Abstract
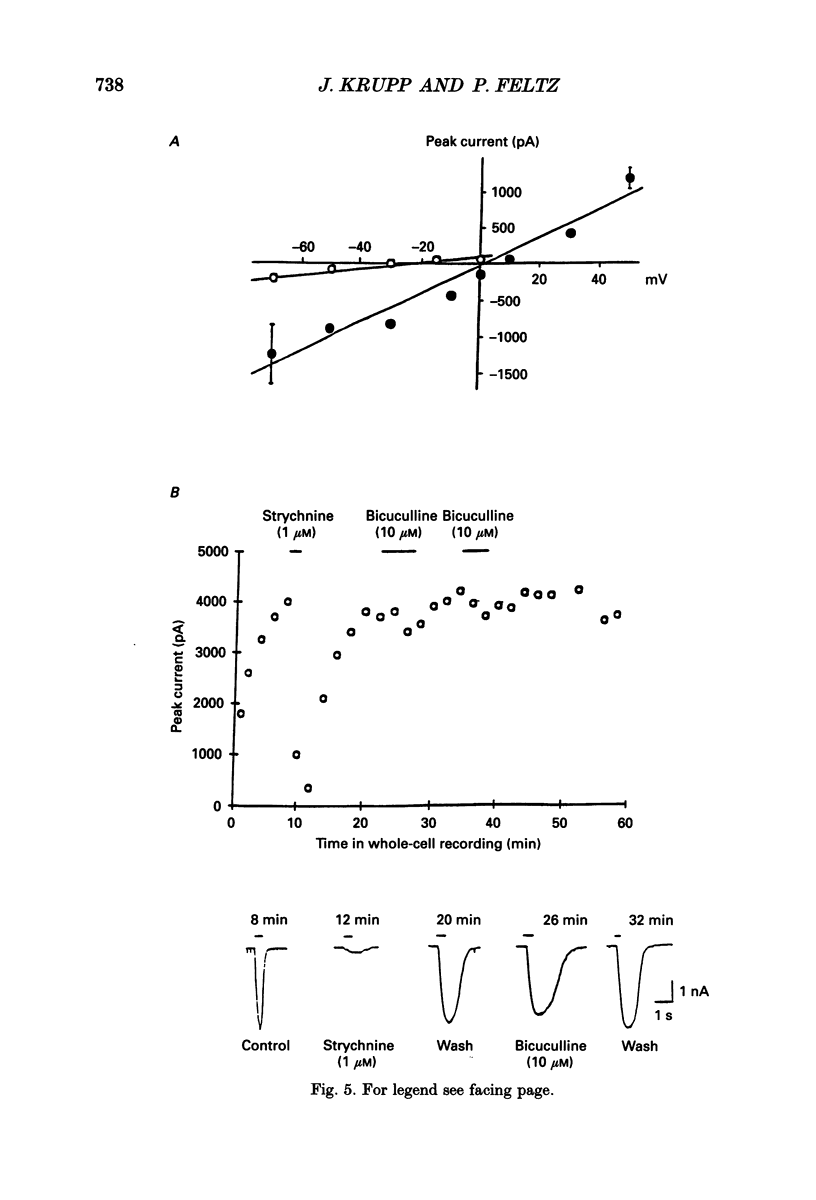
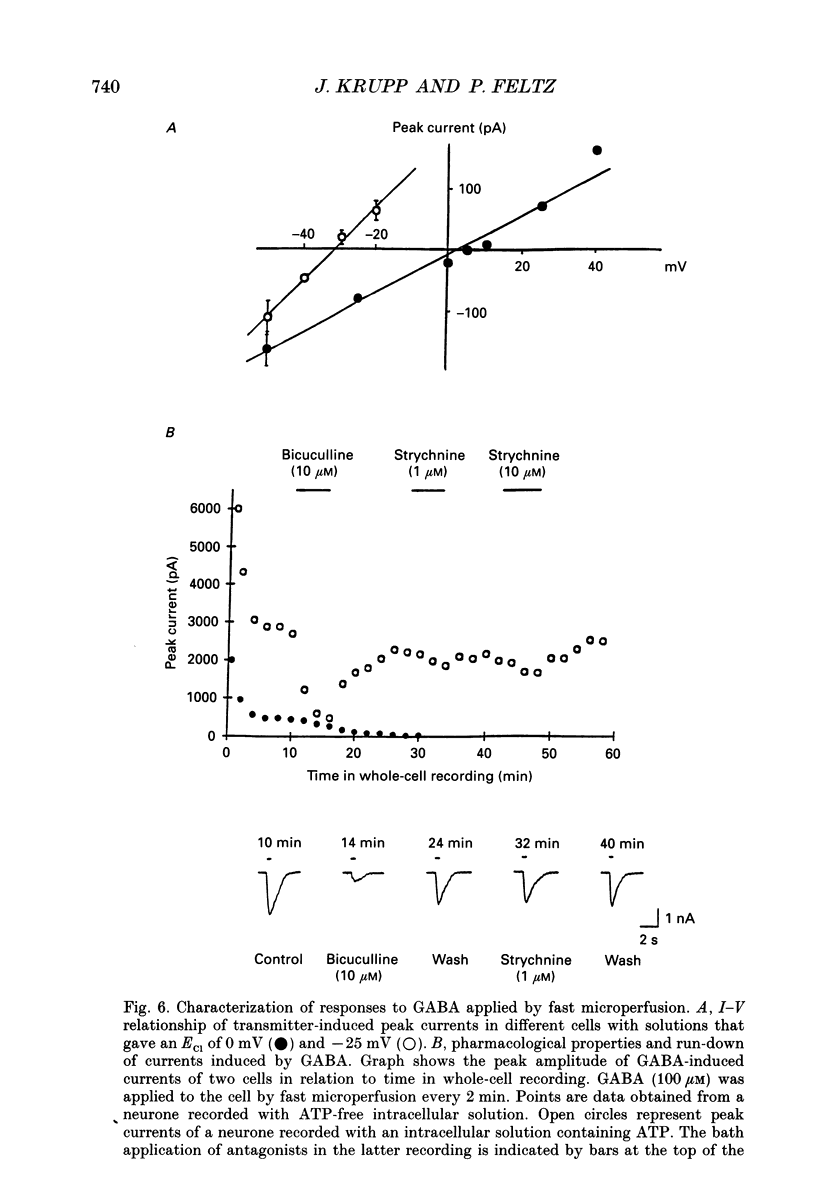
1. By using the whole-cell recording configuration of the patch-clamp technique in a spinal cord slice preparation, we have made recordings from visually identified neurones in the lateral horn of the thoracic and lumbar spinal cord of neonatal rats (newborn to 14 days postnatal). 2. Some of the recorded neurones were labelled with the fluorescent dye Lucifer Yellow (n = 27). Their morphology was typical for sympathetic preganglionic neurones (SPNs). Based on the size of the cell soma and the electrophysiological properties, unlabelled neurones were also regarded as SPNs. 3. Spontaneous synaptic activity of different patterns could be observed in 73% of the recorded neurones (n = 106). It reversed at the chloride equilibrium potential (ECl) and could be reversibly blocked by strychnine (1-10 microM), but not by bicuculline (10 microM) or SR95531 (5-10 microM). 4. Synaptic activity could be elicited by focal electrical stimulation in the vicinity of the recorded neurone. These evoked synaptic events exhibited features similar to the spontaneous synaptic activity. 5. Application of glycine (100 microM-1 mM) by a fast microperfusion system induced a chloride current in twenty-seven out of thirty cells tested. The currents were reversibly blocked by strychnine (1-10 microM), but were only weakly sensitive to bicuculline (10 microM). Stability of current responses to glycine was increased by inclusion of ATP (4 mM) in the intracellular medium. 6. Application of gamma-aminobutyric acid (GABA; 100 microM-1 mM) by the fast microperfusion system induced a chloride current in all twenty neurones tested. These currents were reversibly blocked by bicuculline (10 microM). Strychnine (1-10 microM) blocked this current only weakly. Run-down of GABA-induced currents was prevented to a great extent by inclusion of ATP (4 mM) in the pipette. 7. These results suggest that the inhibitory synaptic activity recorded from SPNs in thin, transverse slices of neonatal rat spinal cord is mediated by glycine receptor-gated Cl- channels. GABAA receptor-gated Cl- channels might be activated by inputs from other spinal segments and/or descending pathways from higher brain regions.
Full text
PDF



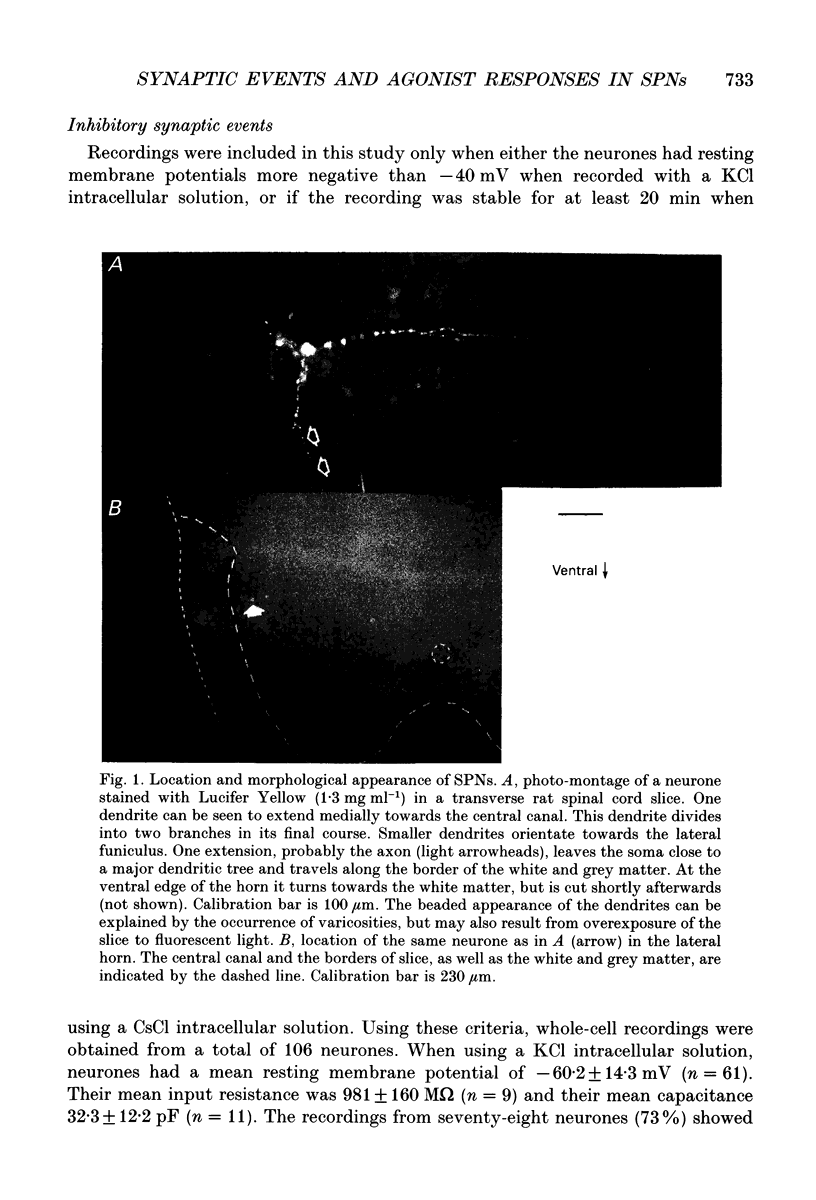
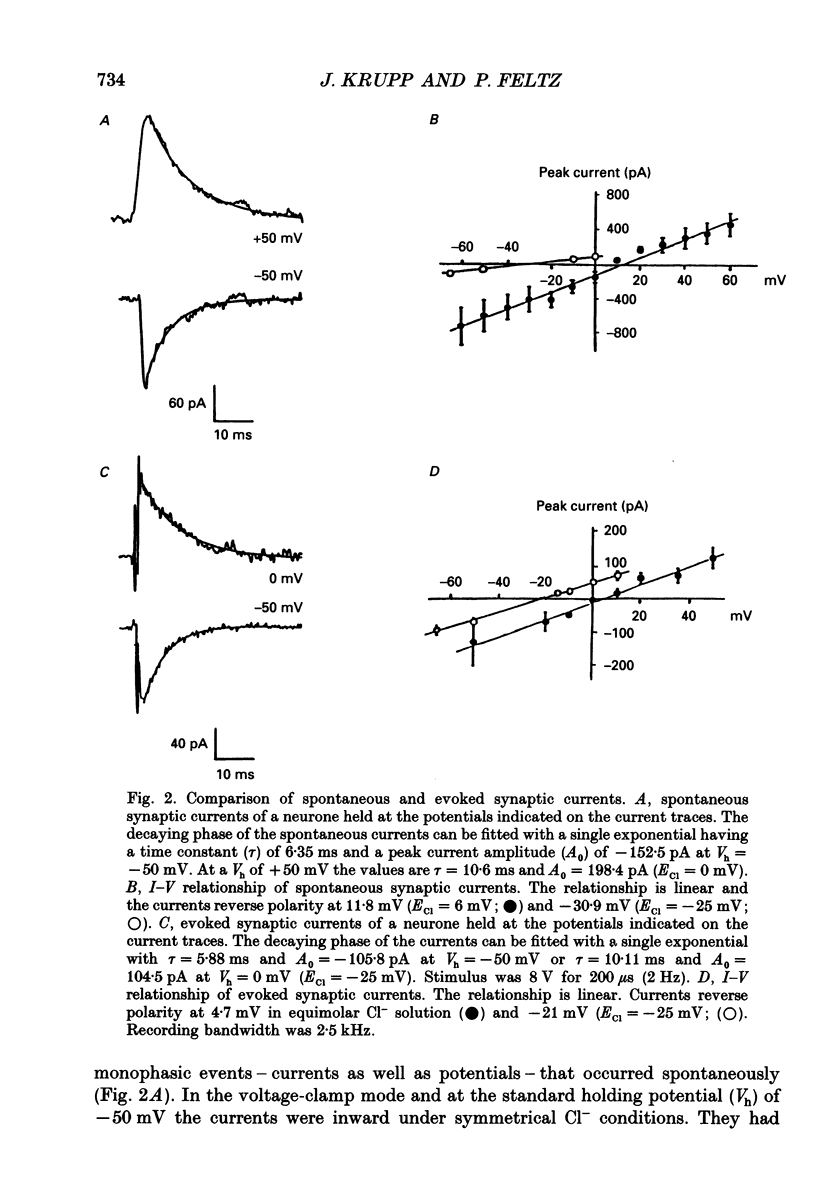
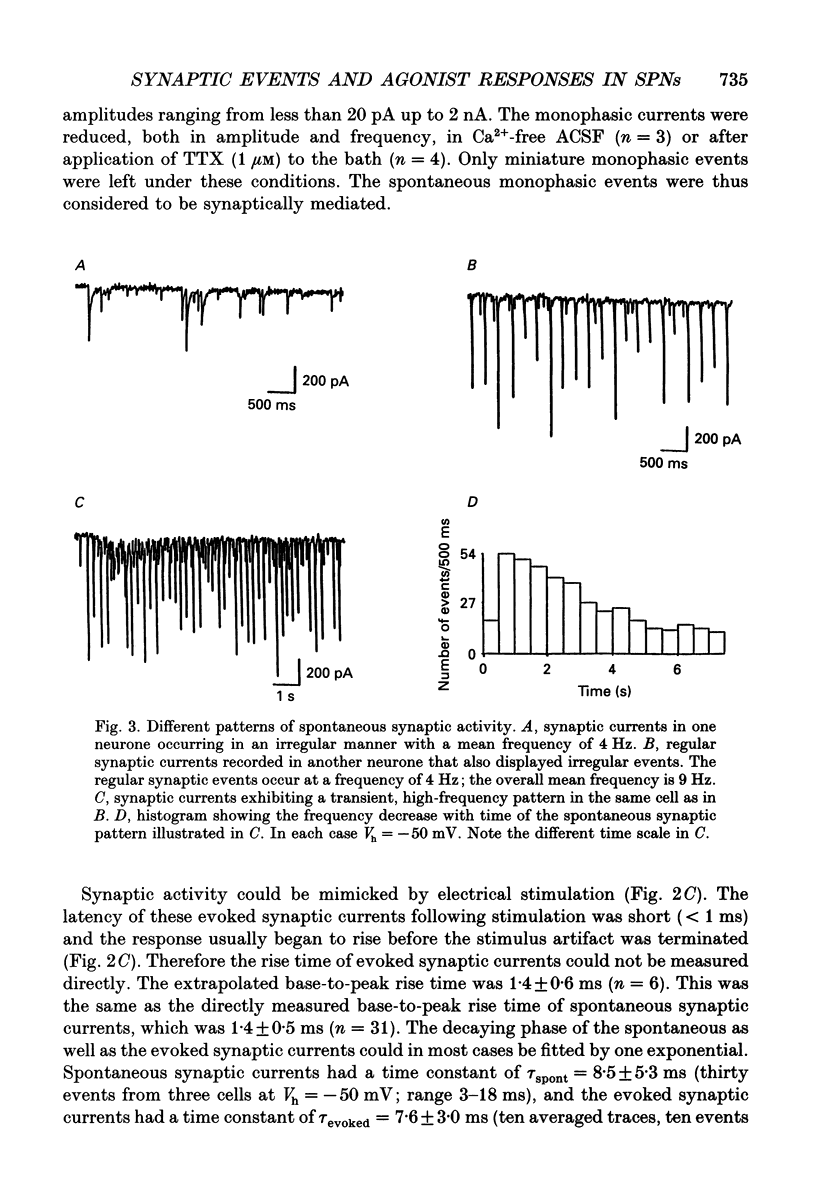
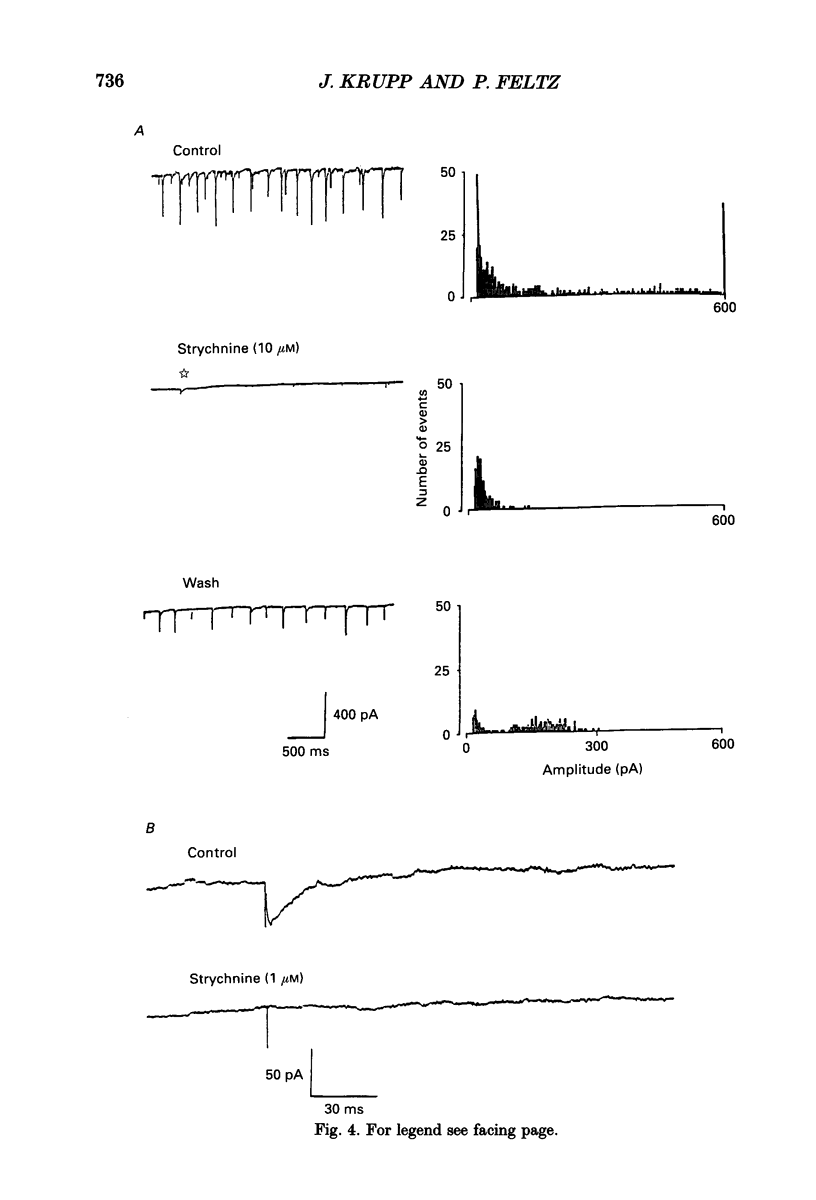










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman S. B., Henry J. L. Effects of GABA and glycine on sympathetic preganglionic neurons in the upper thoracic intermediolateral nucleus of the cat. Brain Res. 1983 Oct 31;277(2):365–369. doi: 10.1016/0006-8993(83)90947-2. [DOI] [PubMed] [Google Scholar]
- Bacon S. J., Smith A. D. Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J Auton Nerv Syst. 1988 Sep;24(1-2):97–122. doi: 10.1016/0165-1838(88)90140-3. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot J. B., Alessi V., Bushnell A. Glycine-like immunoreactive input to sympathetic preganglionic neurons. Brain Res. 1992 Jan 31;571(1):1–18. doi: 10.1016/0006-8993(92)90504-3. [DOI] [PubMed] [Google Scholar]
- Cabot J. B., Bogan N. Light microscopic observations on the morphology of sympathetic preganglionic neurons in the pigeon, Columba livia. Neuroscience. 1987 Feb;20(2):467–486. doi: 10.1016/0306-4522(87)90105-9. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Semba R. Immuno-electronmicroscopic studies on the gamma-aminobutyric acid and glycine receptor in the intermediolateral nucleus of the thoracic spinal cord of rats and guinea pigs. J Auton Nerv Syst. 1991 Dec;36(3):173–181. doi: 10.1016/0165-1838(91)90041-z. [DOI] [PubMed] [Google Scholar]
- Clendening B., Hume R. I. Expression of multiple neurotransmitter receptors by sympathetic preganglionic neurons in vitro. J Neurosci. 1990 Dec;10(12):3977–3991. doi: 10.1523/JNEUROSCI.10-12-03977.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff R. A., Shank R. P., Graham L. T., Jr, Aprison M. H., Werman R. Association of glycine with spinal interneurones. Nature. 1967 May 13;214(5089):680–681. doi: 10.1038/214680a0. [DOI] [PubMed] [Google Scholar]
- Dembowsky K., Czachurski J., Seller H. An intracellular study of the synaptic input to sympathetic preganglionic neurones of the third thoracic segment of the cat. J Auton Nerv Syst. 1985 Jul;13(3):201–244. doi: 10.1016/0165-1838(85)90012-8. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Mo N. Inhibitory postsynaptic potentials in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1989 Mar;410:267–281. doi: 10.1113/jphysiol.1989.sp017532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Forehand C. J. Morphology of sympathetic preganglionic neurons in the neonatal rat spinal cord: an intracellular horseradish peroxidase study. J Comp Neurol. 1990 Aug 15;298(3):334–342. doi: 10.1002/cne.902980306. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Llinás R. Morphological artifacts induced in intracellularly stained neurons by dehydration: circumvention using rapid dimethyl sulfoxide clearing. Neuroscience. 1985 Oct;16(2):461–475. doi: 10.1016/0306-4522(85)90018-1. [DOI] [PubMed] [Google Scholar]
- Hamann M., Desarmenien M., Desaulles E., Bader M. F., Feltz P. Quantitative evaluation of the properties of a pyridazinyl GABA derivative (SR 95531) as a GABAA competitive antagonist. An electrophysiological approach. Brain Res. 1988 Mar 1;442(2):287–296. doi: 10.1016/0006-8993(88)91514-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henry J. L., Calaresu F. R. Pathways from medullary nuclei to spinal cardioacceleratory neurons in the cat. Exp Brain Res. 1974;20(5):505–514. doi: 10.1007/BF00238016. [DOI] [PubMed] [Google Scholar]
- Laskey W., Polosa C. Characteristics of the sympathetic preganglionic neuron and its synaptic input. Prog Neurobiol. 1988;31(1):47–84. doi: 10.1016/0301-0082(88)90022-6. [DOI] [PubMed] [Google Scholar]
- Lebedev V. P., Petrov V. I., Skobelev V. A. Do sympathetic preganglionic neurones have a recurrent inhibitory mechanism? Pflugers Arch. 1980 Jan;383(2):91–97. doi: 10.1007/BF00581868. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., McKellar S., Saper C. B. Direct projections from the A5 catecholamine cell group to the intermediolateral cell column. Brain Res. 1979 Oct 5;174(2):309–314. doi: 10.1016/0006-8993(79)90852-7. [DOI] [PubMed] [Google Scholar]
- Magoul R., Onteniente B., Geffard M., Calas A. Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti-GABA antibodies. Neuroscience. 1987 Mar;20(3):1001–1009. doi: 10.1016/0306-4522(87)90258-2. [DOI] [PubMed] [Google Scholar]
- McCall R. B. Effects of putative neurotransmitters on sympathetic preganglionic neurons. Annu Rev Physiol. 1988;50:553–564. doi: 10.1146/annurev.ph.50.030188.003005. [DOI] [PubMed] [Google Scholar]
- Mo N., Dun N. J. Is glycine an inhibitory transmitter in rat lateral horn cells? Brain Res. 1987 Jan 1;400(1):139–144. doi: 10.1016/0006-8993(87)90661-5. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Pickering A. E., Spanswick D., Logan S. D. Whole-cell recordings from sympathetic preganglionic neurons in rat spinal cord slices. Neurosci Lett. 1991 Sep 16;130(2):237–242. doi: 10.1016/0304-3940(91)90405-i. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. Direct hypothalamo-autonomic connections. Brain Res. 1976 Nov 26;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., López-Barneo J., Konnerth A. Excitatory and inhibitory synaptic currents and receptors in rat medial septal neurones. J Physiol. 1992 Jan;445:261–276. doi: 10.1113/jphysiol.1992.sp018923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E., Dun N. J. Neonate rat sympathetic preganglionic neurons intracellularly labelled with lucifer yellow in thin spinal cord slices. J Auton Nerv Syst. 1990 Mar;29(3):247–254. doi: 10.1016/0165-1838(90)90151-8. [DOI] [PubMed] [Google Scholar]
- Song Y. M., Huang L. Y. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990 Nov 15;348(6298):242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- Spanswick D., Logan S. D. Spontaneous rhythmic activity in the intermediolateral cell nucleus of the neonate rat thoracolumbar spinal cord in vitro. Neuroscience. 1990;39(2):395–403. doi: 10.1016/0306-4522(90)90276-a. [DOI] [PubMed] [Google Scholar]
- Swanson L. W. Immunohistochemical evidence for a neurophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 1977 Jun 10;128(2):346–353. doi: 10.1016/0006-8993(77)91000-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Intracellular recording from visually identified motoneurons in rat spinal cord slices. Proc R Soc Lond B Biol Sci. 1978 Jul 26;202(1148):417–421. doi: 10.1098/rspb.1978.0076. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991 Dec;7(6):965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. J Physiol. 1992 May;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A. J., Sullivan A. C. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990 Jun 15;296(3):496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991 Apr;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Nishi S. Intracellular recordings from lateral horn cells fo the spinal cord in vitro. J Auton Nerv Syst. 1982 Jul;6(1):5–11. doi: 10.1016/0165-1838(82)90017-0. [DOI] [PubMed] [Google Scholar]



