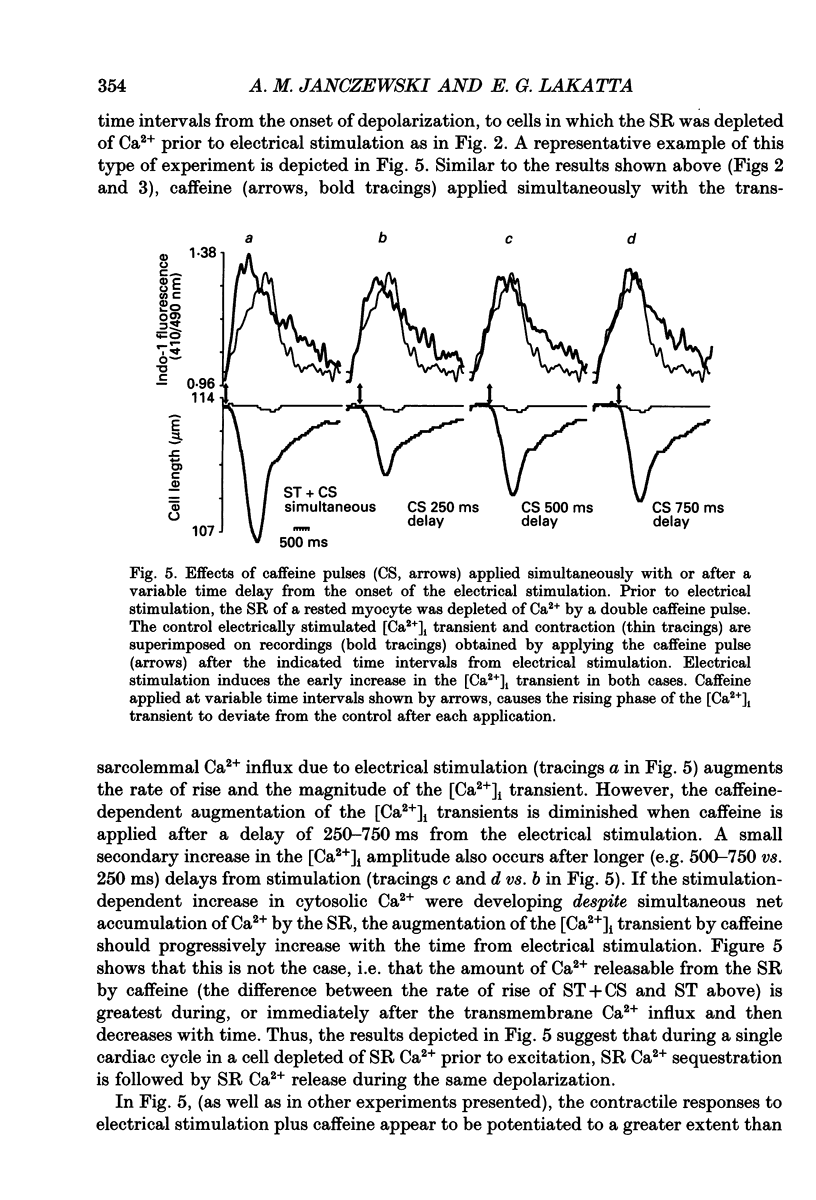
1. Intracellular [Ca2+] ([Ca2+]i) transients, monitored by the fluorescent Ca2+ indicator, indo-1, and twitch contractions elicited by action potentials, by voltage clamp pulses or by rapid, brief pulses of caffeine, were measured in guinea-pig single ventricular myocytes. Experiments were designed to determine whether and to what extent the trans-sarcolemmal Ca2+ influx is immediately sequestered by the sarcoplasmic reticulum (SR). 2. Rapid, brief (100-200 ms) pulses of caffeine onto a rested myocyte elicited a [Ca2+]i transient and a contraction. Following exposure to specific SR inhibitors, ryanodine (100 nM) or thapsigargin (200 nM), the rapid application of caffeine onto a rested myocyte failed to elicit changes in [Ca2+]i or in cell length, indicating that caffeine increases [Ca2+]i by specifically discharging Ca2+ from the SR. In the absence of these inhibitors, a second pulse of caffeine, within 3 min following a prior pulse, failed to elicit a [Ca2+]i transient or contraction, indicating that a caffeine pulse depletes the SR releasable Ca2+ pool. 3. Following Ca2+ depletion of the SR by double caffeine pulses at rest, an electrical stimulation elicited a slow increase in [Ca2+]i, and, after a delay, a small, slow twitch contraction. The simultaneous application of caffeine and electrical stimulation of cells in which the SR was Ca2+ depleted elicited [Ca2+]i transients with an increased rate of rise and a larger amplitude (53 +/- 8 and 63 +/- 9% respectively; mean +/- S.E.M., n = 21) than those elicited by electrical stimulation alone. 4. Whether caffeine affected the L-type calcium current (ICa) elicited by electrical stimulation was determined under whole-cell voltage clamp. A caffeine pulse delivered at the onset of a depolarizing voltage clamp step also increased the rates of rise and the amplitudes of the [Ca2+]i transients and twitch contractions in cells in which the SR was depleted of Ca2+. However, Ca2+ influx via ICa decreased when caffeine was pulsed in conjunction with the voltage clamp, as the peak ICa was either unchanged or decreased while its inactivation was consistently accelerated. 5. Because the stimulation-dependent trans-sarcolemmal Ca2+ influx via ICa is not increased by a caffeine pulse, the augmentation of the rates of rise and the amplitudes of the electrically stimulated [Ca2+]i transients by caffeine pulsed in conjunction with the electrical stimulation in cells in which the SR had been depleted of Ca2+ indicates that a portion of Ca2+ influx during depolarization in the absence of caffeine is rapidly buffered by the SR.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- Bassani R. A., Bassani J. W., Bers D. M. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H., Spitzer K. W. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol. 1989 Oct;417:537–553. doi: 10.1113/jphysiol.1989.sp017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H. Rapid regulation of the 'second inward current' by intracellular calcium in isolated rat and ferret ventricular myocytes. J Physiol. 1988 Dec;407:77–102. doi: 10.1113/jphysiol.1988.sp017404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R., Marengo J. J., Suárez-Isla B. A., Donoso P., Sutko J. L., Hidalgo C. Activation of calcium channels in sarcoplasmic reticulum from frog muscle by nanomolar concentrations of ryanodine. Biophys J. 1989 Oct;56(4):749–756. doi: 10.1016/S0006-3495(89)82722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- Doerr T., Denger R., Doerr A., Trautwein W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflugers Arch. 1990 May;416(3):230–237. doi: 10.1007/BF00392058. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Noble D. Caffeine and tetracaine abolish the slow inward calcium current in sheep cardiac Purkinje fibres [proceedings]. J Physiol. 1979 Aug;293:76P–77P. [PubMed] [Google Scholar]
- Eisner D. A., Valdeolmillos M. The mechanism of the increase of tonic tension produced by caffeine in sheep cardiac Purkinje fibres. J Physiol. 1985 Jul;364:313–326. doi: 10.1113/jphysiol.1985.sp015747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. J., Lipford G. B. Mechanism of action of ryanodine on cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Feb 28;813(1):77–86. doi: 10.1016/0005-2736(85)90347-5. [DOI] [PubMed] [Google Scholar]
- Habuchi Y., Tanaka H., Furukawa T., Tsujimura Y. Caffeine-induced block of Na+ current in guinea pig single ventricular cells. Am J Physiol. 1991 Dec;261(6 Pt 2):H1855–H1863. doi: 10.1152/ajpheart.1991.261.6.H1855. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Wier W. G. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of caffeine on the intracellular [Ca2+] transient, membrane currents, and contraction. J Gen Physiol. 1984 Mar;83(3):417–433. doi: 10.1085/jgp.83.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. D., Hering S., Bolton T. B. The action of caffeine on inward barium current through voltage-dependent calcium channels in single rabbit ear artery cells. Pflugers Arch. 1990 Jun;416(4):462–466. doi: 10.1007/BF00370755. [DOI] [PubMed] [Google Scholar]
- Kirby M. S., Sagara Y., Gaa S., Inesi G., Lederer W. J., Rogers T. B. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem. 1992 Jun 25;267(18):12545–12551. [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Calcium transients caused by calcium entry are influenced by the sarcoplasmic reticulum in guinea-pig atrial myocytes. J Physiol. 1992 Aug;454:321–338. doi: 10.1113/jphysiol.1992.sp019266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L. Transient inward current in guinea-pig atrial myocytes reflects a change of sodium-calcium exchange current. J Physiol. 1988 Mar;397:601–630. doi: 10.1113/jphysiol.1988.sp017021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Electrical activity and contraction in cells isolated from rat and guinea-pig ventricular muscle: a comparative study. J Physiol. 1987 Oct;391:527–544. doi: 10.1113/jphysiol.1987.sp016754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen U., Brøogger Christensen S., Sandberg F. Thapsigargine and thapsigargicine, two new histamine liberators from Thapsia garganica L. Acta Pharm Suec. 1978;15(2):133–140. [PubMed] [Google Scholar]
- Reiter M. Calcium mobilization and cardiac inotropic mechanisms. Pharmacol Rev. 1988 Sep;40(3):189–217. [PubMed] [Google Scholar]
- Reiter M., Vierling W., Seibel K. Excitation-contraction coupling in rested-state contractions of guinea-pig ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):159–169. doi: 10.1007/BF00506196. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989 Feb;256(2 Pt 2):H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991 Jul 25;266(21):13503–13506. [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., duBell W. H., Stern M. D., Sollott S. J., Ziman B. D., Silverman H. S., Capogrossi M. C., Talo A., Lakatta E. G. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol. 1992 Feb;447:83–102. doi: 10.1113/jphysiol.1992.sp018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Wrzosek A., Schneider H., Grueninger S., Chiesi M. Effect of thapsigargin on cardiac muscle cells. Cell Calcium. 1992 May;13(5):281–292. doi: 10.1016/0143-4160(92)90063-x. [DOI] [PubMed] [Google Scholar]
- Zholos A. V., Baidan L. V., Shuba M. F. The inhibitory action of caffeine on calcium currents in isolated intestinal smooth muscle cells. Pflugers Arch. 1991 Oct;419(3-4):267–273. doi: 10.1007/BF00371106. [DOI] [PubMed] [Google Scholar]
- de Beer E. L., Gründeman R. L., Wilhelm A. J., Caljouw C. J., Klepper D., Schiereck P. Caffeine suppresses length dependency of Ca2+ sensitivity of skinned striated muscle. Am J Physiol. 1988 Apr;254(4 Pt 1):C491–C497. doi: 10.1152/ajpcell.1988.254.4.C491. [DOI] [PubMed] [Google Scholar]


