Abstract
Brain metastasis (BrM) is a devastating complication of solid tumors associated with poor outcomes. Immune checkpoint inhibitors (ICI) have revolutionized the treatment of cancer, but determinants of response are incompletely understood. Given rising incidence of BrM, improved understanding of immunobiological principles unique to the CNS and dissection of those that govern activity of ICI is paramount towards unlocking BrM-specific anti-tumor immunity. In this review, we seek to discuss the current clinical landscape of ICI activity in the CNS, CNS immunobiology, and focus, in particular, on the role of glial cells in the CNS immune response to BrM.
Introduction
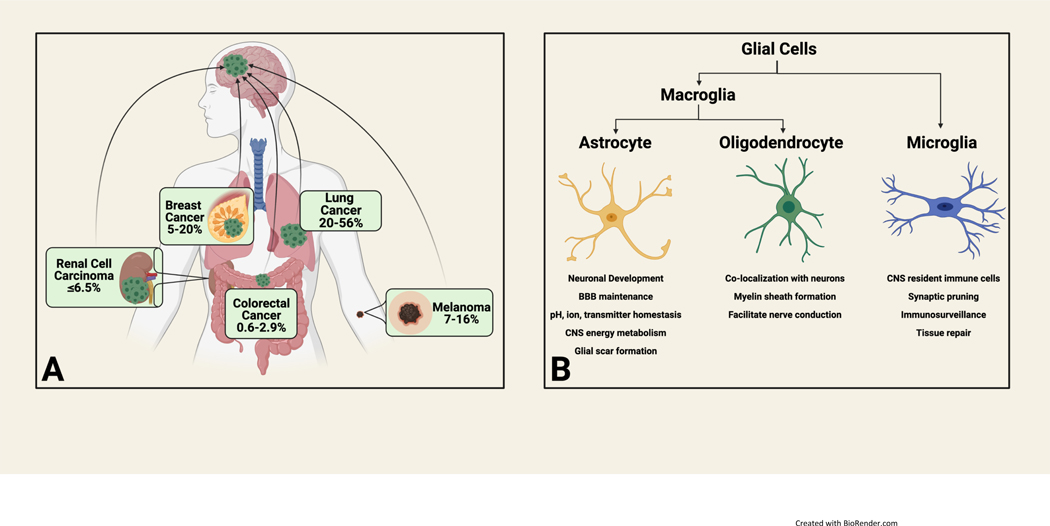
Brain metastasis (BrM) is an increasingly common complication from solid tumor malignancies owing to improved imaging techniques and increasing overall survival among cancer patients secondary to improved systemic therapies(1–3). While various tumor types can develop BrM, primary histologies that metastasize to the brain most frequently include lung cancer, melanoma and breast cancer (figure 1A)(4–7). Even histologies with comparatively lower frequencies of BrM, such as colorectal cancer and renal cell carcinomas, have more recently demonstrated increasing incidence(8, 9). Unfortunately, diagnosis of BrM is often associated with increased morbidity and decreased overall survival, with the majority of patients surviving less than one year after diagnosis(4, 10, 11). Current treatment approaches for BrM include whole brain radiation therapy, stereotactic radiosurgery, and surgical resection. Conventional chemotherapy has traditionally shown limited efficacy in the treatment of BrM, which is largely attributed to poor penetration of the blood brain barrier and transmembrane efflux pumps(12, 13). Thus, there is an urgent need to improve therapeutic paradigms.
Figure 1.
A) Most common primary tumor histologies associated with brain metastasis and frequency B) Glial cells and their biological roles
Although there are currently no approved central nervous system (CNS)-specific systemic therapies available, evolving treatment paradigms such as targeted therapies and immune checkpoint inhibition (ICI) offer new hope for the treatment of BrM. ICI has shown intracranial activity in a subset of patients with CNS disease(14–17); however, the determinants of response to ICI remain incompletely characterized and existing data is conflicting as to whether the anti-tumor immune response is concordant versus discordant across central and peripheral compartments. To improve outcomes for patients with BrM, characterization of the determinants of anti-tumor immunity across these compartments is paramount. To date, efforts have been disproportionately directed towards study of the peripheral compartment, owing in part to technical difficulty and risk associated with CNS tissue sampling. In this review, we focus on the current knowledge and promising investigations underway towards inducing anti-tumor immunity against BrM with a special focus on glial cell role and function (figure 1B). When applicable, we have used examples from the primary brain tumor literature, but, overall, focus specifically on BrM as the tumor immune microenvironment (TIME) of primary brain tumors has been expertly reviewed elsewhere(18, 19).
ICI challenges in the CNS: a clinical snapshot
ICI has revolutionized cancer care; programmed death 1 (PD-1) axis inhibitors are now approved in more than 19 different cancer types and have two tissue-agnostic indications(20). Depending on the underlying disease type, PD-1 pathway inhibitors are being used alone or in combination with chemotherapy, tyrosine kinase inhibitors, or with other ICIs (e.g., cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors). Despite impressive survival benefits observed across cancer types, the extent of intracranial activity of ICI in patients with concomitant brain metastases remains understudied. Attempts to answer this question are hampered by several factors including 1) patients with BrM have been historically excluded from clinical trials of ICIs 2) intracranial clinical activity is typically not a pre-specified endpoint and thus analyses are often limited to subgroups or are post-hoc with limited power 3) patients with BrM that have participated in clinical trials are often highly selected for asymptomatic and/or treated disease and 4) for the small group of patients with symptomatic/progressing BrM that have participated in clinical trials, there is a high frequency of steroid-dependence which may mitigate ICI-induced anti-tumor immunity(21). While the bulk of existing data may not fully reflect the immunobiological landscape of CNS metastatic disease, prospective data for patients with untreated, symptomatic or progressing BrM is growing—and forms the foundation for our current understanding of translational challenges for evaluating ICI efficacy in this patient population.
A phase II trial by Tawbi et al. included patients with asymptomatic, untreated melanoma-derived BrM and demonstrated an impressive intracranial response rate (ICRR) of 54.5% using combined ipilimumab/nivolumab (table 1)(14–16, 22–26). In an updated analysis from the same trial focused solely on patients whose BrM were symptomatic (versus asymptomatic), an ICRR rate of 22.2% was observed, and all intracranial responders demonstrated concordant extracranial responses (22.2%)(25). Median intracranial progression-free survival (IC-PFS) was 1.2 months and overall survival was 8.7 months in these patients. By contrast, with a median of 20.6 months follow up, median PFS and OS were not reached among asymptomatic participants. This landmark trial is perhaps the strongest evidence to date that ICI can induce clinically meaningful anti-tumor activity in the CNS and that the immune response is frequently concordant across central and peripheral compartments (50.5% of asymptomatic patients demonstrated a “global response”). Both of these intriguing observations directly challenge conventional CNS immune privilege dogma that would predict mitigated clinical activity in the CNS. In parallel, these data also suggest impaired ICRR, PFS and OS in patients with symptomatic versus asymptomatic BrM, and that clinical activity is diminished (though power is limited) in patients receiving corticosteroids raising important questions regarding how the biology of a symptomatic lesion may differ compared to clinically asymptomatic disease. Intracranial activity of ICI leading to survival benefits in this population with historically increased morbidity and decreased survival may extend beyond melanoma as well, and thus these questions are in need of urgent exploration(27).
Table 1.
Key prospective investigations evaluating immune checkpoint blockade in patients with untreated and/or progressive brain metastasis
| Reference(s) | Study Design | Primary Histology | Total cohort size | Inclusion criteria and stratification | Stratum cohort size | Treatment regimen | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC RR (%) | EC RR (%) | Median IC PFS (months) | Median EC PFS (months) | Median OS (months) | |||||||||
| Margolin et al24 | Phase II | Melanoma | 72 | Asymptomatic | 51 | ipilimumab | 16 | 14 | 1.9 | 3.3 | 7 | ||
| Symptomatic with stable corticosteroid dose | 21 | 5 | 5 | 1.2 | 1.3 | 3.7 | |||||||
| Goldberg et al14,22 Kluger et al23 | Phase II | Melanoma | 60 | Asymptomatic, ≥1 untreated or progressive brain metastasis, no steroid requirement | 23 | pembrolizumab | 26 | 47 | 2* | 2* | 17 | ||
| NSCLC | 37 | 29.7 | 29.7 | 2.3 | not reported | 9.9 | |||||||
| Long et al15,26 | Phase II | Melanoma | 76 | Asymptomatic, untreated | 35 | ipilimumab + nivolumab | 51 | 57 | not reached | 13.8 | not reached | ||
| 25 | nivolumab | 20 | 29 | 2.5 | 2.6 | 18.5 | |||||||
| Local failure, symptomatic and/or LMD | 16 | nivolumab | 6 | 25 | 2.3 | 2.6 | 5.1 | ||||||
| Tawbi et al16,25 | Phase II | Melanoma | 119 | Asymptomatic | 101 | ipilimumab + nivolumab | 54.5 | 48.5 | not reached | not reached | not reached | ||
| Symptomatic, stable steroid dose | 18 | 22.2 | 22.2 | 1.2 | 2.2 | 8.7 | |||||||
| Brastianos et al17 | Phase II | Mixed | 20 | LMD | - | pembrolizumab | 0 | 0 | 2.6 | 3.6 | 3.6 | ||
PFS was not reported stratified by site ie: intra- versus extracranial PFS therefore median overall PFS (2 months) is reported
IC=intracranial, EC=extracranial, RR=response rate (complete response + partial response), PFS=progression-free survival, OS=overall survival, LMD=leptomeningeal disease, NSCLC=non-small cell lung cancer
Three additional trials have investigated the activity of ICI among patients with active or progressing parenchymal BrM. Margolin et al. conducted a phase II study in patients with melanoma-derived BrM who received ipilimumab monotherapy, which demonstrated an ICRR of 16% and 5% for asymptomatic and symptomatic patients, respectively—again possibly suggesting that corticosteroid-dependence for symptomatic CNS disease may mitigate ICI activity in the CNS(24). Long et al. conducted a multicenter, randomized phase II clinical trial of nivolumab monotherapy versus combined ipilimumab/nivolumab in patients with asymptomatic melanoma-derived BrM(15). Notably, they included an additional nivolumab monotherapy treatment arm for patients with progressing, symptomatic, or leptomeningeal lesions. Only one patient (6%, N=16) demonstrated an intracranial response in this treatment arm. In the trial overall, there appeared to be higher intracranial response rates with combined ipilimumab/nivolumab (46% [95% CI 29–63]—which rose to 51% in an updated long-term analysis)—versus nivolumab monotherapy (20% [95% CI 7–41])(26). It remains unclear in what proportions the lower response rate observed in symptomatic patients was due to immunosuppression from corticosteroid use or perhaps a unique role may exist for combined PD-1 and CTLA-4 inhibition in the setting of CNS metastases. Finally, Goldberg et al. conducted a phase II trial studying pembrolizumab in patients with non-small cell lung cancer (NSCLC) or melanoma with untreated or progressing brain metastases(14). In patients with NSCLC and PDL1 ≥1%, 29.7% (N=11) [95% CI, 15.9–47%] demonstrated an intracranial response which was concordant with extracranial response. Similarly, 26% (N=6) patients with melanoma demonstrated an intracranial response—all of whom demonstrated an extracranial response(23). Data from these trials reinforce earlier findings that there is a higher than expected ICRR to ICI, responses are superior compared to dismal failures noted in several trials studying ICI for glioblastoma (NCT02617589, NCT02667587, NCT02011717)(28), responses tend to be concordant across intracranial and peripheral compartments, and patients with symptomatic BrM have decreased response rates and shorter relapse times compared to asymptomatic patients. However, these select trials are few in number with highly selected patient populations, and do not include correlative work to dissect underlying biology at play. These trends should be interpreted with caution as the field awaits larger, randomized clinical trials across cancer types beyond melanoma and NSCLC with embedded translational plans focused on determinants of immune response.
Overall, there are few prospective clinical data and correlative studies assessing the determinants of ICI response across central and peripheral compartments. One retrospective series comprising 18 patients with lung cancer and BrM who received pembrolizumab or nivolumab showed that intracranial and peripheral responses were discordant. Of eleven (61%) who demonstrated partial response or stable disease extracranially, eight patients (72%) exhibited CNS progression(29). The remaining seven patients (39%) demonstrated concordant intra- and extracranial progression of disease. In a separate cohort with available paired tissue specimens from primary and BrM lesions, Kim et al. showed that BrM specimens harbored statistically significant decreases in PD1+ tumor infiltrating lymphocytes (TILs). Although these data are retrospective, this study is notable because of available correlative data from paired specimens that suggests response rates and duration of response in the CNS may be lower compared to the extracranial compartment—and that the tumor immune microenvironment could account for such discordance which stands in contrast to available clinical data. Given the historical view of an immune privileged CNS and its distinct immunobiology, further investigation into the determinants of anti-tumor immunity in the CNS is necessary towards improved immunotherapy outcomes in patients with BrM.
ICI in corticosteroid-dependent patients with BrM
Given poorer outcomes in patients with symptomatic brain metastasis receiving ICI, and the high frequency of corticosteroid-dependence in this population, an examination of whether corticosteroids play a causal role in iatrogenic immunosuppression is critical. Conversely, it may be possible that symptomatic lesions tend to be larger and have a greater degree of peri-tumoral edema leading to decreased response. Additionally, corticosteroids are frequently used in patients to palliate symptoms such as poor performance status or low appetite—and a higher proportion of patients with BrM would be expected to be in this performance category. A recent meta-analysis reviewed 16 studies involving patients receiving ICI with available data for corticosteroid use(30). ICI treatment and concomitant corticosteroid use for any reason was associated with worse overall survival (HR= 1.54, 95% CI: 1.24–1.91; p=0.0001). However, all studies were retrospective and only 3 included patients with BrM. Interestingly, when stratified by corticosteroid indication on sub-group analysis, “supportive care“ was associated with worse overall survival (HR= 2.51, 95% CI 1.41–4.43; p = <0.01) versus BrM (1.51, 95% CI: 1.22–1.87; p = <0.01). These data suggest that while there may be a signal for reduced ICI efficacy in corticosteroid-dependent patients with BrM, this effect may not be as dominant as advanced disease with poor performance status requiring palliative corticosteroids. Jesserun et al. conducted a meta-analysis of 15 studies of human subjects with BrM who received ICI with available data for corticosteroid use(31). In pooled data, corticosteroid use was associated with worse overall survival (HR=1.84, 95% CI 1.22– 2.77, p=0.007) and worse extracranial (EC)-PFS (HR 2.00, 95% CI 1.37–2.91, p=0.007) but not worse IC-PFS (HR 1.31, 95% CI 0.42–4.07, p=0.500). No difference in ICRR was found between the corticosteroid and non-corticosteroid groups. Limitations to their analysis include significant heterogeneity across studies, conflicting data within the pooled analysis for IC-PFS, and heterogeneity in response criteria. Surprisingly, murine studies assessing the role of corticosteroids on anti-PD1 responses found that intracranial anti-PD1 tumor response was not abrogated by dexamethasone contrary to an observed immunosuppressive effect and impaired immune response against extracranial tumors(32). Prospective studies that include patients taking corticosteroids (most have excluded these patients to date) with pre-specified steroid-use endpoints including careful attention to dose and duration, ICI regimen and local treatments are needed to more definitively dissect the influence of corticosteroids on intracranial activity of ICI. However, data reviewed herein do suggest that corticosteroid use alone is insufficient to explain worse overall survival in patients with symptomatic BrM.
An interesting future direction would be investigation of intracranial activity of ICI combined with corticosteroid-sparing agents in patients with symptomatic BrM. For example, bevacizumab has shown promise in the treatment of refractory BrM-associated edema and clinical trials are ongoing studying this agent as an upfront steroid-sparing strategy in patients with BrM (NCT03175432)(33–35). Promising prospective data exists for the steroid-sparing effect of other agents as well including corticorelin (peptide-mimic of corticotropin-releasing factor), cediranib (vascular endothelial growth factor receptor inhibitor), Boswellia serrata and angiotensin II-converting enzyme inhibitors, however, most of these studies were done in patients with glioblastoma or primary brain tumors(36–40). Prospective trials with BrM-specific patients are needed to adequately elucidate steroid-sparing strategies in this patient population.
CNS immunobiology: unique features and considerations
CNS immune privilege vs. specialization
Immune privilege is defined as an inability to reject heterotypically transplanted tissue(18). CNS immune privilege achieved a conceptual stronghold in the early twentieth century when Shirai et al. demonstrated that implanted rat sarcoma cells grow well in the brain but not in skin or muscle and, later, when Medawar et al. showed that the immune response against a skin homograft implanted into a rabbit brain was dependent on the presence of a concurrent skin homograft(41),(42). Ostensibly, the absence of CNS draining lymph nodes and consequent lack of an afferent immune arm was hypothesized to explain the missing immune response when a skin homograft was implanted into the CNS alone. However, recent evidence builds upon this data and supports an updated model better described as immune specialized versus immune privileged.
Tissue graft rejection in the CNS does occur but is site-specific: rejection has been observed when tissue is implanted into cerebral ventricles but not parenchyma(43, 44). Activated T cells can pass through the blood-brain barrier (BBB) and patrol in the absence of neuroinflammation—a process called “immunosurveillance”(45, 46). Importantly, new insights on CNS routes of lymphatic drainage have also come into focus. In 2012, a fluid exchanger system responsible for moving unwanted byproducts from the parenchymal interstitial fluid into the draining CSF which exits via a network of meningeal lymphatic vessels (MLVs) was described and termed the “glymphatic system”(47, 48). Alitalo and colleagues characterized MLVs in the dura matter of the murine brain that drain out of the skull via the foramina alongside arteries, veins and cranial nerves(49). Using injection tracer experiments, they confirmed that these MLVs absorb both interstitial fluid of the brain parenchyma as well as cerebrospinal fluid from the subarachnoid space for transport into deep cervical lymph nodes. In separate work, Kipnis and colleagues similarly found murine lymphatic vessels that line the dural sinuses and drain preferentially to the deep cervical lymph nodes (dCLNs)(50). Interestingly, they showed that resection of the dCLNs resulted in an increased number of meningeal T cells attributed to an inability of T cells to drain from the meningeal space. More recently, their group demonstrated preferential CSF drainage and stromal-mediated immune cell recruitment results in an immune-CNS interface located specifically at the dural sinuses in the murine brain allowing peripheral surveillance of CNS antigens(51). With these new discoveries, the conceptual CNS immune privilege model has been appropriately revised to one of “CNS immune specialization”, and pre-clinical work towards induction of CNS anti-tumor immunity has commenced.
Hu et al. investigated the roles of meningeal lymphatic vessels (MLVs) in mouse models of glioma and melanoma and found that intracranial tumors induced extensive remodeling of dorsal MLVs and that disruption of these MLVs attenuated the efficacy of combined PD-1/CTLA-4 blockade(52). Lymphangiogenesis of MLVs was mediated by vascular endothelial growth factor C (VEGF-C). Indeed, VEGF-C overexpressing mice receiving combined PD-1/CTLA-4 blockade showed improved overall survival due to a potentiated ICI response. These mice were noted to have increased CD8+ T cell/Treg ratios within tumors and dCLNs. Finally, antibody-mediated blockade of the chemokine-ligand 21 (CCL21)/C-C chemokine receptor 7 (CCR7) pathway abrogated the efficacy of combined ICI suggesting that VEGF-C potentiation of ICI-induced anti-tumor immunity is dependent on CCL21/CCR7 axis signaling. The CCL21/CCR7 axis has been shown to have dual roles in various cancer models; supporting anti-tumor immune responses in immune cells yet promoting tumor cell propagation(53). These results are consistent with separate work that demonstrated therapeutic delivery of VEGF-C potentiated ICI activity in a murine glioblastoma model(54). Further studies and development of CCL21/CCR7 axis inhibitors must proceed cautiously but, more broadly, future development of therapeutic strategies to increase lymphangiogenesis of MLVs and/or optimize lymphatic drainage to dCLNs via VEGF-C signaling is attractive towards improved intracranial immune response to ICI.
CNS anti-tumor immunity: role of extracranial disease
Despite these exciting new discoveries regarding CNS immune specialization, seminal work by Taggart et al.(55) using a murine B16 melanoma tumor transplantation model with extracranial (subcutaneous) plus intracranial tumors (which models the majority of patients who have concurrent disease intracranially and extracranially) suggests Shirai and Medawar’s model of immune privilege, as it pertains to BrM, remains insightful. Extracranial tumors were requisite for induction of intracranial tumor response by combined PD1/CTLA4 blockade and response correlated with 1) increased infiltration of CD8+ T cells, peripheral macrophages and microglia and 2) gene expression changes associated with activation of T cells, NK cells and macrophages/microglia. However, flow cytometry analysis for T cell activation markers confirmed that increased T cell activation gene expression was due to increased intratumoral percentage of CD8+ T cells: thus increased trafficking after peripheral expansion explains the observed T cell activation flux as opposed to activation of already centrally-located T cells. Finally, and of particular translational importance, they showed via gene pathway analysis and immunofluorescence that increased CD8+ T cell trafficking after combined ICI may occur via upregulation of T cell entry receptors on tumor vasculature (ICAM-1/VCAM-1). Inhibitors of ICAM-1/VCAM-1 signaling in humans, mostly monoclonal antibody-based treatments, have been studied in inflammatory and autoimmune contexts but await validation in anti-tumor applications. Interestingly, chimeric antigen receptor (CAR) T cells targeting ICAM-1 have been developed and have shown success in murine and patient-derived xenograft models of anaplastic thyroid and gastric cancer(56, 57). The role for inhibitors of ICAM-1/VCAM-1 in the treatment of BrM and primary brain tumors remains unexplored but represents an attractive target for future investigation.
Clinical data supporting the importance of extracranial disease burden for effective intracranial response secondary to ICI is supported by a retrospective analysis conducted by Rauschenberg et al. studying the impact of radiation and systemic therapy (including PD1/CTLA4 blockade) on survival in patients with melanoma-derived BrM(58). They found that presence of extracranial metastatic disease correlated with improved overall survival on both univariate (HR 0.1, 95% CI: 0.1–0.2; p<0.001) and multivariate analysis (HR=0.1, 95% CI: 0.01–0.35; p<0.001). Taken together with work performed by Taggart et al., preclinical and clinical evidence suggest that burden of extracranial disease may be a determinant of intracranial response of BrM to ICI. Therefore, prospective clinical trial design should account for this variable and ensure precise baseline and serial characterization of extracranial disease response as it pertains to assessment of intracranial treatment response. Additionally, the critical role of the extracranial compartment in orchestrating the priming, activation and trafficking of peripheral T cells to the CNS suggests consideration of delivery to the extracranial space for future development of CNS-specific T cell therapies or personalized cancer vaccines.
Blood-brain barrier and blood-tumor barrier
The BBB is composed of non-fenestrated endothelial cells, pericytes, a basal lamina layer and astrocytic endfeet that form a layer known as the astrocytic glia limitans. This tightly regulated neurovascular bundle serves to maintain CNS homeostasis and protects against unregulated transport of potentially harmful molecules or substances into the CNS(18, 59). Similarly, the BBB restricts antigen presentation and immune cell infiltration in the normal resting state. In order to gain entry into the CNS parenchymal space in the setting of inflammation, T cells must first pass through the endothelial layer followed by passage through the glia limitans(60). In the context of BrM, vascular structures lose integrity such that otherwise restricted entry by peripheral immune cells may be facilitated(61). However, a more modern conceptual framework is that the BBB does not breakdown per se but forms a “brain-tumor barrier” (BTB) in which lymphocytes can traverse the intact BBB via chemokine axes and multistep adhesion processes(62–64). Indeed, the blood-tumor barrier was shown to have heterogeneous permeability (regulated by reactive astrocytes)(65) which is a likely a driver of a variable immune cell infiltrate. The BTB (structure, function, pharmacokinetics, and complementary in vitro/in vivo data) has been expertly reviewed elsewhere(59, 64). Broadly speaking, however, the role of the BTB in modulation of the therapeutic immune response to BrM is largely unknown including patterns of infiltration, immune cell subsets involved, spatial and temporal dynamics, interactions with CNS resident populations and downstream functional consequences.
Recently, thromboinflammation studied in murine models of acute stroke has offered intriguing insight towards possible overlapping biology with respect to BrM and the immune interface of the BBB/BTB. Thromboinflammation results from the pathological interplay between platelets and T cells in response to CNS tissue insult resulting in exacerbation of underlying tissue injury(66). Work by Feinauer et al. has established an early role in BrM intiation and outgrowth dependent on thromboinflammation(67). Using multiphoton laser scanning microscopy to study the brain metastatic cascade in murine models, their group showed that clot formation occurs in brain microvessels preferentially at sites with intravascularly-arrested tumor cells—and that cancer cells embedded in clot had a higher success rate in extravasation and formation of a macrometastasis. While an intriguing etiologic connection between thromboinflammation and BrM initiation/propagation may exist, future examination of the inflammatory component is needed (such as immune cell subsets, cytokine and chemokine signaling, and multicellular interaction at the BBB/BTB interface) before therapeutic strategies targeting thromboinflammation can be developed.
Translational strategies involving development of BBB/BTB disruption methods, permeability-altering receptor agonists, radiosensitizing nanoparticles and novel delivery platforms have rarely progressed past phase I clinical trials to date. Cellular approaches that leverage the high CNS tumor tropism of neural stem cells and mesenchymal stem cells are attractive options for development of therapeutic carriers(68). Cell-mediated delivery of immune-supporting products (i.e: cytokines, chemokines) or oncolytic adenovirus are examples(59, 69). Future studies focusing on further characterization of structure/function, improved model systems representative of (and compared to) human tissue studies, patterns of permeability (both innate and induced secondary to therapeutic modulation) and drug distribution are imperative to bring actionable understanding of the BTB to light. These areas of focus for future investigations are needed not only to increase understanding of the BBB/BTB proper but also to, specifically, characterize the role of BBB/BTB as it pertains to modulating CNS anti-tumor immunity.
CNS Adaptive Immunity and T cells: a deeper dive
Infiltration of brain tumors by CD8+ and CD4+ T cells has been observed(70), the patterns of which are variable and dependent on primary tumor histology(71). In a retrospective study, Berghoff et al. evaluated 116 BrM resection specimens from various cancer types for TIL density and subset patterns(72). Tumors showed frequent tumor penetration by TILs and TIL density was highest in the tumor stroma and tumor-parenchyma border while solid tumor areas were sparsely populated. Interestingly, no associations were found between pre-operative corticosteroid treatment and any differences in TIL subset. Peritumoral edema was inversely associated with TIL density. Higher TIL density, CD8+ T cells, CD45RO+ T cells were associated with improved overall survival. In a separate study, Berghoff and colleagues showed that BrM in small cell lung cancer patients similarly showed frequent TIL rates across cell subtypes, and that improved overall survival was associated with higher density of CD45RO+ TILs(73). Thus, there is heterogenous spatial and immune cell subtype distribution in BrM across multiple cancer types, and further characterization of these patterns is required, especially in patient-matched intra- and extracranial specimens.
One study performed immune gene expression profiling on paired intra- and extracranial samples from 39 patients with NSCLC and found that BrM samples demonstrated reduced T cell infiltration and clonal expansion compared to extra-cranial samples, but that TCR repertoires were largely shared(74). Interestingly, another study also looking at paired samples in a smaller NSCLC patient cohort found that T-cell clonality was largely non-overlapping across paired samples and that BrM harbor contracted T cell diversity(75). Surprisingly, TMB was higher in the BrM samples compared to their respective extracranial samples but this did not correspond to a statistically higher predicted neoantigen load. Proposed reasons for the discrepant results were that the former study focused selectively on abundant clones and that there were largely shared somatic hotspot mutations between samples(74). Further study in larger cohorts is needed but it is possible that divergent underlying genetics between not only extra- and intracranial lesions, but also across distinct intracranial lesions, could result in non-overlapping T cell repertoires(76–78). Collectively, these data suggest that there could be spatiotemporal heterogeneity in the immune response across CNS lesions in the same patient.
CD4+ T cells
CD4+ T cells have diverse and context-dependent “helper” roles in mediating the anti-tumor immune response as well as immunosuppression via T regulatory cell function(79). Thus far, immuno-oncology has disproportionately focused on CD8+ T cell biology. However, there is a growing body of preclinical and clinical evidence that CD4+ T cells play a critical role in supporting anti-tumor immunity outside of the brain (reviewed elsewhere by Tay et al.)(79). However, the anti-tumor role of CD4+ T cells in BrM remains to be defined.
In contrast to the anti-tumor role of CD4+ T cells, CD4+CD25+FOXP3+ T regulatory cells may play pro-tumor roles acting as drivers of immunosuppression in the BrM TIME(80). Kim et al.(81) performed a comprehensive single cell transcriptomic characterization of 208,506 cells obtained from normal, intra- and extracranial tumors from 44 patients with NSCLC, (29,060 cells were from BrM) and showed that, at all stages of progression, a shift towards a pro-tumoral and an immunosuppressive TME marked by replacement of myeloid populations with monocyte-derived macrophages and increased T cell exhaustion was observed. On the whole, further characterization of the BrM-specific role of CD4+ T cell subtypes should be prioritized going forward and is likely to yield novel opportunities to enhance efficacy of ICI as well as further develop cancer vaccine approaches. One pre-clinical example of modulating CD4+ T cell activity in brain tumors to enhance anti-tumor immunity comes from work by Bunse et al(82). They studied the role of the oncometabolite R-2-hydroxyglutarate (R-2-HG) accumulation in gliomas and its influence on T cells and the tumor microenvironment(82). They demonstrated that R-2-HG impairs antigen-specific T cell activation and that, specifically, CD4+ T cells were shown more susceptible to R-2-HG-mediated inhibition. They also validated in IDH1-mutant glioma murine models that R-2-HG impairs anti-tumor immunity induced by IDH1-specific vaccination, adoptive T cell transfer and checkpoint inhibition. Their study showcases that T cell-specific therapies can be developed towards primary brain tumors and underscore the need to further dissect CD4+ T cell biology in the treatment of BrM.
CNS anti-tumor response
The main driver for BrM-specific, activated CD8+ T cells remains to be defined. One possibility is that BrM outgrowth results in tissue injury leading to release of endogenous peptides known as danger associated molecular patterns (DAMPs) or alarmins which are recognized by pattern-recognition receptors (PRRs) on microglia, neurons and astrocytes and lead to innate immune system activation(83). Subsequent cytokine-release results in an inflammatory cascade and immune cell infiltrate including both innate and adaptive immune cells(84–86). However, if DAMP/alarmin release was the sole determinant of activated T cells with receptor-specificity towards newly-liberated CNS antigens, we might expect to see evidence of CNS-specific autoimmunity in patients that develop BrM—a very uncommon scenario. Rather, it seems more probable that BrM harbor tumor neo-antigen profiles that have both overlapping and non-overlapping components when compared to the periphery.
A unifying hypothesis is that local tissue injury secondary to BrM outgrowth leads to DAMP/alarmin release and PRR activation which drives, in part, a T cell-mediated adaptive immune response via two mechanisms (figure 2A-M). First, T cells with receptor specificity for a peripheral neo-antigen shared with BrM lesion will subsequently traffic from the periphery to the CNS to execute an effector response. Second, BrM-specific neo-antigens could drain to peripheral lymph nodes resulting in T cells with BrM-unique receptor specificity that then subsequently traffic to the CNS. However, many questions remain pertaining to what proportion these T cell activation patterns might play out, anatomical considerations such as how T cells might overcome an immunosuppressive milieu on arrival, and the role of the BBB/BTB in adaptive anti-tumor immunity.
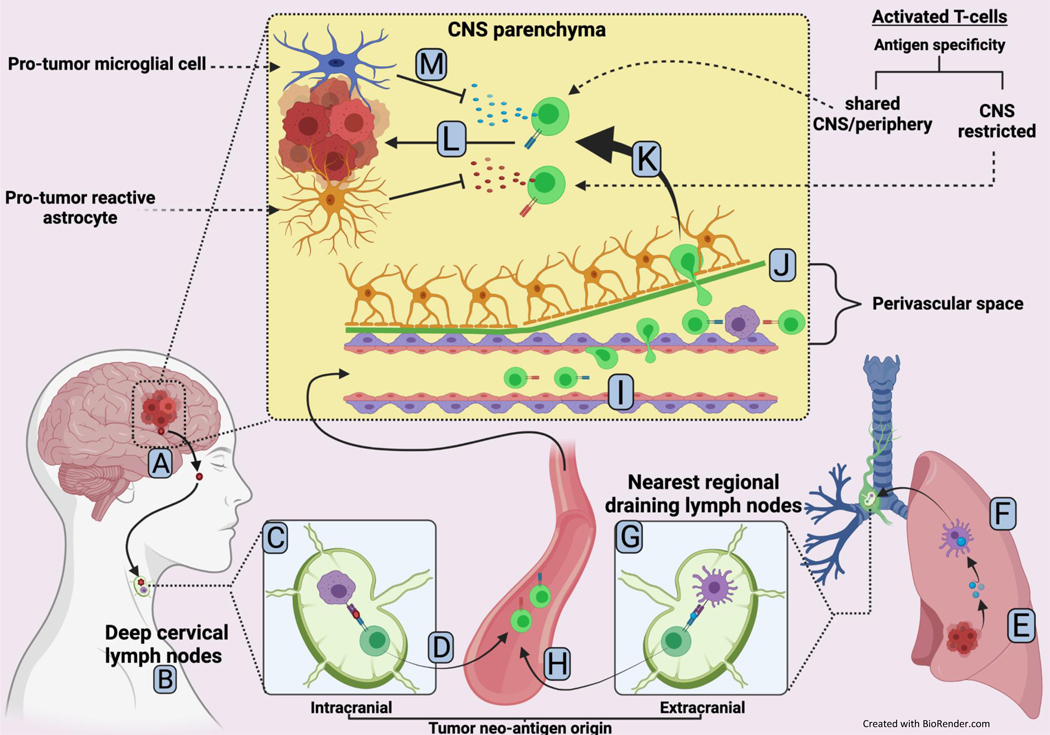
Figure 2.
A model for T cell-mediated adaptive immune response towards CNS-specific and centrally/peripherally shared tumor neo-antigens
A. CNS-specific tumor neo-antigen (red) is shed from tumor cell(s) making up the brain metastasis
B. Lymphatic drainage to the nearest regional draining lymph nodes ie: deep cervical nodes occurs
C. CNS specific tumor neo-antigen is phagocytosed and presented to a naïve T-cell by an antigen-presenting cell
D. T cell is primed and activated entering the bloodstream en route to the brain
E. Centrally/peripherally-shared tumor neo-antigen is shed from an extracranial metastatic tumor
F. Patrolling dendritic cell recognizes and phagocytoses tumor neo-antigen followed by trafficking to nearest draining lymph node (afferent immunity)
G. In the lymph node, dendritic cell presents the centrally/peripherally-shared tumor neo-antigen to a naïve T cell
H. T cell is primed and activated entering the bloodstream en route to the brain
I. Activated T cells slow, roll and crawl as they begin extravasation into the perivascular space
J. Activated T cells encounter perivascular macrophages which induce re-stimulation of the T-cells
K. Extravasation across the glia limitans (basal lamina and astrocyte end feet layers) into the CNS parenchyma is completed
L. Activated T-cells encounter respective target tumor cells and execute a cytotoxic attack
M. Pro-tumor glial cells in the tumor microenvironment drive immunosuppression and mitigate T-cell attack
CNS Innate Immunity
Neuroinflammation that results from CNS injury (such as tumor outgrowth), infection or neurodegenerative disease activates the innate immune system via complex interplay of CNS resident cells, centrally-recruited peripheral immune cells, cytokine signaling and complement(87). Neuroinflammation in the short term is considered neuroprotective while prolonged neuroinflammation can have deleterious consequences such as support/promotion of underlying pathophysiology. Complement was initially thought to be an absent component of neuroinflammtion but we now understand that neuronal and glial cells have complement receptors and can produce complement(87). Further, cancer cells may hijack complement signaling resulting in outgrowth in the CSF in the setting of leptomeningeal disease(88). The presence of functional dendritic cells within the CNS has been a topic of debate. Historically, lack of evidence for dendritic cells exhibiting antigen uptake and processing, cell surface display via MHC class II, afferent lymph node trafficking and naïve T cell activation has formed the cellular basis of immune privilege. However, dendritic cells can be found in the human brain and increases in DC infiltration in the setting of neuroinflammation has been observed (although it is unclear to what degree these are peripheral in origin given perceived BBB disruption)(89). Dendritic cell role and function in the CNS remains incompletely characterized, especially in the context of BrM. Finally, glial cells (figure 1B) act as CNS resident innate immune cells, yet their diverse functions extend far beyond innate immunity.
Next, we choose to focus in depth on glial cells such as astrocytes and microglia given their multilateral roles in BrM propagation in addition to innate and adaptive immune signaling. For detailed examinations of non-glial cells and their role in the TIME of brain tumors, see expert work by Quail et al., Doron et al. and Klemm et al.(19, 90, 91).
Glial cells: CNS resident immune cells and beyond
Astrocytes
Astrocytes, the most abundant cell type in the CNS, participate in a wide variety of homeostatic functions in the normal brain including maintenance of the BBB, immune signaling, modulation of neuronal networks and maintenance of ion, pH and transmitter balance in synaptic, interstitial fluid(92–95) (figure 1B). Given the wide-ranging role of astrocytes, it is unsurprising that significant functional and phenotypic heterogeneity exists for the resident sentinels of the CNS. Classically, astrocyte functional phenotype has been defined within the confines of binary polarization states: the neuro-inflammatory and anti-tumor “A1” state versus the neuro-protective and tumor supportive “A2” state(96, 97). It is increasingly recognized, however, that these functional states exist on a dynamic continuum and that these states are temporally and contextually determined(98–100). In the presence of a tissue insult such as tumor or infection, astrocytes activate into “reactive astrocytes” undergoing morphological and phenotypic changes –a process known as astrogliosis which culminates in formation of a glial scar (figure 3A-C)(93).
Figure 3.
Phenotypic and functional continuum for astrocytes and microglial in the setting of brain metastasis-mediated neuroinflammation
A) Outgrowth of brain metastasis results in local tissue injury resulting in DAMP and alarmin release which drive a neuroinflammation cascade
B) Glial cells in resting state adopt phenotypic and functional changes resulting in a reactive state in the setting of acute inflammation
C) In the chronic setting of neuroinflammation, glial cells adopt a suppressed phenotype thought to be neuroprotective and limit damage from an acute insult and resulting immune response
Role for astrocytes in BrM
Initiation of micro-metastasis
In early development of BrM, sometimes referred to as micro-metastatic initiation, astrocytes appear hostile to BrM-initiating cells (figure 4A). Valiente and colleagues demonstrated in lung and breast cancer models that reactive astrocytes are a major source of plasminogen activator (PA) leading to the production of plasmin which plays a role in stromal response to injury(101). Plasmin accumulation inhibited development of BrM by triggering Fas ligand-dependent apoptosis in BrM-initiating cells. To overcome this, breast and lung cancer cells (human and murine) were found to secrete serpins with inhibitory activity against PA; thereby, promoting survival against tumor-inhibiting effects of plasmin(101). In contrast, Lorger et al demonstrated in a murine breast cancer model that astrocytes preferentially co-localized with invading tumor cells followed by activation and secretion of pro-angiogenic and growth-promoting matrix metalloproteinase-9 (MMP-9) suggesting a supportive role for astrocytes in the early BrM initiation phase(102). Doron et al. showed in murine models of melanoma-derived BrM that astrocyte-secreted CXCL10 resulted in chemoattraction of CNS-tropic melanoma cells expressing CXCR3 (CXCL10 receptor), and that targeted inhibition of CXCR3 decreased BrM formation(103). Taken together, available data conflicts on whether astrocytes aid or impede BrM-initiation despite detailed mechanistic work underscoring the overall complexity of the functional role of astrocytes and BrM. Further, whether astrocytes assume reactive functional states due to cancer cells directly or indirectly as a result of tissue-injury associated with BrM outgrowth is unknown(104), however Chen et al. demonstrated that the former is highly likely(105). In co-culture and BrM murine experiments, they showed that BrM cells containing cytosolic DNA and cyclic GMP engage astrocytes in Cx43-based gap junctions resulting in co-option of astrocyte-produced cytokines that support cancer cell growth and survival. Thus, future characterization of the role of astrocytes in early BrM formation should focus on precise timing of the BrM initiator cell entry into the CNS microenvironment.
Figure 4.
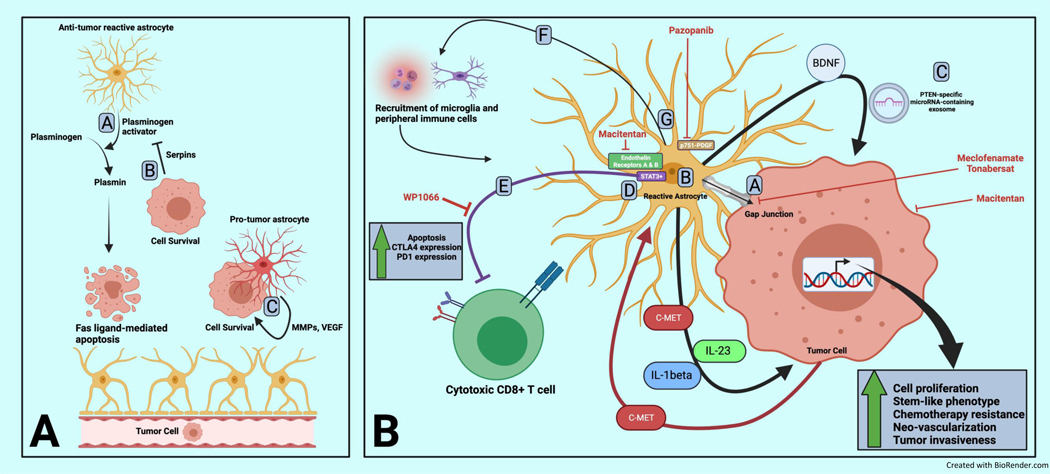
Astrocyte role in brain metastasis initiation and propagation including pro-tumor immunosuppression
Panel A:
Astrocytes have been shown to be both hostile and supportive to invading brain metastasis-initiating cells.
A. Astrocytes secrete plasminogen activator resulting in plasmin accumulation which can induce FAS ligand-dependent apoptosis of tumor cells
B. Tumor cells can secrete serpins which inhibit astrocyte-secreted plasminogen activator resulting in tumor cell survival
C. Reactive astrocytes can adopt pro-tumor roles associated with co-localization with tumor cells and secretion of pro-tumor factors such as matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF) and others.
Panel B:
A. Tumor cells benefit from formation of gap junctions with tumor cells in which astrocyte-secreted metabolites lead to downstream expression of tumor survival genes conferring resistance to chemotherapy. Direct targeting of gap junctions (with inhibitors such as meclofenamate and tonabersat) as well as dual inhibition of endothelin receptors with macitentan (approved for the treatment of renal cell carcinoma and soft tissue sarcoma) has been shown to decrease astrocyte-mediated survival gene expression in tumor cells and reduce outgrowth.
B. Astrocytes have diverse secretory programs including brain derived neurotropic factor (BDNF), c-MET, Jagged 1 (JAG1), interleukin-23 (IL-23), and interleukin-1 beta (IL1-beta) resulting in pro-tumor proliferation, adoption of stem-like features, vascular reprogramming and increased invasiveness.
C. Astrocytes also secrete PTEN-specific micro-RNA containing exosomes which result in decreased tumor cell expression of PTEN shown to be unique to brain metastasis tumors compared to paired extracranial lesions
D. STAT3+ astrocytes co-localize with and shield tumor cells from immune attack by mitigating cytotoxic attack by T cells. WP1066 is one example of a STAT3 pathway inhibitor that has shown pre-clinical efficacy in treating brain metastasis.
E. Astrocytes oppose the adaptive immune response via direct induction of apoptosis and up regulation of exhaustion markers in T cells.
F. Astrocytes appears to harbor the ability to recruit peripheral immune cells as well as macrophages/microglia to the local tumor environment, however, it is unclear in what proportion this recruitment results in an anti- versus pro-brain metastasis effect.
G. Platelet-derived growth factor receptor phosphorylated at residue 751 was found to be a marker of a pro-tumor astrocyte sub-population and inhibition using pazopanib (which is approved for use in renal cell carcinoma and soft tissue sarcoma) results in decreased brain metastasis outgrowth
Propagation/outgrowth of macro-metastasis
Astrocytes have been shown to surround BrM and promote propagation and outgrowth via co-option of astrocyte gene expression programs resulting in tumor supportive programming(101, 102, 105–108). For example, co-culture of astrocytes with tumor cells of breast, lung and skin origin led to astrocyte-induced up-regulation of survival genes in tumor cells, resulting in protection from various chemotherapeutic agents(107, 108). Underscoring this mechanism, work by Kim et al. demonstrated in a murine BrM model that dual targeting of endothelin receptors resulted in decreased survival gene expression in tumor cells and re-sensitization to anti-neoplastic therapy(109).
Astrocytes also have many pro-tumor secretory functions. Astrocytes were found to secrete brain-derived neurotrophic factor (BDNF) leading to heterodimerization of tumor cell tropomyosin-related kinase B (Trkb) and human epidermal growth factor receptor 2 (HER2) in breast cancer cells resulting in increased cell proliferation(110). An auto-paracrine feedback loop was discovered in which tumor cells with mesenchymal-epithelial transition factor (c-MET) expression leading to pro-tumor vascular reprogramming and interleukin 1-beta (IL-1beta) expression, which induced tumor-associated astrocytes to secrete additional c-met ligand thus supporting tumor neo-vascularization(110). In a separate study, Xing et al. found that breast cancer cells secreted Il-1beta which was found to induce astrocyte-production of Jagged 1 (JAG1)(111). JAG1 is a transmembrane protein that facilitates Notch signaling and its production led to an increase of a stem-like phenotype of cancer cells via Notch-Hes5(111). In a melanoma brain metastasis model, Klein et al. demonstrated that tumor cells induced IL-23 secretion by astrocytes which resulted in increased MMP2-mediated tumor invasion(112). Lastly, in a notable study by Zhang et al, the relationship between tumor microenvironment across anatomic compartments was explored(113). Downregulation of PTEN expression was found to be unique to murine and human breast cancer-derived BrM specimens compared to paired primary and other extracranial metastases in a process mediated by microRNA-containing exosomes secreted by astrocytes. Collectively, these results across multiple BrM tumor models suggest that astrocytes modulate the tumor microenvironment through various tumor supportive secretory functions that promote tumor initiation as well as outgrowth.
Astrocytes and CNS anti-tumor immunity
Astrocytes play a role in modulating the innate and adaptive immunity. Priego et al. identified a subpopulation of signal transducer and activator of transcription 3-positive (STAT3+) reactive astrocytes that surround brain metastases and elegantly demonstrated that not only was outgrowth of human and murine lung-, breast- and melanoma-derived BrM dependent on this reactive astrocyte subpopulation, but that these cells directly influenced components of innate and adaptive immunity towards favoring tumor survival (figure 4B)(114). In the same study, Priego and colleagues treated patients with metastatic lung cancer with BrM (N=18) with oral legasil, an available silibinin-containing nutraceutical with STAT3 inhibitory activity, in combination with varying palliative chemotherapy regimens and demonstrated intracranial clinical responses with an overall response rate of 75%. Their work nominated STAT3+ reactive astrocytes as putative targets for achieving CNS-specific therapeutic activity. Further, their demonstration that culture media from STAT3+ astrocytes is sufficient alone to abrogate CD8+ T cell anti-tumor activity further underscores that this astrocyte subtype may be a promising target that could sensitize BrM to concurrent treatment with ICI.
Astrocytes appear to harbor the ability to recruit immune cells locally from the periphery in response to tissue damage (figure 4B). In a murine model to simulate intracranial tissue damage and inflammation, IL-1beta was injected intracranially and it was demonstrated that astrocyte-shed extracellular vesicles are responsible for recruiting peripheral leukocytes into the CNS(115). However, astrocyte-mediated recruitment patterns may be dependent on astrocyte subtype and location. Juxtavascular astrocytes, for example, were shown to negatively regulate invasion of peripheral monocytes at the vascular interface in murine models(116). Although the interactions between astrocytes and other immune cells such as microglia/macrophages remain underexplored(70), Priego et al. showed that an increased number of CD74+ microglia/macrophages surrounding BrM was astrocyte-dependent(114).
Astrocytes and T cells appear to have bi-directional mechanisms for suppressive signaling. For example, astrocytes are targeted by regulatory T-cells (Treg) resulting in attenuated signals that otherwise threaten neuronal viability, a mechanism to limit further tissue damage in the setting of an existing inflammatory insult(70). However, astrocytes also harbor some ability to induce FAS ligand-mediated apoptosis of T-cells in the setting of neuroinflammation, similarly thought to be a neuroprotective mechanism(117). Other work has shown astrocytes in co-culture with T cells induce CTLA-4 and PD-1 upregulation on activated T-cells resulting in attenuated CD8+ T cell activity suggestive of a driving role in CNS immune escape (figure 4B)(118, 119). Thus, there is strong preclinical and clinical evidence that astrocytes play a direct role in modulating the adaptive immune response in the setting of BrM. However, astrocyte subtype, location and interactions with other immune cells form a complex network that must be characterized before a clear target that could enhance anti-tumor immunity in patients receiving ICI can be identified.
Astrocytes as therapeutic targets
Given growing evidence for multifaceted roles of some astrocyte subpopulations in supporting BrM initiation and outgrowth, these cells are attractive therapeutic targets (figure 4B). Sarmiento and colleagues showed in rat models of BrM that STAT3+ reactive astrocytes drove cerebral vascular dysfunction which was reversible by treatment with WP1066, a STAT3 pathway small molecule inhibitor(120). Macitentan, an approved drug for treating pulmonary hypertension, targets endothelin receptors which, when combined with paclitaxel in a murine lung and breast cancer-derived BrM models, showed a reduced tumor cell division, increased apoptosis in both tumor and endothelial cells, and increased overall survival in treated-mice(121). Given pre-clinical evidence of efficacy, a phase I clinical trial (NCT01499251) was conducted to determine the tolerability of combined macitentan and temozolomide in recurrent glioblastoma and/or gliosarcoma but was terminated early due to lack of efficacy. It remains unclear whether endothelin receptors may offer a viable astrocyte-specific target in the treatment of BrM, and therefore BrM-specific clinical trials are needed.
A murine breast-derived BrM model was used to identify a previously unidentified subpopulation of astrocytes characterized by phosphorylated tyrosine 751 platelet-derived growth factor receptor (p751-PDGFR) that preferentially co-localized with BrM lesions in the perivascular space(65). Gril et al. validated this association in human BrM samples and demonstrated in their murine model that pazopanib, a multikinase inhibitor with activity against PDGFR, depleted the p751-PDGFR astrocytes which was associated with decreased BrM outgrowth(65, 122). Validation of targeting p751-PDGFR in humans with BrM from breast cancer and other histologies is needed, but it is encouraging that there is growing clinical experience with pazopanib in the treatment of renal cell carcinoma and advanced soft tissue sarcoma(123),(124). Further, there are case reports of RCC-derived BrM demonstrating an intracranial response to pazopanib treatment(125, 126).
Re-education of astrocytes by tumor cells into tumor supportive phenotypes via established gap junctions has led to interest in targeting these cell-to-cell signaling structures. Chen et al. demonstrated in murine models of BrM that protocadherin 7 (PCDH7) promotes assembly of tumor cell-astrocyte gap junctions(105). They demonstrated that meclofenamate and tonabersat, two approved oral drugs targeting gap junctions, were able to effectively break the tumor supportive paracrine loop resulting in inhibition of BrM outgrowth. Results from an ongoing single arm, phase II clinical trial (NCT02429570) studying meclofenamate in patients with recurrent or progressive BrM from solid tumors are eagerly awaited.
Microglia/Macrophages
Microglia are myeloid cells of the CNS parenchyma derived from the yolk sac that colonize the CNS during early embryonic development relying on local self-renewal thereafter(127, 128). In steady state, microglia have long processes that execute constant monitoring of healthy neural tissue and the local microenvironment—a process called “immunosurveillance”(129, 130). To maintain a resting, surveillant state, microglia are thought to be repressed by healthy neurons through diverse mechanisms(131). The dependent relationship microglia have on neurons is logical for cells whose fate is to spring into action at the first signs of local neuronal injury and/or death which would result in loss of neuron-mediated inhibitory inputs. Additionally, this mechanism suggests that microglial activation may be induced by removal of neuronal inhibition-an attractive guiding principle for discovery of potential modulatory targets. In certain disease states, such as stroke, infection or malignancy, microglial activation triggers morphological and cell surface marker changes resulting in the ability to secrete inflammatory signals, phagocytose, and participate in both oxidative burst and antigen presentation (figure 5A-F)(127). Although microglia have been shown to be activated via CX3CR1(132) and plasma-derived fibrinogen(133), much work remains to gain understanding of the activation and effector states of these cells due to their highly heterogenous and dynamic transcriptional programs(134, 135).
Figure 5.
Role of microglia and bone-derived macrophages in brain metastasis
A. Presence of invading tumor cells in the CNS parenchyma are sufficient to activate microglia from their resting state
B. Activated or “M1-like” microglia play a role in the immune response to neuroinflammation and are capable of oxidative burst and antigen presentation and recruiting cytotoxic T cells
C.Tumor cells can co-opt the functional state of nearby microglial resulting in an anti-inflammatory phenotype (“M2”) that promotes angiogenesis, tumor growth, and immunosuppression by recruitment of additional PDL1+/VISTA+ microglia. Selective targeting for this functional state has been demonstrated experimentally with mannosylated clodronate, for example.
D. Bone-marrow derived macrophages infiltrate the neuro-inflamed CNS and, similar to microglia, adopt a functional continuum ranging from anti-tumor to pro-survival tumor-associated macrophages (TAMs)
E. TAMs protect tumor cells by secretion of immunosuppressive factors such as inducible nitric oxide (iNOS) and tumor necrosis factor (TNF) which mitigates the overall immune response. Colony-stimulating factor-one axis signaling drives additional TAM recruitment and M2 polarization resulting in further support and protection of tumor cells. In mice, CSF1 axis inhibitors such as BLZ945 and PLX3397 have demonstrated reduced tumor outgrowth.
F. Brain metastasis have been shown to have enriched phosphoinositide-3 kinase (PI3K) signaling activity and that PI3K was a master regulator of metastasis-promoting microglia/macrophages. PI3K is an attractive therapeutic target with a growing list of CNS-penetrant inhibitors available such as GDC-0084.
It has been proposed that microglia—despite their competence as APCs—cannot leave the CNS thus barring their participation in afferent (and consequently) efferent immunity(127). Rather, it is believed that non-parenchymal macrophages (choroid plexus, meninges, perivascular) are responsible for T cell re-stimulation upon their arrival (figure 2J)(127, 136). While work remains to differentiate and functionally characterize CNS-native microglia and macrophages, another dimension of complexity is added by infiltrating monocytes/macrophages that traffic to the CNS from the circulation in the setting of neuroinflammation(137). The challenge of differentiating microglia and resident CNS macrophages from peripheral, monocyte-derived, CNS-infiltrating macrophages has been vast owing to substantial phenotypic and cell surface marker overlap. Only recently have markers such as TMEM119, MS4A7, Gpr56 and CD49d have been shown to differentiate between microglia and bone marrow-derived macrophages (BMDMs)—a critical distinction when trying to study the heterogenous mix of CNS-native microglia/macrophages and bone marrow-derived myeloid cells that have infiltrated the CNS in the disease state(91, 138, 139).
Role for microglia/macrophages in BrM
In vitro data suggest invasion of BrM-initiating cells can be rapidly sensed by microglia, and the presence of a single tumor cell is sufficient to activate and recruit microglia(102, 140). Both microglia as well as BMDMs have been shown to infiltrate and persist within malignant brain lesions and undergo unique, cell-specific tumor education that results in a tumor supportive phenotype (figure 5A-F)(139). Interestingly, most tumor associated macrophages (TAMs) in brain metastases appear to be derived from peripheral monocytes and not from resident microglia(139, 141). Microglia/macrophages appear to polarize in highly context dependent manner along a continuum between two extremes(141). One extreme, commonly referred to as “M1”, is defined as a pro-inflammatory phenotype characterized by increased levels of inflammatory cytokines and the ability to elicit a T cell-mediated anti-tumor response. Another extreme, commonly referred to as “M2”, is defined as an anti-inflammatory phenotype which promotes angiogenesis and tumor growth(141). Work by Andreou et al. has shown inducible nitric oxide (iNOS) and cyclooxygenase-2 (COX2) to be surface markers for a microglial pro-inflammatory state, and mannose receptor c-type 1 (MRC1) and arginase 1 (ARG1) to denote anti-inflammatory state in a murine model of breast cancer-derived BrM(141). Recent work by Gulder et al. used single cell profiling technologies to reveal that CNS-native myeloid cells appear to support BrM outgrowth by driving an immunosuppressive TME via CXCL10 signaling and that this contribution dominates compared to that of BMDMs(142). Other mechanisms that promote microglia/BMDM mediated tumor growth include secretion of immunosuppressive factors, decreased cytotoxic activity, TNF, iNOS expression(143).
Microglia as therapeutic targets
Colony-stimulating factor-1 receptor and its cognate ligand (CSF1/CSF1R) have been shown to regulate macrophage survival, proliferation, differentiation, chemotaxis, and signaling through this axis appears to mediate TAM recruitment and survival(144). Pyonteck et al. demonstrated that inhibition of macrophage/microglia via CSF1R inhibition with BLZ945 in a murine model of glioblastoma resulted in increased survival and tumor regression (figure 5A-F). Interestingly, inhibition led to microglial—but not macrophage—depletion(145). Further, CSF1R inhibition led to a loss of M2 marker expression suggesting at least partial re-polarization towards the pro-inflammatory, anti-tumor M1 state. In a murine melanoma orthotopic BrM model, CSF1R inhibition with PLX3397 was shown to decrease BrM initiation and lead to reduced tumor burden(140). A phase I/II clinical trial (NCT02452424) investigating the combination of PLX3397 and pembrolizumab in a cohort of patients with metastatic melanoma and other solids tumors was unfortunately terminated early due to lack of efficacy underscoring the challenge of translating findings from murine models to humans.
The fractalkine receptor (CX3CR1) has been shown to be unique to microglia and modulates microglial response to neuronal injury(132). Guldner et al. found that CNS-myeloid cells in brain metastases uniquely downregulated CX3CR1 leading to increased CXCL10 resulting in recruitment of PD1- and V-domain Ig suppressor of T cell activation (VISTA)-expressing cells and a shift towards an immunosuppressive program(142). Targeting of PD1 and VISTA resulted in impaired BrM outgrowth paving the way for potential future therapeutic strategies that may be translatable clinically.
Blazquez et al. found a cohort of human breast cancer-derived brain metastases to be enriched in phosphoinositide-3 kinase (PI3K) signaling activity and that PI3K was a master regulator of metastasis-promoting microglia/macrophages(146). BrM outgrowth in mice was impaired when using a CNS-penetrant small molecule inhibitor of PI3K. The promise of targeting PI3K in the CNS is further supported by the high frequency of PI3K alterations observed in human BrM and the availability of CNS-penetrant PI3K inhibitors(76, 147). Clinical trials investigating PI3K as a putative target in patients with BrM are ongoing (NCT03994796, NCT04192981). Finally, Andreou et al. found in a murine breast cancer orthotopic BrM model that selectively depleting anti-inflammatory/M2 microglial cells with mannosylated clodronate liposomes resulted in decreased BrM burden. This finding suggests that targeting microglia polarized to a tumor supportive state may be a viable therapeutic strategy(141). Further work should focus on identifying specific targets of the anti-inflammatory microglial phenotype and corresponding high affinity small molecule inhibitors.
Conclusion
BrM is a devastating and increasingly common complication in patients with cancer. While ICI has revolutionized the treatment of various cancers, the determinants of response remain incompletely understood, especially within the CNS. Increased understanding of the unique anatomical, cellular and immune architecture of the CNS is paramount in developing novel CNS-directed immunotherapy strategies. Critical roles for astrocytes and microglia have emerged in helping drive growth of BrM as well as interacting with the innate and adaptive immune system. There is substantial pre-clinical evidence that these glial cells may be rationale targets for treating BrM and/or enhancing anti-tumor immunity in patients treated with ICI.
Translation of promising therapeutic strategies based on pre-clinical in vitro and in vivo work does remain a major challenge(148). Specifically, the identification of therapeutic targets that may sensitize a host to ICI depends on an intact immune system. Therefore, data from syngeneic murine models is difficult to generalize to humans given interspecies differences in innate and adaptive immune responses. To this end, patient-derived organotypic spheroid (PDOTS) models that allow for ex vivo studies with an intact immune infiltrate are an exciting tool currently in use(149). Development of such a model with viable astrocytes and microglia would offer an opportunity to rapidly translate understanding of glial cell, BrM and TIME interactions into early phase clinical trials. Another model system that may be advantageous is immunodeficient mice engrafted with human immune cells or tissues i.e., “human immune system (HIS) mice” for which development is ongoing(150) Additionally, a significant portion of the current pre-clinical evidence for targeting glial cells comes with the use of already approved agents, thus providing immediate opportunity to develop clinical trials studying agents with astrocyte-specific activity. With implementation of clinical trials investigating therapies with potential CNS-activity, ongoing refinement of existing model systems and increasingly available tools such as single cell RNA sequencing to combat tissue heterogeneity, the future is promising for therapies that will effectively unlock anti-tumor immunity in the CNS.
Statement of significance.
There is an urgent need to improve patient selection for and clinical activity of immune checkpoint inhibitors in cancer patients with concomitant brain metastases. Increased understanding of the unique immunobiological principles that govern response to immune checkpoint inhibitors in the central nervous system is critical towards identifying targets in the tumor microenvironment that may potentiate anti-tumor immunity.
Acknowledgments:
PKB receives funding from National Institutes of Health (5R01CA227156-021, R21CA220253-0A01 and 1R01CA244975-01), Damon Runyon Cancer Research Foundation, the Ben and Catherine Ivy Foundation, Breast Cancer Research Foundation, Demetra fund of the Hellenic Women’s Association and the Terry and Jean de Gunzburg MGH Research Scholar Award.
Footnotes
Disclosures
MRS and CAB have no disclosures.
JFG has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech/Roche, Ariad/Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, Moderna, AstraZeneca, Pfizer, Novartis, iTeos, Nuvalent, Karyopharm, Beigene, Silverback Therapeutics, Merck, and GlydeBio; research support from Novartis, Genentech/Roche, and Ariad/Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. PKB has consulted for Angiochem, Genentech-Roche, Lilly, Tesaro, Voyager Therapeutics, ElevateBio, Pfizer (Array), Pfizer, SK Life Sciences and Dantari, received grant/research support (to Massachusetts General Hospital) from Merck, BMS, Mirati and Lilly and honoraria from Merck, Pfizer, Genentech-Roche and Lilly.
References
- 1.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barajas RF, Jr., Cha S. Imaging diagnosis of brain metastasis. Prog Neurol Surg. 2012;25:55–73. [DOI] [PubMed] [Google Scholar]
- 3.Svokos KA, Salhia B, Toms SA. Molecular biology of brain metastasis. Int J Mol Sci. 2014;15(6):9519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 5.Lauko A, Rauf Y, Ahluwalia MS. Medical management of brain metastases. Neuro-Oncology Advances. 2020;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. Journal of Clinical Oncology. 2004;22(14):2865–72. [DOI] [PubMed] [Google Scholar]
- 7.Berghoff AS, Schur S, Fureder LM, Gatterbauer B, Dieckmann K, Widhalm G, et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open. 2016;1(2):e000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117(11):2505–12. [DOI] [PubMed] [Google Scholar]
- 9.Christensen TD, Spindler K-LG, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer—Incidence and patient characteristics. BMC Cancer. 2016;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5. [DOI] [PubMed] [Google Scholar]
- 11.D’Andrea G, Palombi L, Minniti G, Pesce A, Marchetti P. Brain Metastases: Surgical Treatment and Overall Survival. World Neurosurg. 2017;97:169–77. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Sun H, Yakisich JS. Overcoming the blood-brain barrier for chemotherapy: limitations, challenges and rising problems. Anticancer Agents Med Chem. 2014;14(8):1085–93. [DOI] [PubMed] [Google Scholar]
- 13.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. The Lancet Oncology. 2016;17(7):976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–81. [DOI] [PubMed] [Google Scholar]
- 16.Tawbi HA, Chung C, Margolin K. Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379(22):2178. [DOI] [PubMed] [Google Scholar]
- 17.Brastianos PK, Lee EQ, Cohen JV, Tolaney SM, Lin NU, Wang N, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nature Medicine. 2020;26(8):1280–4. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quail DF, Joyce JA. The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 2017;31(3):326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twomey JD, Zhang B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. The AAPS Journal. 2021;23(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel RR, Verma V, Miller AB, Lin TA, Jethanandani A, Espinoza AF, et al. Exclusion of patients with brain metastases from cancer clinical trials. Neuro Oncol. 2020;22(4):577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. The Lancet Oncology. 2020;21(5):655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. Journal of Clinical Oncology. 2019;37(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–65. [DOI] [PubMed] [Google Scholar]
- 25.Tawbi HA, Forsyth PA, Hodi FS, Lao CD, Moschos SJ, Hamid O, et al. Safety and Efficacy of the Combination of Nivolumab Plus Ipilimumab in Patients With Melanoma and Asymptomatic or Symptomatic Brain Metastases (CheckMate 204). Neuro-Oncology. 2021;23(11):1961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long GV, Atkinson VG, Lo S, Sandhu SK, Brown M, Gonzalez M, Guminski A, Scolyer RA, Emmet L, Menzies AM, McArthur GA 13110-Long term outcomes from the randomized phase II study of nivolumab (nivo) or nivo + ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets): Anti-PD1 brain collaboration (ABC). Annals of Oncology. 2019;30:534. [Google Scholar]
- 27.Amin S, Baine MJ, Meza JL, Lin C. Association of Immunotherapy With Survival Among Patients With Brain Metastases Whose Cancer Was Managed With Definitive Surgery of the Primary Tumor. JAMA Network Open. 2020;3(9):e2015444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma. JAMA Oncology. 2020;6(7):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim R, Keam B, Kim S, Kim M, Kim SH, Kim JW, et al. Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: therapeutic implications for immune checkpoint inhibitors. BMC Cancer. 2019;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers. 2020;12(3):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessurun CAC, Hulsbergen AFC, de Wit AE, Tewarie IA, Snijders TJ, Verhoeff JJC, et al. The combined use of steroids and immune checkpoint inhibitors in brain metastasis patients: a systematic review and meta-analysis. Neuro Oncol. 2021;23(8):1261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell R, Luksik AS, Garzon-Muvdi T, Hung AL, Kim ES, Wu A, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. OncoImmunology. 2018;7(12):e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks PD, Lasocki A, Lau PKH, Sandhu S, McArthur G, Shackleton M. Bevacizumab as a steroid-sparing agent during immunotherapy for melanoma brain metastases: A case series. Health Science Reports. 2019;2(3):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascha MS, Wang JF, Kumthekar P, Sloan AE, Kruchko C, Barnholtz-Sloan JS. Bevacizumab for the treatment of non-small cell lung cancer patients with synchronous brain metastases. Scientific Reports. 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berghoff AS, Breckwoldt MO, Riedemann L, Karimian-Jazi K, Loew S, Schlieter F, et al. Bevacizumab-based treatment as salvage therapy in patients with recurrent symptomatic brain metastases. Neuro-Oncology Advances. 2020;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III Randomized Trial Comparing the Efficacy of Cediranib As Monotherapy, and in Combination With Lomustine, Versus Lomustine Alone in Patients With Recurrent Glioblastoma. Journal of Clinical Oncology. 2013;31(26):3212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpentier AF, Ferrari D, Bailon O, Ursu R, Banissi C, Dubessy AL, et al. Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur J Neurol. 2012;19(10):1337–42. [DOI] [PubMed] [Google Scholar]
- 38.Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, et al. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors. Cancer. 2011;117(16):3788–95. [DOI] [PubMed] [Google Scholar]
- 39.Recht L, Mechtler LL, Wong ET, O’Connor PC, Rodda BE. Steroid-Sparing Effect of Corticorelin Acetate in Peritumoral Cerebral Edema Is Associated With Improvement in Steroid-Induced Myopathy. Journal of Clinical Oncology. 2013;31(9):1182–7. [DOI] [PubMed] [Google Scholar]
- 40.Arvold ND, Armstrong TS, Warren KE, Chang SM, Deangelis LM, Blakeley J, et al. Corticosteroid use endpoints in neuro-oncology: Response Assessment in Neuro-Oncology Working Group. Neuro-Oncology. 2018;20(7):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirai Y. On the transplantation of the rat sarcoma in adult heterogenous animals. Jap Med World. 1921;1:14–5. [Google Scholar]
- 42.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 43.Mason DW, Charlton HM, Jones AJ, Lavy CB, Puklavec M, Simmonds SJ. The fate of allogeneic and xenogeneic neuronal tissue transplanted into the third ventricle of rodents. Neuroscience. 1986;19(3):685–94. [DOI] [PubMed] [Google Scholar]
- 44.Nicholas MK, Antel JP, Stefansson K, Arnason BG. Rejection of fetal neocortical neural transplants by H-2 incompatible mice. J Immunol. 1987;139(7):2275–83. [PubMed] [Google Scholar]
- 45.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. [DOI] [PubMed] [Google Scholar]
- 46.Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nature Neuroscience. 2012;15(8):1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mestre H, Mori Y, Nedergaard M. The Brain’s Glymphatic System: Current Controversies. Trends in Neurosciences. 2020;43(7):458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–31. [DOI] [PubMed] [Google Scholar]
- 49.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–16 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Research. 2020;30(3):229–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizeq B, Malki MI. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers. 2020;12(4):1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577(7792):689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, et al. Anti–PD-1/anti–CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proceedings of the National Academy of Sciences. 2018;115(7):E1540–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung M, Yang Y, McCloskey JE, Zaman M, Vedvyas Y, Zhang X, et al. Chimeric Antigen Receptor T Cell Therapy Targeting ICAM-1 in Gastric Cancer. Molecular Therapy - Oncolytics. 2020;18:587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min IM, Shevlin E, Vedvyas Y, Zaman M, Wyrwas B, Scognamiglio T, et al. CAR T Therapy Targeting ICAM-1 Eliminates Advanced Human Thyroid Tumors. Clinical Cancer Research. 2017;23(24):7569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rauschenberg R, Bruns J, Brutting J, Daubner D, Lohaus F, Zimmer L, et al. Impact of radiation, systemic therapy and treatment sequencing on survival of patients with melanoma brain metastases. Eur J Cancer. 2019;110:11–20. [DOI] [PubMed] [Google Scholar]
- 59.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67(12):1113–21. [DOI] [PubMed] [Google Scholar]
- 61.Schulz M, Salamero-Boix A, Niesel K, Alekseeva T, Sevenich L. Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis. Front Immunol. 2019;10:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu SY, Watabe K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front Biosci (Landmark Ed). 2017;22:1805–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steeg PS. The blood–tumour barrier in cancer biology and therapy. Nature Reviews Clinical Oncology. 2021;18(11):696–714. [DOI] [PubMed] [Google Scholar]
- 65.Gril B, Palmieri D, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, et al. Pazopanib Inhibits the Activation of PDGFRβ-Expressing Astrocytes in the Brain Metastatic Microenvironment of Breast Cancer Cells. The American Journal of Pathology. 2013;182(6):2368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuhmann MK, Stoll G, Bieber M, Vögtle T, Hofmann S, Klaus V, et al. CD84 Links T Cell and Platelet Activity in Cerebral Thrombo-Inflammation in Acute Stroke. Circulation Research. 2020;127(8):1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feinauer MJ, Schneider SW, Berghoff AS, Robador JR, Tehranian C, Karreman MA, et al. Local blood coagulation drives cancer cell arrest and brain metastasis in a mouse model. Blood. 2021;137(9):1219–32. [DOI] [PubMed] [Google Scholar]
- 68.Mooney R, Hammad M, Batalla-Covello J, Abdul Majid A, Aboody KS. Concise Review: Neural Stem Cell-Mediated Targeted Cancer Therapies. Stem Cells Transl Med. 2018;7(10):740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mooney R, Majid AA, Batalla-Covello J, Machado D, Liu X, Gonzaga J, et al. Enhanced Delivery of Oncolytic Adenovirus by Neural Stem Cells for Treatment of Metastatic Ovarian Cancer. Mol Ther Oncolytics. 2019;12:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Priego N, Valiente M. The Potential of Astrocytes as Immune Modulators in Brain Tumors. Frontiers in Immunology. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 2015;6(38):40836–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. OncoImmunology. 2016;5(1):e1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berghoff AS, Ricken G, Wilhelm D, Rajky O, Widhalm G, Dieckmann K, et al. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). J Neurooncol. 2016;130(1):19–29. [DOI] [PubMed] [Google Scholar]
- 74.Kudo Y, Haymaker C, Zhang J, Reuben A, Duose DY, Fujimoto J, et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Annals of Oncology. 2019;30(9):1521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mansfield AS, Ren H, Sutor S, Sarangi V, Nair A, Davila J, et al. Contraction of T cell richness in lung cancer brain metastases. Scientific Reports. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015;5(11):1164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paik PK, Shen R, Won H, Rekhtman N, Wang L, Sima CS, et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discovery. 2015;5(6):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shih DJH, Nayyar N, Bihun I, Dagogo-Jack I, Gill CM, Aquilanti E, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020;52(4):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Therapy. 2021;28(1–2):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lesniak. Regulatory T cells actively infiltrate metastatic brain tumors. International Journal of Oncology. 2009;34(6). [DOI] [PubMed] [Google Scholar]
- 81.Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi JW, et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nature Communications. 2020;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nature Medicine. 2018;24(8):1192–203. [DOI] [PubMed] [Google Scholar]
- 83.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11(7):615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang D, Han Z, Oppenheim JJ. Alarmins and immunity. Immunological Reviews. 2017;280(1):41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353(6301):766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kigerl KA, De Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Experimental Neurology. 2014;258:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shastri A, Bonifati DM, Kishore U. Innate Immunity and Neuroinflammation. Mediators of Inflammation. 2013;2013:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 2017;168(6):1101–13.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. Journal of Clinical Investigation. 2012;122(4):1164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doron H, Pukrop T, Erez N. A Blazing Landscape: Neuroinflammation Shapes Brain Metastasis. Cancer Research. 2019;79(3):423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell. 2020;181(7):1643–60 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences. 2009;32(12):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nature Neuroscience. 2019;22(2):154–66. [DOI] [PubMed] [Google Scholar]
- 96.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46(6):957–67. [DOI] [PubMed] [Google Scholar]
- 97.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Reports. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelley KW, Nakao-Inoue H, Molofsky AV, Oldham MC. Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nature Neuroscience. 2018;21(9):1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell. 2018;174(4):1015–30 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic Analysis of Reactive Astrogliosis. Journal of Neuroscience. 2012;32(18):6391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valiente M, Anna, Jin X, Chen Q, Xiang, Derek, et al. Serpins Promote Cancer Cell Survival and Vascular Co-Option in Brain Metastasis. Cell. 2014;156(5):1002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, et al. Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis. Cell Reports. 2019;28(7):1785–98.e6. [DOI] [PubMed] [Google Scholar]
- 104.Wasilewski D, Priego N, Fustero-Torre C, Valiente M. Reactive Astrocytes in Brain Metastasis. Frontiers in Oncology. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clinical & Experimental Metastasis. 2008;25(7):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13(3):286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia. 2010;12(9):748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim SW, Choi HJ, Lee H-J, He J, Wu Q, Langley RR, et al. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro-Oncology. 2014;16(12):1585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choy C, Ansari KI, Neman J, Hsu S, Duenas MJ, Li H, et al. Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases. Breast Cancer Research. 2017;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Molecular Medicine. 2013;5(3):384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klein A, Schwartz H, Sagi-Assif O, Meshel T, Izraely S, Ben Menachem S, et al. Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. J Pathol. 2015;236(1):116–27. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24(7):1024–35. [DOI] [PubMed] [Google Scholar]
- 115.Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frik J, Merl-Pham J, Plesnila N, Mattugini N, Kjell J, Kraska J, et al. Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Rep. 2018;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X, Haroon F, Karray S, Martina D, Schluter D. Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. Eur J Immunol. 2013;43(1):115–24. [DOI] [PubMed] [Google Scholar]
- 118.Gimsa U, A OR, Pandiyan P, Teichmann D, Bechmann I, Nitsch R, et al. Astrocytes protect the CNS: antigen-specific T helper cell responses are inhibited by astrocyte-induced upregulation of CTLA-4 (CD152). J Mol Med (Berl). 2004;82(6):364–72. [DOI] [PubMed] [Google Scholar]
- 119.Schachtele SJ, Hu S, Sheng WS, Mutnal MB, Lokensgard JR. Glial cells suppress postencephalitic CD8+ T lymphocytes through PD-L1. Glia. 2014;62(10):1582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarmiento Soto M, Larkin JR, Martin C, Khrapitchev AA, Maczka M, Economopoulos V, et al. STAT3-Mediated Astrocyte Reactivity Associated with Brain Metastasis Contributes to Neurovascular Dysfunction. Cancer Research. 2020;80(24):5642–55. [DOI] [PubMed] [Google Scholar]
- 121.Lee HJ, Hanibuchi M, Kim SJ, Yu H, Kim MS, He J, et al. Treatment of experimental human breast cancer and lung cancer brain metastases in mice by macitentan, a dual antagonist of endothelin receptors, combined with paclitaxel. Neuro Oncol. 2016;18(4):486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gril B, Palmieri D, Qian Y, Smart D, Ileva L, Liewehr DJ, et al. Pazopanib Reveals a Role for Tumor Cell B-Raf in the Prevention of HER2+ Breast Cancer Brain Metastasis. Clinical Cancer Research. 2011;17(1):142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. [DOI] [PubMed] [Google Scholar]
- 124.Van Der Graaf WT, Blay J-Y, Chawla SP, Kim D-W, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2012;379(9829):1879–86. [DOI] [PubMed] [Google Scholar]
- 125.Gooch ME, Nader K, Kubicek GJ, Somer RA. Brain Metastasis Responsive to Pazopanib in Renal Cell Carcinoma: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2016;14(4):e401–4. [DOI] [PubMed] [Google Scholar]
- 126.Hingorani M, Dixit S, Maraveyas A. Pazopanib-Induced Regression of Brain Metastasis After Whole Brain Palliative Radiotherapy in Metastatic Renal Cell Cancer Progressing on First-Line Sunitinib: A Case Report. World Journal of Oncology. 2014;5(5–6):223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–62. [DOI] [PubMed] [Google Scholar]
- 128.de Groot CJ, Huppes W, Sminia T, Kraal G, Dijkstra CD. Determination of the origin and nature of brain macrophages and microglial cells in mouse central nervous system, using non-radioactive in situ hybridization and immunoperoxidase techniques. Glia. 1992;6(4):301–9. [DOI] [PubMed] [Google Scholar]
- 129.Weinhard L, Di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Communications. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. [DOI] [PubMed] [Google Scholar]
- 131.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94. [DOI] [PubMed] [Google Scholar]
- 132.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–24. [DOI] [PubMed] [Google Scholar]
- 133.Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204(3):571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity. 2018;48(3):599. [DOI] [PubMed] [Google Scholar]
- 135.Sankowski R, Bottcher C, Masuda T, Geirsdottir L, Sagar, Sindram E, et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci. 2019;22(12):2098–110. [DOI] [PubMed] [Google Scholar]
- 136.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11(3):335–9. [DOI] [PubMed] [Google Scholar]
- 137.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20(2):136–44. [DOI] [PubMed] [Google Scholar]
- 138.Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, et al. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron. 2018;98(6):1170–83 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep. 2016;17(9):2445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qiao S, Qian Y, Xu G, Luo Q, Zhang Z. Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. Journal of Neuroinflammation. 2019;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andreou KE, Soto MS, Allen D, Economopoulos V, De Bernardi A, Larkin JR, et al. Anti-inflammatory Microglia/Macrophages As a Potential Therapeutic Target in Brain Metastasis. Frontiers in Oncology. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guldner IH, Wang Q, Yang L, Golomb SM, Zhao Z, Lopez JA, et al. CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10. Cell. 2020;183(5):1234–48 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vilariño N, Bruna J, Bosch-Barrera J, Valiente M, Nadal E. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treatment Reviews. 2020;89:102067. [DOI] [PubMed] [Google Scholar]
- 144.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blazquez R, Wlochowitz D, Wolff A, Seitz S, Wachter A, Perera-Bel J, et al. PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. Glia. 2018;66(11):2438–55. [DOI] [PubMed] [Google Scholar]
- 147.Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019;21(11):1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–8. [PMC free article] [PubMed] [Google Scholar]
- 149.Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, et al. Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018;8(2):196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Allen TM, Brehm MA, Bridges S, Ferguson S, Kumar P, Mirochnitchenko O, et al. Humanized immune system mouse models: progress, challenges and opportunities. Nature Immunology. 2019;20(7):770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]