Abstract
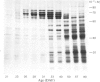
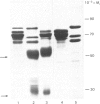
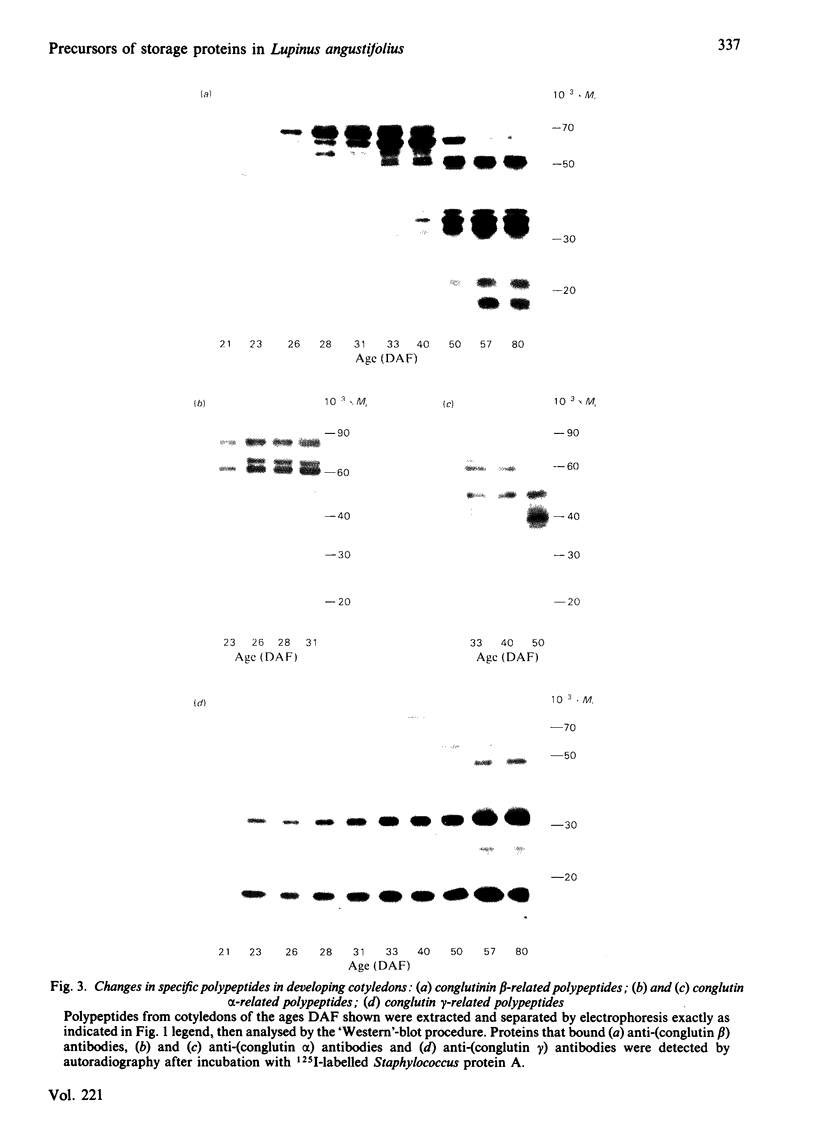
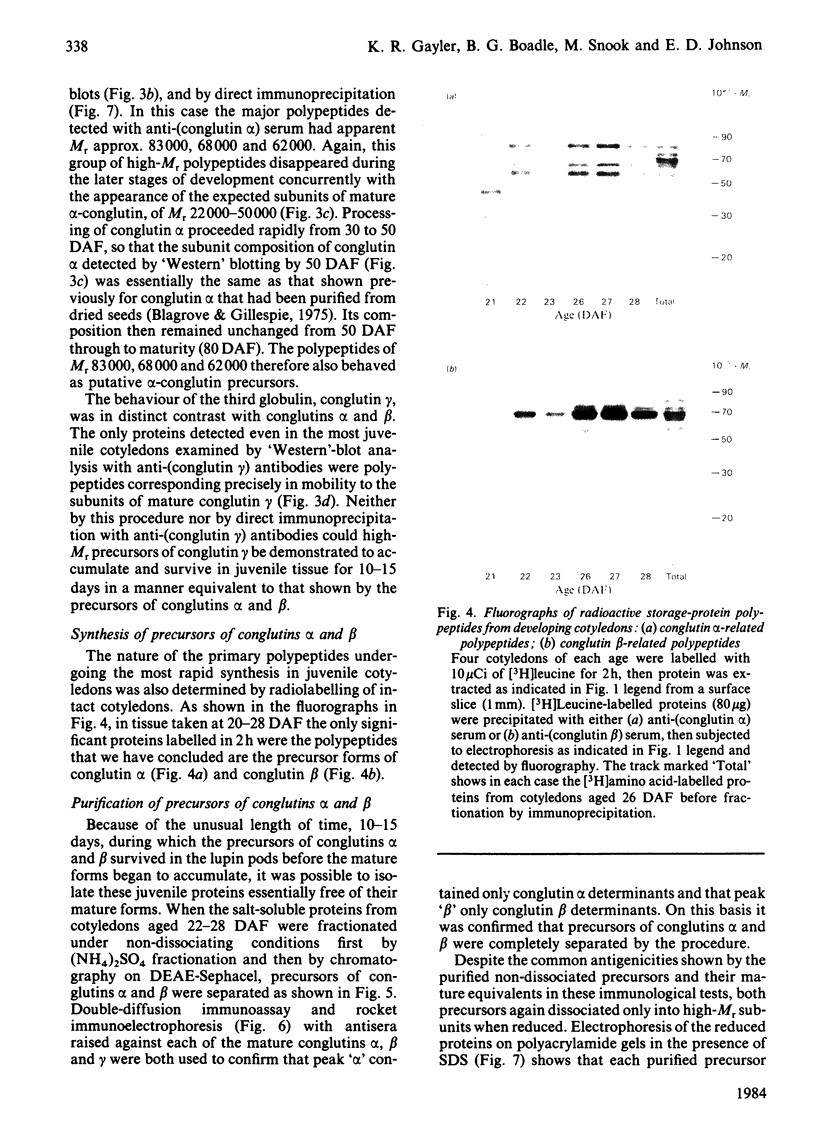
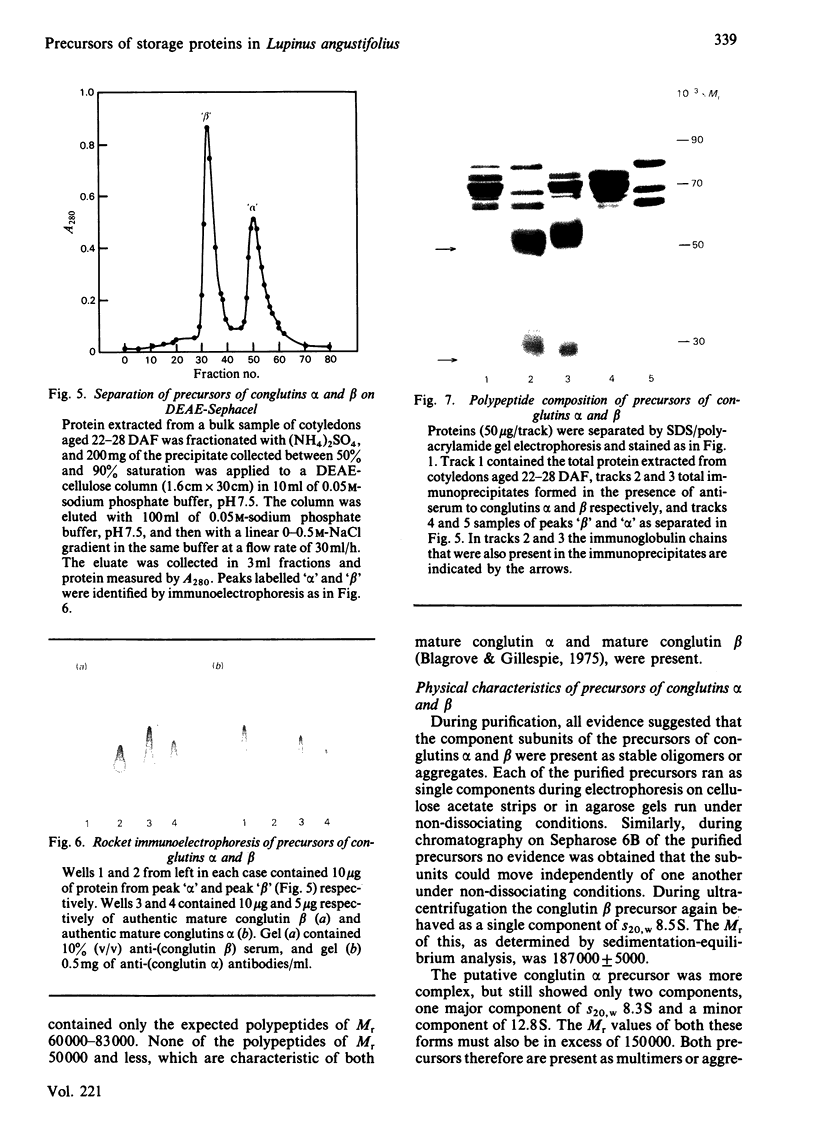
The proteins that are synthesized during differentiation and development in the cotyledons of Lupinus angustifolius L. were characterized both in situ and after purification. The proteins present in situ were separated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and subjected to 'Western'-blot analysis to identify immunologically related polypeptides. The major storage proteins of the lupin, conglutins alpha and beta, were both present in juvenile tissue only as higher Mr precursors. For conglutin beta, a family of at least three polypeptides of Mr 66 000-72 000 accumulated during the earliest phases of protein synthesis in the developing cotyledon (20-28 days after flowering). Later in development each of these polypeptides disappeared and there was the concurrent appearance in the cotyledon of the lower-Mr fragments characteristic of mature conglutin beta. For conglutin alpha, an equivalent family of precursor polypeptides of Mr 60 000-83 000 was detected. Multiple internal sites for proteolytic cleavage of all these precursors appeared to be present. However, processing of the precursors was sufficiently slow to allow them to accumulate to over 50% of total soluble protein in juvenile tissue. The precursors were purified by column chromatography under non-dissociating conditions and shown by ultracentrifugation to be multimeric proteins with Mr values in the range 150 000-200 000.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley R. A., Atkinson D., Hauser H., Oldani D., Green J. P., Stubb J. M. The structure, physical and chemical properties of the soy bean protein glycinin. Biochim Biophys Acta. 1975 Dec 15;412(2):214–228. doi: 10.1016/0005-2795(75)90036-7. [DOI] [PubMed] [Google Scholar]
- Barton K. A., Thompson J. F., Madison J. T., Rosenthal R., Jarvis N. P., Beachy R. N. The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J Biol Chem. 1982 Jun 10;257(11):6089–6095. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Casey R. Immunoaffinity chromatography as a means of purifying legumin from Pisum (pea) seeds. Biochem J. 1979 Feb 1;177(2):509–520. doi: 10.1042/bj1770509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Higgins T. J., Randall P. J., Spencer D. Regulation of Legumin Levels in Developing Pea Seeds under Conditions of Sulfur Deficiency: Rates of Legumin Synthesis and Levels of Legumin mRNA. Plant Physiol. 1983 Jan;71(1):47–54. doi: 10.1104/pp.71.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereken-Tumer N., Richter J. D., Nielsen N. C. Structural characterization of the glycinin precursors. J Biol Chem. 1982 Apr 25;257(8):4016–4018. [PubMed] [Google Scholar]
- Gatehouse J. A., Croy R. R., Morton H., Tyler M., Boulter D. Characterisation and subunit structures of the vicilin storage proteins of pea (Pisum sativum L.). Eur J Biochem. 1981 Sep 1;118(3):627–633. doi: 10.1111/j.1432-1033.1981.tb05565.x. [DOI] [PubMed] [Google Scholar]
- Gatehouse J. A., Evans I. M., Bown D., Croy R. R., Boulter D. Control of storage-protein synthesis during seed development in pea (Pisum sativum L.). Biochem J. 1982 Oct 15;208(1):119–127. doi: 10.1042/bj2080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse J. A., Lycett G. W., Croy R. R., Boulter D. The post-translational proteolysis of the subunits of vicilin from pea (Pisum sativum L.). Biochem J. 1982 Dec 1;207(3):629–632. doi: 10.1042/bj2070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse J. A., Lycett G. W., Delauney A. J., Croy R. R., Boulter D. Sequence specificity of the post-translational proteolytic cleavage of vicilin, a seed storage protein of pea (Pisum sativum L.). Biochem J. 1983 May 15;212(2):427–432. doi: 10.1042/bj2120427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayler K. R., Sykes G. E. beta-Conglycinins in Developing Soybean Seeds. Plant Physiol. 1981 May;67(5):958–961. doi: 10.1104/pp.67.5.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lycett G. W., Delauney A. J., Gatehouse J. A., Gilroy J., Croy R. R., Boulter D. The vicilin gene family of pea (Pisum sativum L.): a complete cDNA coding sequence for preprovicilin. Nucleic Acids Res. 1983 Apr 25;11(8):2367–2380. doi: 10.1093/nar/11.8.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Partial characterization of the acidic and basic polypeptides of glycinin. J Biol Chem. 1979 Oct 10;254(19):9921–9926. [PubMed] [Google Scholar]
- Pusztai A., Stewart J. C. Molecular size, subunit structure and microheterogeneity of glycoprotein II from the seeds of kidney bean (Phaseolus vulgaris L.). Biochim Biophys Acta. 1980 Jun 26;623(2):418–428. doi: 10.1016/0005-2795(80)90271-8. [DOI] [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes G. E., Gayler K. R. Detection and characterization of a new beta-conglycinin from soybean seeds. Arch Biochem Biophys. 1981 Sep;210(2):525–530. doi: 10.1016/0003-9861(81)90217-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]