Abstract
It is uncertain whether the prognostic power of white matter hyperintensity (WMH) on post-stroke outcomes is modulated as a function of initial neurological severity, a critical determinant of outcome after stroke. This multi-center MRI study tested if higher WMH quintiles were associated with 3-month poor functional outcome (modified Rankin Scale ≥ 3) for mild versus moderate-to-severe ischemic stroke. Mild and moderate-to-severe stroke were defined as admission National Institute of Health Stroke Scale scores of 1–4 and ≥ 5, respectively. Mean age of the enrolled patients (n = 8918) was 67.2 ± 12.6 years and 60.1% male. The association between WMH quintiles and poor functional outcome was modified by stroke severity (p-for-interaction = 0.008). In mild stroke (n = 4994), WMH quintiles associated with the 3-month outcome in a dose-dependent manner for the 2nd to 5th quintile versus the 1st quintile, with adjusted-odds-ratios (aOR [95% confidence interval]) being 1.29 [0.96–1.73], 1.37 [1.02–1.82], 1.60 [1.19–2.13], and 1.89 [1.41–2.53], respectively. In moderate-to-severe stroke (n = 3924), however, there seemed to be a threshold effect: only the highest versus the lowest WMH quintile was significantly associated with poor functional outcome (aOR 1.69 [1.29–2.21]). WMH burden aggravates 3-month functional outcome after mild stroke, but has a lesser modulatory effect for moderate-to-severe stroke, likely due to saturation effects.
Subject terms: Neuro-vascular interactions, Neuroscience, Neurology, Neurological disorders, Neurological disorders
Introduction
White matter hyperintensity (WMH), which is a common neuroradiological biomarker of brain health, increases the risks of both stroke occurrence and recurrence1. We previously demonstrated that WMH volume is an independent predictor of 3-month functional outcome and 1-year stroke recurrence and mortality after ischemic stroke2,3. Clearly, both initial stroke severity and WMH burden impact stroke outcomes3,4, but the interaction between these two important prognosticators, is as yet unknown. We hypothesized that a significant interaction effect exists between stroke severity and WMH burden in affecting stroke outcomes.
A few studies showed correlations between WMH burden and outcomes in patients with mild stroke5,6. However, these studies had limitations, such as a small sample size and insufficient adjustment for covariates or unavailable information on long-term functional outcome. The relationship between WMH burden and outcomes in patients with moderate-to-severe stroke is also poorly understood, although a subgroup analysis in a recent study showed that WMH burden was associated with functional outcome in patients with NIHSS (National institutes of Health Stroke Scale) score of 7 or more6. Thus, it is still not clear if the impact of WMH burden on outcomes is consistent regardless of initial stroke severity.
In this nationwide multi-center study using an imaging-based stroke database, we investigated whether the influence of WMH on post-stroke outcomes is modified by the initial neurological severity. In contrast to current literature, this study has: large numbers, good follow-up data on functional outcome, and allows for the adjustment of multiple covariates including the interaction term ‘WMH burden × stroke severity’.
Methods
Study participants
This multi-center study, involving 11 academic and regional stroke centers, utilized the Korean Nationwide Image-based Stroke Database, encompassing data from 14,070 ischemic stroke patients who visited the participating centers within 7 days of symptom onset from May 2011 to February 20142,3,7–10. This project is linked to a prospective multicenter stroke registry: Clinical Research Collaboration for Stroke-Korea (CRCS-K). As for stroke severity, we defined, a priori, (a) mild stroke as magnetic resonance imaging (MRI)-confirmed ischemic stroke with the admission NIHSS scores of 1–4, and (b) moderate-to-severe stroke as the one with the admission NIHSS score of ≥ 5. A total of 8918 patients were finally included after excluding 5152 for the following reasons: (1) no matching data in the clinical (CRCS-K) database (n = 533), (2) admission NIHSS score of 0 (n = 1453), (3) no or inadequate fluid attenuated inversion recovery (FLAIR) images for measuring WMH volume (n = 1171), (4) pre-stroke modified Rankin Scale (mRS) score of 2 or more (n = 1747), and (5) follow-up loss at 3 months after the index stroke (n = 248; Supplementary Figure S1). All patients underwent a standard evaluation, treatment, and rehabilitation, adhering to pre-specified guidelines for ischemic stroke11.
Standard protocol approval, registration, and patient consent
The institutional review boards of all participating centers approved the study (DUIH 2010–01-083–020). All patients or their legally authorized representatives provided a written informed consent for study participation. This study adhered to the Helsinki declaration and study results were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology statement12.
Clinical data and definition
We prospectively collected admission NIHSS score, pre-stroke mRS score, and mRS score at 3 months and 1 year after stroke. We trained and certified physicians and nurses at participating centers for the assessment of NIHSS scores and mRS scores through a web-based education system. We also collected demographic data, prior medication history, laboratory data, and the presence of vascular risk factors, including hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, atrial fibrillation, and smoking history, as per standard protocol2,3,7. Stroke subtypes were determined by the consensus of experienced neurologists in each participating center, using a validated MRI-based algorithm built on the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria13 as follows: large artery atherosclerosis (LAA), small vessel occlusion (SVO), cardioembolism (CE), undetermined, and other-determined etiologies.
MRI registration and analysis
Brain MRI was performed on 1.5 Tesla (n = 7140) or 3.0 Tesla (n = 1778) MRI systems. FLAIR image protocols were as follows: echo time 76–160 ms; repetition time 6000–11,000 ms; voxel size 1 × 1 × 3 ~ 1 × 1 × 7 mm3; interslice gap 0–2.25 mm; field of view 250 mm; and matrix size 256 × 256. Diffusion-weighted imaging (DWI) protocols were as follows: b-values of 0 and 1000 s/mm2; echo time 50–99 ms; repetition time 2400–9000 ms; voxel size 1 × 1 × 3 ~ 1 × 1 × 5 mm3; and interslice gap 0–2 mm. We quantified WMH volume on FLAIR and infarct volume on DWI after transferring the images into a patient-independent quantitative visual format as previously reported7,14. In brief, brain template images (1 × 1 × 1mm3 voxels) were chosen from the Montreal Neurological Institute template within the range of 63.5 to 74.5 mm in the Z-axis of Talairach space. Each patient's lesions with high signal intensity on FLAIR and DWI were semi-automatically segmented and registered onto the brain templates under close supervision by vascular neurologists using a validated in-house program7,8. WMH volume on FLAIR and acute infarct volume on DWI were calculated as a percentage of brain volume by dividing the number of voxels in the lesions over the total number of brain voxels, with corrections applied to account for the differences in scan slice thicknesses by adjusting the denominators7. As previously reported, inter-observers and intra-observer agreement ranged from 0.987 to 0.995 for WMH volume on FLAIR and 0.836 to 0.977 for infarct volume on DWI7.
Statistical analysis
Data are presented as mean ± standard deviation, median (interquartile range), and number (percentage), as appropriate. Quantified WMH volumes for the entire study population were categorized a priori into quintiles, and this WMH burden was used for all relevant statistical analyses. Either Student t test or ANOVA was used for continuous variables with normal distribution. For continuous variables with skewed distribution, we used either Wilcoxon Rank-Sum test or Kruskal–Wallis test. For categorical variables, we used χ2 test. Missing data for infarct volume on DWI (n = 67), fasting glucose (n = 449), hemoglobin (n = 13), and total cholesterol (n = 175) were replaced with the median of the entire population. There were no missing values for the other variables.
To examine the independent association of WMH with poor functional outcome (defined as mRS score of ≥ 3) at 3 months, we used a mixed-effects logistic regression model to account for clustering by hospital. To test if WMH affects outcomes differently in mild vs. moderate-to-severe strokes, an interaction term (WMH burden × stroke severity) was included in the logistic model. An odds ratio (OR) and its 95% confidence interval (CI) for each quintile of WMHs (vs. the lowest quintile) were obtained. Pre-defined covariates potentially associated with the functional outcome3,7,15 were entered into the multivariable model: age, sex, admission NIHSS score, stroke severity, pre-stroke mRS score, previous history of stroke, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, stroke subtype, prior use of statin, prior use of antiplatelet agent(s), revascularization therapy, hemoglobin, fasting blood glucose, total cholesterol, and initial infarct volume on DWI. NIHSS scores for the moderate-to-severe stroke severity range from 5 to 41, requiring further adjustment using the NIHSS score as a continuous variable to reduce residual confounding. Secondary outcomes that were associated with WMH burden in mild vs. moderate-to-severe strokes were (a) poor functional outcome at 1 year and (b) mRS score of ≥ 2 at 3 months and at 1 year.
Subgroup analyses for LAA, SVO, and CE strokes were performed using mixed-effects logistic regression analyses with three-way interactions among WMH burden, stroke severity, and stroke subtype. For sensitivity analyses, we conducted mixed-effects logistic regression analyses after (i) dichotomizing stroke severity into NIHSS scores of either 1–5 vs. ≥ 6 or 0–4 vs. ≥ 5 (where we additionally included 1219 patients with an NIHSS score of 0 who met all the other inclusion/exclusion criteria) or (ii) trichotomizing it into mild, moderate, and severe (NIHSS score of 1–4, 5–15, and ≥ 16, respectively)16. Additionally, we analyzed the association of WMH with poor functional outcome, including an interaction term (WMH burden × stroke severity), in patients admitted within 48 h of symptom onset. Excluding late-arrival strokes accounts for the variation in NIHSS scores over time; that is, admission NIHSS scores measured on days 1–2 and days 3–7 may differ. Moreover, we investigated the association of WMH volume as a continuous variable with poor functional outcome using restricted cubic spline curve models with 4 knots at 20th, 40th, 60th, and 80th percentile of WMH volume. In addition, we assessed the relationship of admission NIHSS score and WMH volume, both as continuous variables, with poor functional outcome using a mixed-effects logistic regression model.
Statistical power was calculated for the sample size of 8918 and an alpha of 0.1. We explored several scenarios involving correlations among WMH, stroke severity, and poor functional outcome at 3 months, with regression coefficients drawn from our previous studies1,2. We found that we had far greater than 80% power to detect interactions between WMH and stroke severity in relation to the poor functional outcome, at various interaction coefficients, including knock-out and attenuated interactions. Data were analyzed using STATA software 17.0 (STATA Corp., College Station, Texas, USA), SAS version 9.4 (SAS Institute Inc., Cary, NC), MATLAB R2021b (Mathworks, Natick, MA), and R version 4.0.5 (R Foundation for Statistical Computing). p < 0.05 and p for interaction < 0.10 were considered statistically significant.
Results
Baseline characteristics
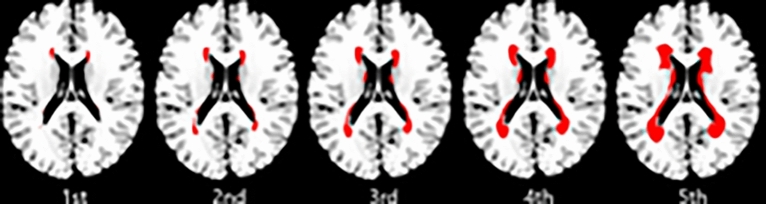
Mean age of 8918 enrolled patients was 67.2 ± 12.6 years, and 60.1% (n = 5362) male. Median WMH volumes for the first to fifth quintile (for representative images, see Fig. 1) were respectively 0.27, 0.48, 0.79, 1.34, and 2.72% of parenchymal brain volume of the Montreal Neurological Institute templates. WMH volumes were not significantly different between the 1.5 T and 3.0 T groups (median 1.18 [interquartile range 0.42–1.55] versus 1.21 [0.43–1.54], respectively; p = 0.89). Compared to patients with the lowest quintile, patients with the highest quintile were more likely to be older, female, had (a) more cerebrovascular risk factors such as hypertension, atrial fibrillation, and previous stroke, (b) a higher admission NIHSS score, (c) more frequent CE strokes, (d) a higher frequency of a history of pre-stroke statin or antiplatelet medication, and e) a less frequent history of smoking (Table 1). Patients with mild stroke comprised 56.0% of all study participants. The median NIHSS score in patients with mild vs. moderate-to-severe stroke was 2 (interquartile range 1–3) and 10 (6–15), respectively. Patients with moderate-to-severe stroke tended to be older, female, had coronary artery disease, atrial fibrillation more, and a history of smoking less frequently, compared with those with mild stroke (Supplementary Table S1). In addition, patients with moderate-to-severe stroke had (a) more frequent CE strokes, (b) less frequent SVO strokes, (c) a higher WMH quintile, (d) a larger infarct volume, and received (e) revascularization therapy more often, when compared to those with mild stroke.
Fig. 1.
Representative images for each quintile of white matter hyperintensity volume. Topographical frequency-volume maps were generated by using the quantitative magnetic resonance data of the 8918 patients of this study, as previously reported3,7. First to fifth quintile of white matter hyperintensity volume correspond to 10, 30, 50, 70, and 90 percentile maps, respectively, which provide a quantitative estimation of the severity of white matter hyperintensity.
Table 1.
Baseline characteristics according to WMH volume quintiles in 8918 patients with acute ischemic stroke.
| WMH volume quintiles | p† | |||||
|---|---|---|---|---|---|---|
| First (n = 1784) | Second (n = 1784) | Third (n = 1784) | Fourth (n = 1783) | Fifth (n = 1783) | ||
| WMH volume, % of brain | 0.27 (0.21–0.32) | 0.48 (0.42–0.55) | 0.79 (0.70–0.89) | 1.34 (1.16–1.55) | 2.72 (2.20–3.62) | < 0.001‡ |
| WMH volume range, % of brain | 0.01–0.37 | 0.37–0.62 | 0.62–1.01 | 1.01–1.82 | 1.82–12.63 | NA |
| Age, y | 56.4 ± 13.0 | 64.4 ± 11.5 | 69.0 ± 10.6 | 72.3 ± 9.4 | 73.9 ± 9.5 | < 0.001 |
| Male | 1195 (67.0) | 1160 (65.0) | 1072 (60.1) | 1026 (57.5) | 909 (51.0) | < 0.001 |
| Admission NIHSS score | 3 (2–8) | 4 (2–8) | 4 (2–8) | 4 (2–9) | 4 (2–10) | < 0.001‡ |
| Pre-stroke mRS score | < 0.001 | |||||
| 0 | 1725 (96.7) | 1683 (94.3) | 1644 (92.2) | 1617 (90.7) | 1523 (85.4) | |
| 1 | 59 (3.3) | 101 (5.7) | 140 (7.9) | 166 (9.3) | 260 (14.6) | |
| Previous stroke | 99 (5.6) | 175 (9.8) | 294 (16.5) | 351 (19.7) | 557 (31.2) | < 0.001 |
| Hypertension | 941 (52.8) | 1102 (61.8) | 1267 (71.0) | 1352 (75.8) | 1387 (77.8) | < 0.001 |
| Diabetes | 493 (27.6) | 598 (33.5) | 634 (35.5) | 621 (34.8) | 588 (33.0) | < 0.001 |
| Hyperlipidemia | 550 (30.8) | 603 (33.8) | 595 (33.4) | 570 (32.0) | 580 (32.5) | 0.35 |
| Smoking | 905 (50.7) | 836 (46.9) | 708 (39.7) | 695 (39.0) | 562 (31.5) | < 0.001 |
| Coronary artery disease | 93 (5.2) | 128 (7.2) | 163 (9.1) | 173 (9.7) | 161 (9.0) | < 0.001 |
| Atrial fibrillation | 286 (15.2) | 357 (18.9) | 412 (21.8) | 392 (20.8) | 441 (23.4) | < 0.001 |
| Stroke subtype | < 0.001 | |||||
| Large artery atherosclerosis | 661 (37.1) | 702 (39.4) | 665 (37.3) | 698 (39.2) | 644 (36.1) | |
| Small vessel occlusion | 313 (17.5) | 318 (17.8) | 327 (18.3) | 319 (17.9) | 319 (17.9) | |
| Cardioembolism | 355 (19.9) | 359 (20.1) | 412 (23.1) | 396 (22.2) | 430 (24.1) | |
| Undetermined | 370 (20.7) | 374 (20.9) | 353 (19.8) | 354 (19.9) | 369 (20.7) | |
| Other determined | 85 (4.8) | 31 (1.7) | 27 (1.5) | 16 (0.9) | 21 (1.2) | |
| Previous use of statin | 217 (12.2) | 228 (12.8) | 289 (16.2) | 282 (15.8) | 327 (18.3) | < 0.001 |
| Previous use of antiplatelet | 349 (19.6) | 374 (21.0) | 500 (28.0) | 520 (29.2) | 621 (34.8) | < 0.001 |
| Recanalization therapy | 407 (22.8) | 384 (21.5) | 327 (18.3) | 313 (17.6) | 293 (16.4) | < 0.001 |
| Infarct volume, % of brain | 0.19 (0.04–0.93) | 0.16 (0.04–0.89) | 0.13 (0.03–0.81) | 0.16 (0.04–0.77) | 0.16 (0.04–0.85) | 0.21‡ |
| Fasting glucose, mg/dL | 118.1 ± 49.6 | 123.7 ± 50.0 | 124.8 ± 54.4 | 121.4 ± 49.2 | 119.6 ± 46.8 | < 0.001 |
| Hemoglobin, g/dL | 14.2 ± 1.9 | 14.0 ± 1.9 | 13.6 ± 1.9 | 13.4 ± 1.9 | 13.4 ± 1.9 | < 0.001 |
| Total cholesterol, mg/dL | 181.9 ± 42.7 | 180.7 ± 42.9 | 177.5 ± 42.8 | 176.4 ± 42.3 | 173.4 ± 40.7 | < 0.001 |
Data are mean ± standard deviation, number (percentage), or median (interquartile range). Some data were missing for infarct volume on diffusion-weighted MRI (n = 67), fasting glucose (n = 449), hemoglobin (n = 13), and total cholesterol (n = 175) and replaced with the median of entire population.
† p-values by ANOVA or χ2 test, unless otherwise indicated.
‡ Kruskal–Wallis test.
MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; WMH, white matter hyperintensity.
Impact of WMH burden on 3-month and 1-year functional outcome after stratification with the initial stroke severity
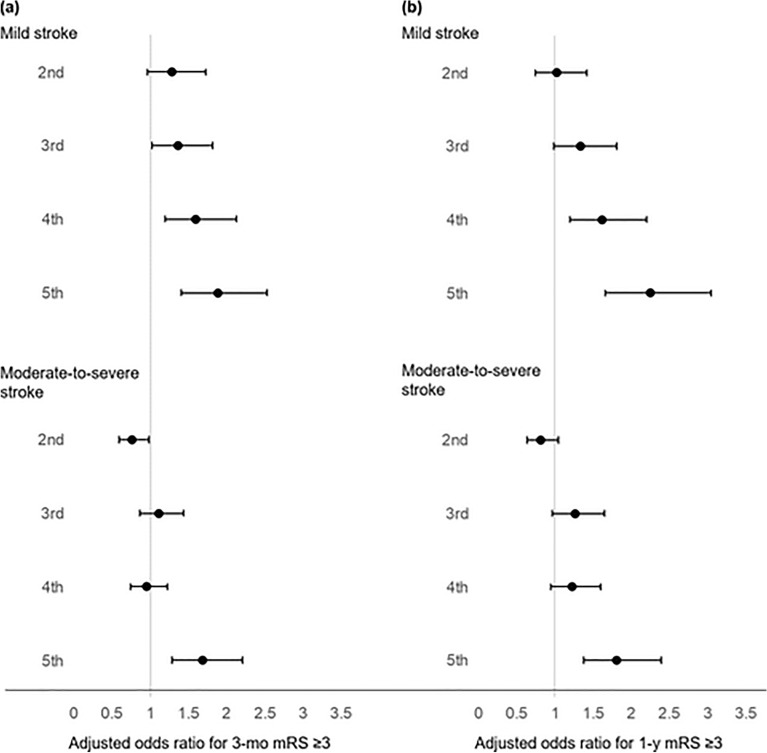
At 3 months after the index ischemic stroke (Table 2), 848 (17.0%) of 4994 patients with mild stroke and 2413 (61.5%) of 3924 patients with moderate-to-severe stroke had poor functional outcome. In the mild stroke group, univariate analysis to examine the relationship between WMH burden and poor functional outcome showed that ORs for the second to fifth WMH quintiles (vs. the first WMH quintile) were 1.75 (95% CI 1.32–2.33), 2.30 (1.75–3.03), 3.05 (2.32–4.00), and 3.79 (2.90–4.97), respectively (all p < 0.001). The dose-dependent relationship remained significant after adjusting for pre-defined covariates: adjusted OR 1.29 (95% CI 0.96–173, p = 0.09), 1.37 (1.02–1.82, p = 0.04), 1.60 (1.19–2.13, p = 0.001), and 1.89 (1.41–2.53, p < 0.001), respectively (Fig. 2a, Table 2, and Supplementary Table S2). A significant, albeit relatively weak, dose-dependent relationship was also observed in the subgroup of patients admitted within 48 h of symptom onset (Supplementary Table S3).
Table 2.
Associations of WMH volume quintiles with 3-month mRS score of ≥ 3 after acute ischemic stroke stratified by stroke severity†
| WMH volume quintiles | |||||
|---|---|---|---|---|---|
| First | Second | Third | Forth | Fifth | |
| Mild Stroke (n = 4994) | |||||
| 3-mo mRS ≥ 3, n (%) | 86 (8.2) | 141 (13.7) | 186 (17.7) | 207 (21.8) | 228 (25.1) |
| Crude OR (95% CI) | Reference | 1.75 (1.32–2.33) | 2.30 (1.75–3.03) | 3.05 (2.32–4.00) | 3.79 (2.90–4.97) |
| p value | Reference | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 1.29 (0.96–1.73) | 1.37 (1.02–1.82) | 1.60 (1.19–2.13) | 1.89 (1.41–2.53) |
| p value | Reference | 0.09 | 0.04 | 0.001 | < 0.001 |
| Moderate-to-Severe Stroke (n = 3924) | |||||
| 3-mo mRS ≥ 3, n (%) | 375 (51.4) | 404 (53.4) | 457 (62.5) | 518 (62.2) | 659 (75.4) |
| Crude OR (95% CI) | Reference | 1.05 (0.86–1.30) | 1.56 (1.26–1.93) | 1.59 (1.29–1.95) | 2.89 (2.33–3.59) |
| p value | Reference | 0.61 | < 0.001 | < 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 0.76 (0.59–0.98) | 1.11 (0.86–1.44) | 0.95 (0.74–1.23) | 1.69 (1.29–2.21) |
| p value | Reference | 0.03 | 0.43 | 0.70 | < 0.001 |
† p for interaction effect between WMH volume quintiles and stroke severity were 0.002 (crude) and 0.008 (adjusted), respectively.
‡ Data were adjusted for age, sex, admission NIHSS score, stroke severity (NIHSS score of 1–4 for mild and ≥ 5 for moderate-to-severe stroke), pre-stroke mRS score, previous history of stroke, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, stroke subtype, prior use of statin, prior use of antiplatelet agent(s), revascularization therapy, hemoglobin, fasting blood glucose, total cholesterol, and infarct volume (on diffusion-weighted MRI). The adjusted OR for admission NIHSS score was 1.19 (95% CI 1.17–1.22, p < 0.001).
CI, confidence interval; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; WMH, white matter hyperintensity.
Fig. 2.
Associations of WMH burden with 3-month and 1-year mRS score (≥ 3) in mild versus moderate-to-severe ischemic stroke. (a-b) Adjusted odds ratios (with 95% confidence intervals) for the 2nd to 5th WMH quintiles relative to the 1st WMH quintiles are presented in patients with mild vs. moderate-to-severe stroke at 3 months (a) and 1 year (b). Mixed-effects logistic regression analyses were performed with adjustment for age, sex, admission NIHSS score, stroke severity (NIHSS score of 1–4 for mild and ≥ 5 for moderate-to-severe stroke), pre-stroke mRS score, previous history of stroke, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, stroke subtype, prior use of statin, prior use of antiplatelet agent(s), revascularization therapy, hemoglobin, fasting blood glucose, total cholesterol, and infarct volume (on diffusion-weighted MRI) with an interaction term WMH volume quintiles × stroke severity. MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; WMH, white matter hyperintensity.
In the moderate-to-severe stroke group, univariate analysis showed ORs of 1.05 (95% CI 0.86–1.30, p = 0.61), 1.56 (1.26–1.93, p < 0.001), 1.59 (1.29–1.95, p < 0.001) and 2.89 (2.33–3.59, p < 0.001) for respectively the 2nd to 5th quintiles (vs. the first quintile), with only the upper three WMH quintiles being significantly associated with poor functional outcome. However, after adjusting for covariates, only the second and fifth WMH quintiles showed statistically significant relationships with poor functional outcome: adjusted ORs for the 2nd to 5th quintiles were 0.76 (0.59–0.98, p = 0.03), 1.11 (0.86–1.44, p = 0.43), 0.95 (0.74–1.23, p = 0.70), and 1.69 (1.29–2.21, p < 0.001), respectively, without showing a clear dose-dependent relationship (Fig. 2a, Table 2, and Supplementary Table S2). Similar findings were observed in the subgroup of patients admitted within 48 h of symptom onset (Supplementary Table S3). Thus, the modulatory effect of WMH burden on 3-month outcome differed for initial mild vs. moderate-to-severe stroke severity (p for interaction = 0.008), with a much stronger and more consistent effect for mild stroke.
mRS scores at 1 year were available in 8600 of the 8918 patients. 812 (16.7%) of 4875 patients with mild stroke and 2121 (56.9%) of 3725 patients with moderate-to-severe stroke had poor functional outcome (Table 3). Univariate analyses showed similarly increasing trends of poor functional outcome with higher WMH quintiles in both groups. After adjusting for covariates, a dose-responsive trend was again observed in mild stroke: adjusted ORs were 1.03 (0.74–1.42, p = 0.03), 1.34 (0.99–1.81, p = 0.06), 1.62 (1.20–2.20, p = 0.002), and 2.25 (1.66–3.04, p < 0.001), respectively for the second to fifth WMH quintiles (Fig. 2b and Table 3).
Table 3.
Associations of WMH volume quintiles with 1-year mRS score of ≥ 3 after acute ischemic stroke stratified by stroke severity†
| WMH volume quintiles | |||||
|---|---|---|---|---|---|
| First | Second | Third | Forth | Fifth | |
| Mild Stroke (n = 4875) | |||||
| 1-y mRS ≥ 3, n (%) | 74 (7.2) | 109 (10.8) | 174 (17.0) | 203 (22.1) | 252 (28.1) |
| Crude OR (95% CI) | Reference | 1.52 (1.11–2.08) | 2.53 (1.90–3.38) | 3.57 (2.68–4.75) | 5.24 (3.96–6.94) |
| p value | Reference | 0.008 | < 0.001 | < 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 1.03 (0.74–1.42) | 1.34 (0.99–1.81) | 1.62 (1.20–2.20) | 2.25 (1.66–3.04) |
| p value | Reference | 0.87 | 0.06 | 0.002 | < 0.001 |
| Moderate-to-Severe Stroke (n = 3725) | |||||
| 1-y mRS ≥ 3, n (%) | 301 (42.8) | 339 (47.4) | 411 (58.9) | 477 (60.8) | 593 (72.0) |
| Crude OR (95% CI) | Reference | 1.18 (0.95–1.46) | 1.91 (1.54–2.37) | 2.12 (1.72–2.62) | 3.47 (2.79–4.30) |
| p value | Reference | 0.14 | < 0.001 | < 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 0.81 (0.63–1.05) | 1.27 (0.97–1.65) | 1.23 (0.95–1.60) | 1.81 (1.38–2.39) |
| p value | Reference | 0.12 | 0.08 | 0.12 | < 0.001 |
† p for interaction effect between WMH volume quintiles and stroke severity were 0.053 (crude) and 0.53 (adjusted), respectively.
‡ Data were adjusted for age, sex, admission NIHSS score, stroke severity (NIHSS score of 1–4 for mild and ≥ 5 for moderate-to-severe stroke), pre-stroke mRS score, previous history of stroke, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, stroke subtype, prior use of statin, prior use of antiplatelet agent(s), revascularization therapy, hemoglobin, fasting blood glucose, total cholesterol, and infarct volume (on diffusion-weighted MRI). The adjusted OR for admission NIHSS score was 1.18 (95% CI 1.15–1.20, p < 0.001).
CI, confidence interval; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; WMH, white matter hyperintensity.
In the moderate-to-severe stroke group, however, only the fifth quantile was associated with poor functional outcome: adjusted OR for the second to fifth quintiles were 0.81 (0.63–1.05, p = 0.12), 1.27 (0.97–1.65 p = 0.08), 1.23 (0.95–1.60, p = 0.12), and 1.81 (1.38–2.39, p < 0.001), respectively (Fig. 2b and Table 3). Although there seems to be a threshold effect for moderate-to-severe stroke (as opposed to the dose-responsive effect seen in mild stroke), WMH burden-related effects on the 1-year functional outcome did not differ significantly depending on the stroke severity (p for interaction = 0.53).
When we dichotomized 3-month mRS score 0 to 1 vs. 2 or higher, 1882 (37.7%) of 4994 patients with mild stroke and 3075 (78.4%) of 3924 patients with moderate-to-severe stroke showed mRS score ≥ 2. Stroke severity-dependent relationships between WMH burden and the 3-month outcome were similar to those for the mRS cut-off of ≥ 3. Moreover, WMH seemed to show clearer dose-responsive effects on the functional outcome in mild ischemic stroke than in moderate-to-severe ischemic stroke (showing threshold effects), albeit no statistically significant difference (p for interaction = 0.43; Supplementary Table S4). Furthermore, a similar relationship was observed for WMH and 1-year mRS score of ≥ 2 in patients with mild and moderate-to-severe stroke (p for interaction = 0.07; Supplementary Table S5).
Association between WMH burden and 3-month functional outcome after stratification with the initial stroke severity and stroke subtype
In mild LAA stroke, the 3rd to 5th WMH quintiles (vs. the 1st quintile) were associated with poor functional outcomes at 3 months (p = 0.006, 0.002, and 0.001, respectively). However, no significant WMH-outcome associations were observed in moderate-to-severe LAA stroke (all p > 0.05; Table 4).
Table 4.
Associations of WMH volume quintiles with 3-month mRS score of ≥ 3 stratified by stroke subtype and severity†
| WMH volume quintiles | |||||
|---|---|---|---|---|---|
| First | Second | Third | Forth | Fifth | |
| Mild Stroke (n = 4994) | |||||
| Large artery atherosclerosis (n = 1980) | |||||
| Crude OR (95% CI) | Reference | 2.07 (1.34–3.19) | 3.13 (2.06–4.75) | 3.77 (2.49–5.71) | 4.13 (2.71–6.30) |
| p value | Reference | 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 1.55 (0.99–2.43) | 1.84 (1.19–2.84) | 1.96 (1.27–3.02) | 2.08 (1.34–3.24) |
| p value | Reference | 0.054 | 0.006 | 0.002 | 0.001 |
| Small vessel occlusion (n = 1299) | |||||
| Crude OR (95% CI) | Reference | 2.14 (1.05–4.37) | 1.83 (0.89–3.77) | 3.67 (1.85–7.28) | 5.26 (2.72–10.18) |
| p value | Reference | 0.04 | 0.10 | < 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 1.62 (0.78–3.35) | 1.18 (0.57–2.46) | 2.03 (1.01–4.09) | 2.50 (1.27–4.91) |
| p value | Reference | 0.19 | 0.66 | 0.048 | 0.008 |
| Cardioembolism (n = 660) | |||||
| Crude OR (95% CI) | Reference | 1.55 (0.68–3.54) | 1.82 (0.84–3.94) | 2.02 (0.92–4.44) | 5.22 (2.50–10.91) |
| p value | Reference | 0.30 | 0.13 | 0.08 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 1.11 (0.48–2.59) | 1.04 (0.47–2.30) | 1.03 (0.46–2.31) | 2.69 (1.26–5.77) |
| p value | Reference | 0.81 | 0.93 | 0.95 | 0.01 |
| Moderate-to-Severe Stroke (n = 3924) | |||||
| Large artery atherosclerosis (n = 1390) | |||||
| Crude OR (95% CI) | Reference | 1.04 (0.74–1.47) | 1.60 (1.12–2.30) | 1.74 (1.23–2.45) | 2.50 (1.75–3.57) |
| p value | Reference | 0.81 | 0.009 | 0.001 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 0.71 (0.48–1.06) | 1.13 (0.75–1.70) | 0.97 (0.65–1.44) | 1.43 (0.95–2.16) |
| p value | Reference | 0.09 | 0.56 | 0.87 | 0.09 |
| Small vessel occlusion (n = 297) | |||||
| Crude OR (95% CI) | Reference | 0.93 (0.38–2.29) | 2.33 (0.98–5.56) | 3.27 (1.50–7.11) | 4.27 (1.89–9.63) |
| p value | Reference | 0.87 | 0.06 | 0.002 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 0.73 (0.28–1.85) | 1.78 (0.71–4.43) | 1.76 (0.78–3.99) | 2.07 (0.88–4.87) |
| p value | Reference | 0.50 | 0.22 | 0.17 | 0.09 |
| Cardioembolism (n = 1292) | |||||
| Crude OR (95% CI) | Reference | 1.15 (0.78–1.69) | 1.57 (1.08–2.30) | 1.42 (0.98–2.07) | 2.56 (1.74–3.74) |
| p value | Reference | 0.48 | 0.02 | 0.06 | < 0.001 |
| Adjusted OR (95% CI)‡ | Reference | 0.83 (0.52–1.33) | 1.17 (0.73–1.87) | 0.90 (0.57–1.43) | 1.43 (0.89–2.30) |
| p value | Reference | 0.45 | 0.51 | 0.66 | 0.13 |
† p for interaction effect among WMH volume quintiles, stroke subtype, and stroke severity were 0.20 (crude) and 0.10 (adjusted), respectively.
‡ Data were adjusted for age, sex, admission NIHSS score, stroke severity (NIHSS score of 1–4 for mild and ≥ 5 for moderate-to-severe stroke), pre-stroke mRS score, previous history of stroke, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, stroke subtype, prior use of statin, prior use of antiplatelet agent(s), revascularization therapy, hemoglobin, fasting blood glucose, total cholesterol, and infarct volume (on diffusion-weighted MRI). The adjusted OR for admission NIHSS score was 1.20 (95% CI 1.18–1.22, p < 0.001).
CI, confidence interval; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; WMH, white matter hyperintensity.
In mild SVO stroke, the 4th and 5th WMH quintiles (vs. the 1st quintile) were associated with poor functional outcome at 3 months (p = 0.048 and 0.008, respectively). However, no significant WMH-outcome associations were observed in moderate-to-severe SVO stroke (all p > 0.05; Table 4).
In mild CE stroke, only the 5th WMH quintile showed an association with the functional outcome when compared to the 1st quintile (p = 0.01). In moderate-to-severe CE stroke, however, no significant WMH-outcome associations were observed (all p > 0.05; Table 4). There were marginally significant 3-way interactions among the WMH quintiles, stroke subtypes, and stroke severity (p = 0.10).
Sensitivity analysis using different definitions of stroke severity
When mild vs. moderate-to-severe stroke was defined as admission NIHSS score of 1–5 versus 6 or higher, a dose-dependent relationship was again observed between WMH burden and poor functional outcome at 3 months in mild stroke. Moreover, a threshold effect was again observed for WMH to worsen outcome in moderate-to-severe stroke (p for interaction = 0.01; Supplementary Table S6). When mild vs. moderate-to-severe stroke was defined as admission NIHSS score of 0–4 vs. 5 or higher, similar results were observed (Supplementary Table S7). Furthermore, when we trichotomized stroke severity into mild, moderate, and severe (admission NIHSS score of 1–4, 5–15, and ≥ 16, respectively), different impacts of WMH burden on poor functional outcome after strokes of different severity were retained: a dose-dependent relationship and a threshold effect respectively in mild and moderate stroke vs. no independent relationship with the outcome in severe stroke (p for interaction = 0.04; Supplementary Table S8).
Sensitivity analysis using WMH volume as WMH burden
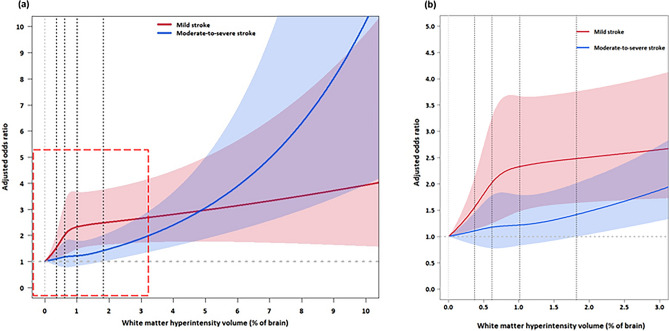
A restricted cubic spline curve model was used to demonstrate how WMH volume affects 3-month functional outcome depending on the initial stroke severity (Fig. 3). In mild stroke, the odds for poor functional outcome increased steeply as WMH volume increased up to approximately 1% of parenchymal brain volume, which corresponded to the WMH burden of approximately upper margin of the 3rd quintile, which corresponds to about 60% of our cohort patients. It increased further, although less steeply, as WMH volume increased higher than 1%. In moderate-to-severe stroke, the increase in WMH volume up to about 1.8% (that corresponds to the upper margin of the 4th quintile WMH burden) was not associated with a significant increase in the odds for poor functional outcome, again showing a threshold effect for WMH to affect functional outcome after moderate-to-severe stroke (p for interaction = 0.01). It should be noted that high WMH volume (e.g., over 3%) represents small subsets of patients and the CIs of ORs are wide. Thus, cautions should be made for the interpretation of the spline curves, particularly for the high WMH volume-related portions.
Fig. 3.
Adjusted odds ratios for 3-month mRS score (≥ 3) in mild versus moderate-to-severe ischemic stroke at different WMH volumes. (a-b) Restricted cubic spline curves (a), with an enlarged view of the graph’s red dashed square section (b), depict associations between WMH volume and poor functional outcome at 3 months after mild vs. moderate-to-severe ischemic stroke. The solid red and blue lines show the adjusted odds ratios, and the shaded regions represent the 95% confidence intervals. Vertical dotted lines represent 20th, 40th, 60th, and 80th percentiles of WMH volumes in this study cohort. Logistic regression analysis was performed with adjustment for age, sex, admission NIHSS score, stroke severity (NIHSS score of 1–4 for mild and ≥ 5 for moderate-to-severe stroke), pre-stroke mRS score, previous history of stroke, hypertension, diabetes, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease, stroke subtype, prior use of statin, prior use of antiplatelet agent(s), revascularization therapy, hemoglobin, fasting blood glucose, total cholesterol, and infarct volume (on diffusion-weighted MRI) with an interaction term WMH volume × stroke severity. The adjusted OR for admission NIHSS score was 1.18 (95% CI 1.16–1.20, p < 0.001). CI, confidence interval; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; WMH, white matter hyperintensity.
Sensitivity analysis using admission NIHSS score as stroke severity and WMH volume as WMH burden
Supplementary Figure S2 depicts how the probability of poor functional outcome at 3 months change as admission NIHSS score and WMH volume increase. With increasing admission NIHSS score, there was a greater risk of poor functional outcome and a lesser modulatory effect of WMH volume on the outcome (p for interaction between WMH volume and admission NIHSS score = 0.01).
Discussion
Our multicenter quantitative MRI study of 8918 consecutive patients with acute ischemic stroke demonstrated that the impact of WMH burden on stroke outcomes differed depending on initial stroke severity. In mild stroke, as WMH volume increased from the 2nd quintile to 5th quintile, the odds of poor functional outcome increased monotonically from about 1.3 to 1.9, when compared to the 1st quintile, demonstrating a progressively increasing harmful impact. In moderate-to-severe stroke, only the 5th WMH quintile (vs. the 1st quintile; OR of ~ 1.7) was associated with poor functional outcome, a much weaker relationship that could be interpreted as a threshold effect at very high WMH burden. The stroke severity-related interaction in the relationship between WMH volume and post-stroke functional outcome was retained in pre-planned supplementary and sensitivity analyses, which supports the robustness of our results.
Previous research with relatively limited sample sizes reported inconsistent findings regarding the impact of WMH burden on functional outcomes following mild ischemic stroke5,6,17,18. Some studies showed relationships between WMH burden and functional outcome5,6, while others did not18 or did so only in the presence of intracranial occlusion/stenosis17. In the current investigation, large clinical and imaging datasets of consecutive patients with acute ischemic stroke allowed for thorough adjustment for relevant confounders, and biases may have been better prevented. Subgroup analyses made possible by the large sample size suggested that the association between WMH burden and poor functional outcome in mild stroke held particularly true for LAA stroke. Compared with CE strokes, LAA strokes have better functional outcomes due to more extensive collateral flow19–22. A longer-lasting development of LAA (vs. CE) allows for more gradual development of collateral flow, which may well be inhibited by gradually developing WMH that is accompanied by a decrease in cerebrovascular density. Thus, WMH-mediated reduction in collateral blood flow, which could facilitate infarct growth2,22, may have a higher impact in LAA than in CE.
In moderate-to-severe stroke, both crude and covariate-adjusted analyses showed that only the highest (vs. lowest) WMH quintile was consistently and significantly associated with poor functional outcome. There seems to be a small window of opportunity for non-advanced WMH (2nd to 4th quintile) to contribute to a worse functional outcome when the initial neurological manifestation is severe. However, advanced WMH (5th quintile)-related white matter disconnection could reduce ischemic resilience23 and brain plasticity24,25, worsening the already severe neurological impairment seen in moderate-to-severe stroke. The covariate-adjusted risk reduction in the 2nd quintile (vs. the 1st quintile) was unexpected and relatively small, suggesting it might be an incidental finding that requires future confirmation. We previously suggested that the effects of WMH on mortality after ischemic stroke may be mediated primarily by the consequences of WMH such as poststroke depression20, frequent falls22, and aspiration pneumonia21, all of which would have a greater impact on functional outcomes in moderate-to-severe stroke.
More than half of patients with ischemic stroke have mild symptom severity on initial presentation (up to 56.0% in our study cohort)26. However, a considerable proportion of these mild patients does not achieve functional independence27,28. Our study showed that 17.0% of mild stroke patients did not regain functional independence at 3 months. Moreover, the proportion was higher (25.1%) in those with highest WMH burden, indicating clinical importance of outcome prediction in this stroke population for patient-centered care. Unfortunately, it is difficult to measure the effect of a subtle moderator of stroke outcome in the face of a neurological disaster. Patients with moderate-to-severe stroke may have moved onto a portion of our measurement scales, where subtle effects may no longer be detectable or clinically meaningful.
Our study has several limitations. First, different baseline characteristics between the included and excluded patients could have affected the study results (Supplementary Table S1). Second, although the proportions of missing data are relatively small (< ~ 5%), the simple (median) imputation method that we used for these missing values may not sufficiently account for the uncertainty related to the imputation step, potentially underestimating data variability. Third, this study may not be generalizable to other ethnic groups since we enrolled only Korean stroke patients. The predominant cause of ischemic stroke (LAA vs SVO vs CE) may vary geographically or ethnically29, leading other investigators to reach different conclusions, as stroke outcomes may be affected by WMH differentially depending on stroke subtypes3. Extracranial atherosclerosis is a more common cause of LAA strokes in Western countries, whereas intracranial atherosclerosis is more prevalent in Asian populations. CE is a more common cause of ischemic stroke in Western countries compared to Asia, where the incidence of CE strokes is rising due to aging populations and improved detection of atrial fibrillation. The prevalence of SVO can be affected by risk factors such as hypertension and diabetes, which are prevalent in many populations, including Asian communities. Previously, we showed that the stroke subtype that was affected the most by increased WMH burden seemed to be LAA stroke, followed by SVO stroke, with a weaker effect observed in CE stroke3. Thus, our subgroup analysis by stroke etiology should provide a pointer for the generalization of WMH as a prognosticator in other parts of the world/populations.
Conclusions
In conclusion, higher WMH burden affects functional outcome adversely after mild stroke in a dose-responsive manner, but the moderating effect is much less strong for moderate-to-severe stroke where only advanced WMH burden seems to matter. This information may be helpful for stroke prognostication and customized treatment.
Supplementary Information
Acknowledgements
The authors appreciate the contributions of all members of the Clinical Research Collaboration for Stroke-Korea to this study.
Author contributions
D-SG and W-SR, the co-first author, established the study protocol, analyzed and interpreted the data, and wrote the manuscript; DS drafted the manuscript including medical writing for content; JC, H-RK, S-WJ, BJK, J-TK, K-SH, J-MP, M-SP, K-HC, T-HP, KL, S-SP, KK, Y-JC, H-KP, B-CL, K-HY, M-SO, SJL, JGK, J-KC, D-HK, JL, M-KH, and H-JB collected and analyzed the data; JSL analyzed the data; and D-EK, the corresponding author, established the study idea, interpreted the data, drafted the manuscript, and made critical revisions in the manuscript with intellectual input.
Funding
This study was supported by the National Priority Research Center Program Grant (NRF- 2021R1A6A1A03038865), the Basic Science Research Program Grant (NRF- 2020R1A2C3008295), and the Multi-ministry Grant for Medical Device Development (KMDF_PR_20200901_0098) of National Research Foundation, funded by Korean government.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request after the approval by the CRCS-K steering committee.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dong-Seok Gwak and Wi-Sun Ryu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71936-9.
References
- 1.Wardlaw, J. M., Valdes Hernandez, M. C. & Munoz-Maniega, S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc.4, 001140. 10.1161/JAHA.114.001140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu, W. S. et al. White matter hyperintensity load on stroke recurrence and mortality at 1 year after ischemic stroke. Neurology93, e578–e589. 10.1212/WNL.0000000000007896 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Ryu, W. S. et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain140, 158–170. 10.1093/brain/aww259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams, H. P. Jr. et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology53, 126–131. 10.1212/wnl.53.1.126 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Onteddu, S. R., Goddeau, R. P. Jr., Minaeian, A. & Henninger, N. Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J. Neurol. Sci.359, 418–423. 10.1016/j.jns.2015.10.005 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Hong, S. et al. Excessive white matter hyperintensity increases susceptibility to poor functional outcomes after acute ischemic stroke. Front. Neurol.12, 700616. 10.3389/fneur.2021.700616 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu, W. S. et al. Grading and interpretation of white matter hyperintensities using statistical maps. Stroke45, 3567–3575. 10.1161/STROKEAHA.114.006662 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Ryu, W. S. et al. Hemispheric asymmetry of white matter hyperintensity in association with lacunar infarction. J. Am. Heart Assoc.7, e010653. 10.1161/JAHA.118.010653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, D. E. et al. Estimation of acute infarct volume with reference maps: a simple visual tool for decision making in thrombectomy cases. J. Stroke21, 69–77. 10.5853/jos.2018.03202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, D. E. et al. Mapping the supratentorial cerebral arterial territories using 1160 large artery infarcts. JAMA Neurol.76, 72–80. 10.1001/jamaneurol.2018.2808 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, B. J. et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J. Stroke17, 38–53. 10.5853/jos.2015.17.1.38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern Med.147, 573–577. 10.7326/0003-4819-147-8-200710160-00010 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Ko, Y. et al. MRI-based algorithm for acute ischemic stroke subtype classification. J. Stroke16, 161–172. 10.5853/jos.2014.16.3.161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, D. E. et al. A new image-based stroke registry containing quantitative magnetic resonance imaging data. Cerebrovasc. Dis.32, 567–576. 10.1159/000331934 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Jampathong, N., Laopaiboon, M., Rattanakanokchai, S. & Pattanittum, P. Prognostic models for complete recovery in ischemic stroke: a systematic review and meta-analysis. BMC Neurol.18, 26. 10.1186/s12883-018-1032-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muchada, M. et al. Baseline National Institutes of Health stroke scale-adjusted time window for intravenous tissue-type plasminogen activator in acute ischemic stroke. Stroke45, 1059–1063. 10.1161/STROKEAHA.113.004307 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Zerna, C. et al. Association of white matter hyperintensities with short-term outcomes in patients with minor cerebrovascular events. Stroke49, 919–923. 10.1161/STROKEAHA.117.017429 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Xu, Y. Y. et al. The association of white matter hyperintensities with stroke outcomes and antiplatelet therapy in minor stroke patients. Ann. Transl. Med.8, 331. 10.21037/atm.2020.02.137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guglielmi, V. et al. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke50, 3360–3368. 10.1161/STROKEAHA.119.026299 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, X. et al. Distinct predictive role of collateral status on clinical outcome in variant stroke subtypes of acute large arterial occlusion. Eur. J. Neurol.25, 293–300. 10.1111/ene.13493 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Rebello, L. C. et al. Stroke etiology and collaterals: atheroembolic strokes have greater collateral recruitment than cardioembolic strokes. Eur. J. Neurol.24, 762–767. 10.1111/ene.13287 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. J. et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J. Cereb. Blood Flow Metab.29, 1138–1145. 10.1038/jcbfm.2009.38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantoni, L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol.9, 689–701. 10.1016/S1474-4422(10)70104-6 (2010). [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan, M. et al. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J. Neurol. Neurosurg. Psych.75, 441–447. 10.1136/jnnp.2003.014910 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Sullivan, M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke41, S154-158. 10.1161/STROKEAHA.110.595314 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Reeves, M. et al. Distribution of national institutes of health stroke scale in the cincinnati/northern kentucky stroke study. Stroke44, 3211–3213. 10.1161/STROKEAHA.113.002881 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romano, J. G. et al. Distinct short-term outcomes in patients with mild versus rapidly improving stroke not treated with thrombolytics. Stroke47, 1278–1285. 10.1161/STROKEAHA.115.011528 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Smith, E. E. et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from Get With The Guidelines-Stroke. Stroke42, 3110–3115. 10.1161/STROKEAHA.111.613208 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Kim, B. J. & Kim, J. S. Ischemic stroke subtype classification: an asian viewpoint. J. Stroke16, 8–17. 10.5853/jos.2014.16.1.8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request after the approval by the CRCS-K steering committee.