Abstract
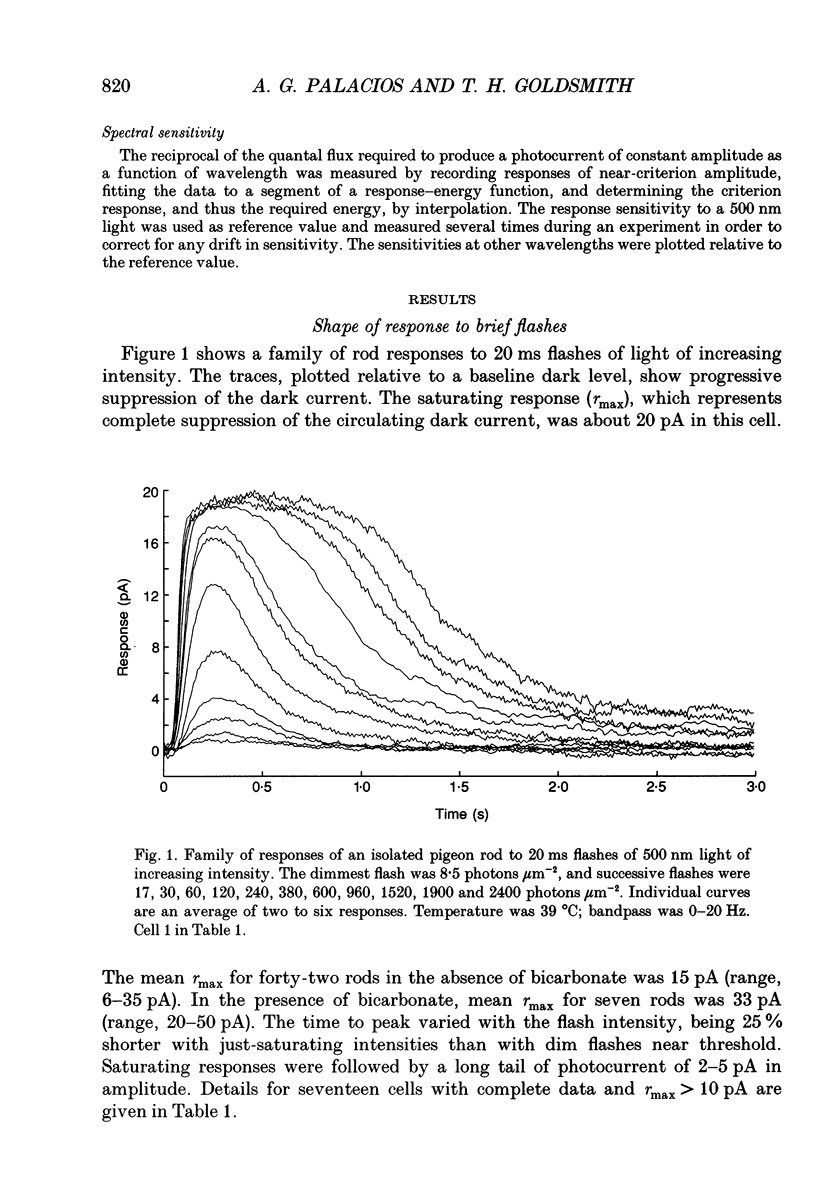
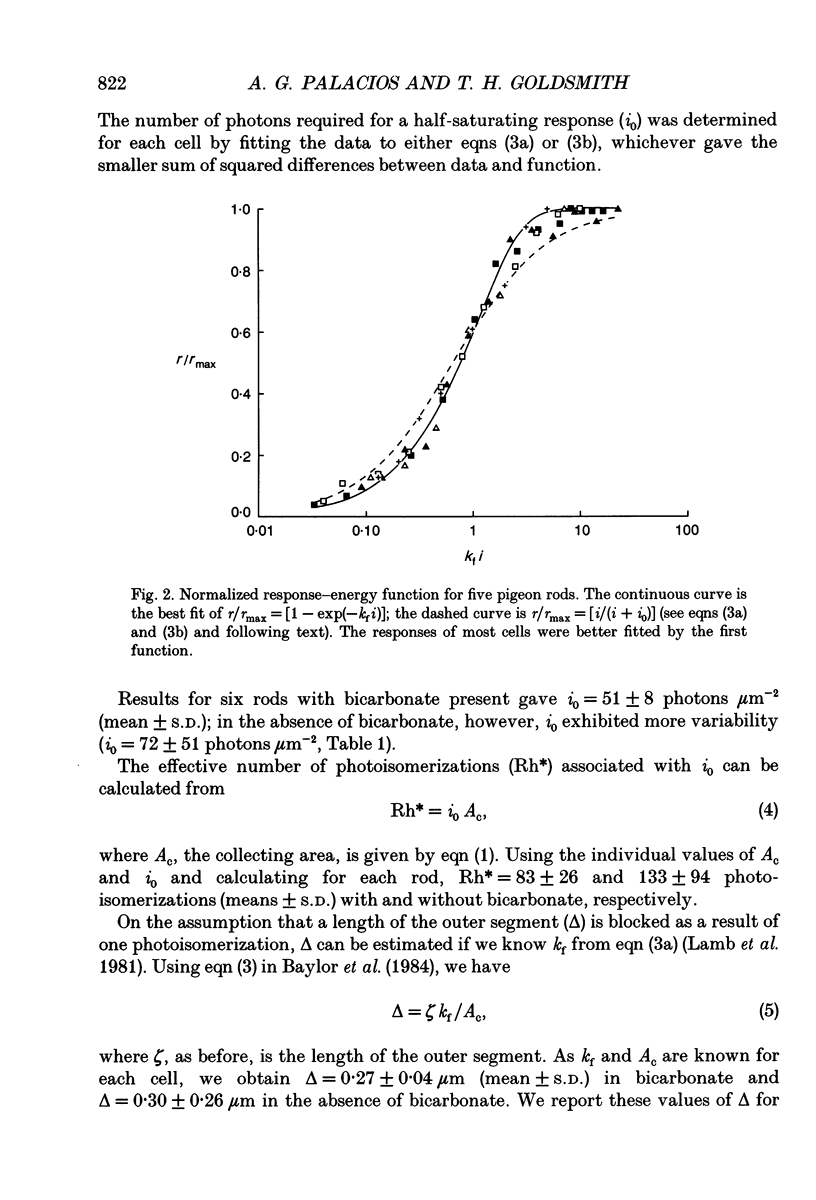
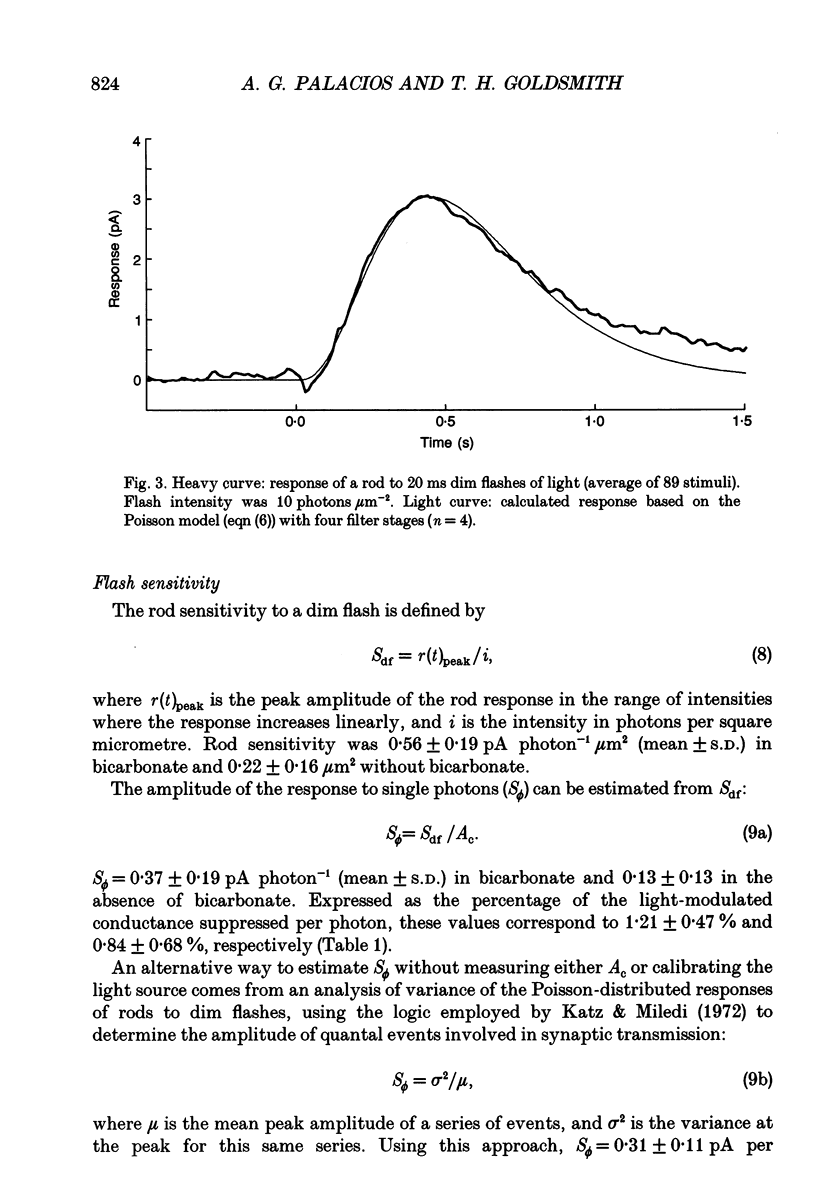
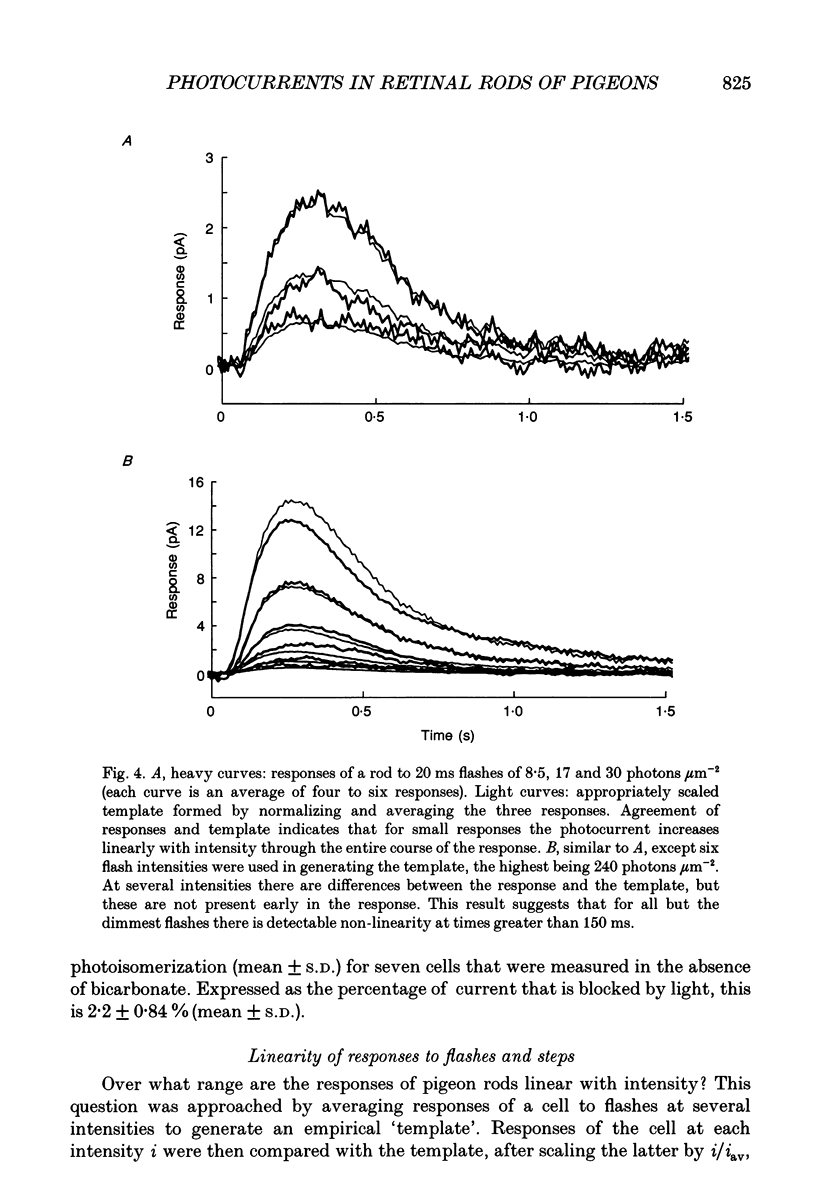
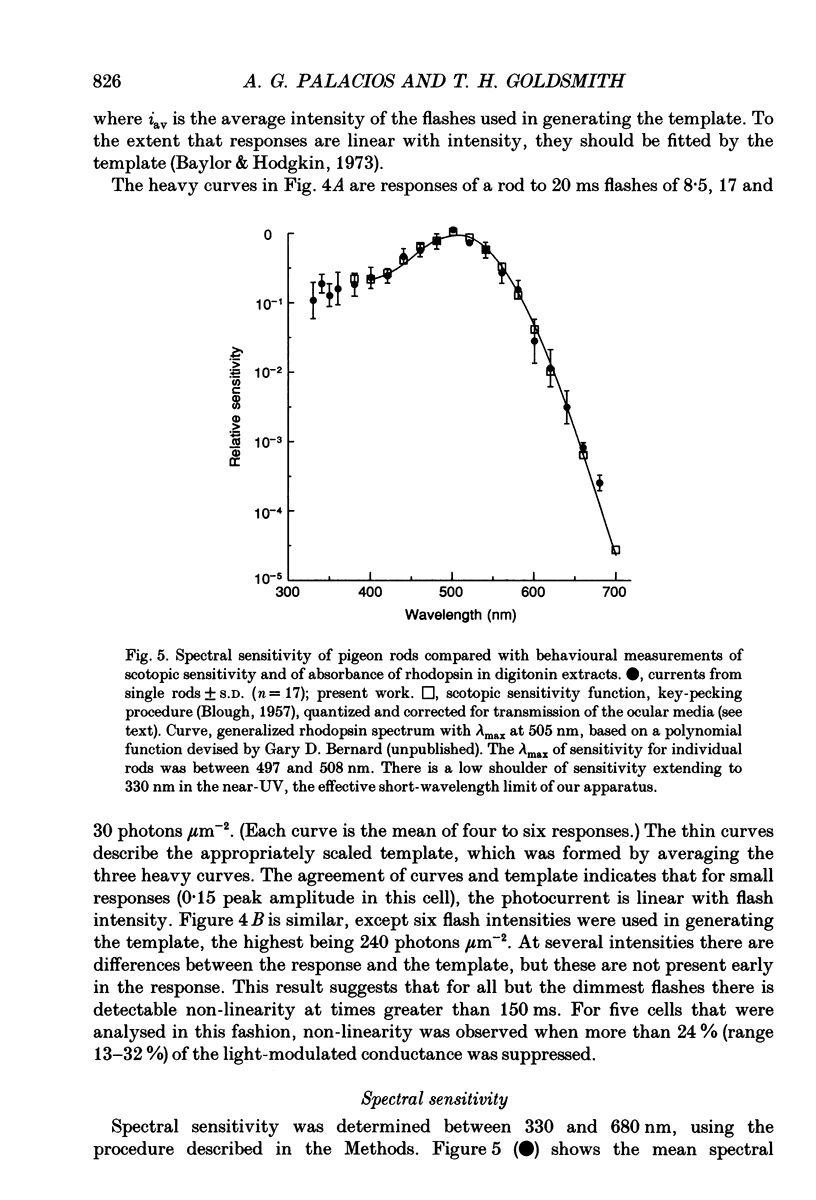
1. Membrane photocurrents were recorded from outer segments of isolated retinal rods of pigeons (Columba livia), the first such measurements on the photoreceptors of a bird. The amplitude of the response to 20 ms flashes of narrow wavelength bands of light increases linearly with intensity at low photon fluxes and saturates at higher intensities. The maximum (saturating) photocurrent observed in forty-nine rod cells was 50 pA. Larger responses with less variability in the intensity for half-maximal responses were observed when the physiological saline contained 20 mM bicarbonate (in addition to Hepes buffer). 2. The dependence of peak amplitude on intensity is well fitted by an exponential function; it is usually less well fitted by the Michaelis-Menten (Naka-Rushton) equation. 3. In the presence of bicarbonate, the average sensitivity of pigeon rods to dim flashes was 0.56 pA photon-1 microns -2. The effective collecting area per photon was 1.8 microns 2. About 83 +/- 26 (mean +/- S.D.) photoisomerizations were required for a half-saturating response. 4. The response kinetics of rods to dim flashes can be reasonably well described by a series of four to five either Poisson or independent filters. The time to peak, measured from the mid-point of a 20 ms flash, was 319 +/- 83 ms (mean +/- S.D.). The integration time of the response was 851 +/- 86 ms (mean +/- S.D.) with bicarbonate present and 572 +/- 126 ms in the absence of bicarbonate. The responses of pigeon rods appear to be slower than those of mammals at the same temperature. The fraction of current suppressed by a single photoisomerization is smaller in pigeon than in mammalian rods by a factor of at least two. 5. The spectral sensitivity function was measured between 680 and 330 nm. The maximum at about 505 nm (range 497-508 nm) corresponds to the alpha-band of a vertebrate rhodopsin and agrees with previous behavioural measurements of scotopic sensitivity of pigeons as well as the absorption spectrum of extracts of pigeon rhodopsin. There was no pronounced beta-band in the near-ultraviolet wavelengths.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOUGH D. S. Spectral sensitivity in the pigeon. J Opt Soc Am. 1957 Sep;47(9):827–833. doi: 10.1364/josa.47.000827. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K. The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vision Res. 1977;17(10):1129–1138. doi: 10.1016/0042-6989(77)90147-x. [DOI] [PubMed] [Google Scholar]
- COHEN A. I. The fine structure of the visual receptors of the pigeon. Exp Eye Res. 1963 Jan;2:88–97. doi: 10.1016/s0014-4835(63)80028-7. [DOI] [PubMed] [Google Scholar]
- Chen D. M., Goldsmith T. H. Four spectral classes of cone in the retinas of birds. J Comp Physiol A. 1986 Oct;159(4):473–479. doi: 10.1007/BF00604167. [DOI] [PubMed] [Google Scholar]
- Emmerton J., Schwemer J., Muth I., Schlecht P. Spectral transmission of the ocular media of the pegion (Columba livia). Invest Ophthalmol Vis Sci. 1980 Nov;19(11):1382–1387. [PubMed] [Google Scholar]
- Goldsmith T. H. Optimization, constraint, and history in the evolution of eyes. Q Rev Biol. 1990 Sep;65(3):281–322. doi: 10.1086/416840. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovic K. N. A new method of non-enzymatic dissociation of the Bufo retina. J Neurosci Methods. 1986 Feb;15(4):301–306. doi: 10.1016/0165-0270(86)90143-3. [DOI] [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Tamura T., Yau K. W. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991 Mar;97(3):413–435. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991 Jul;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Light adaptation in cat retinal rods. Science. 1989 Aug 18;245(4919):755–758. doi: 10.1126/science.2772634. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]


