Abstract
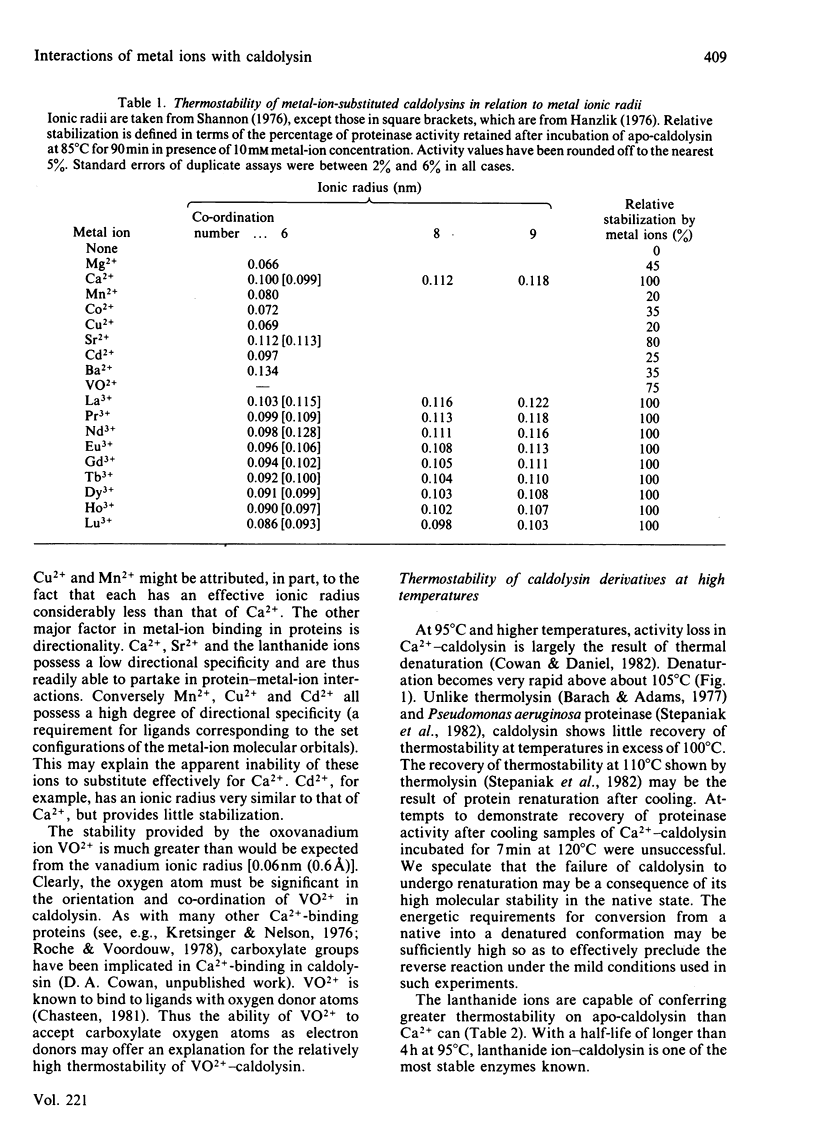
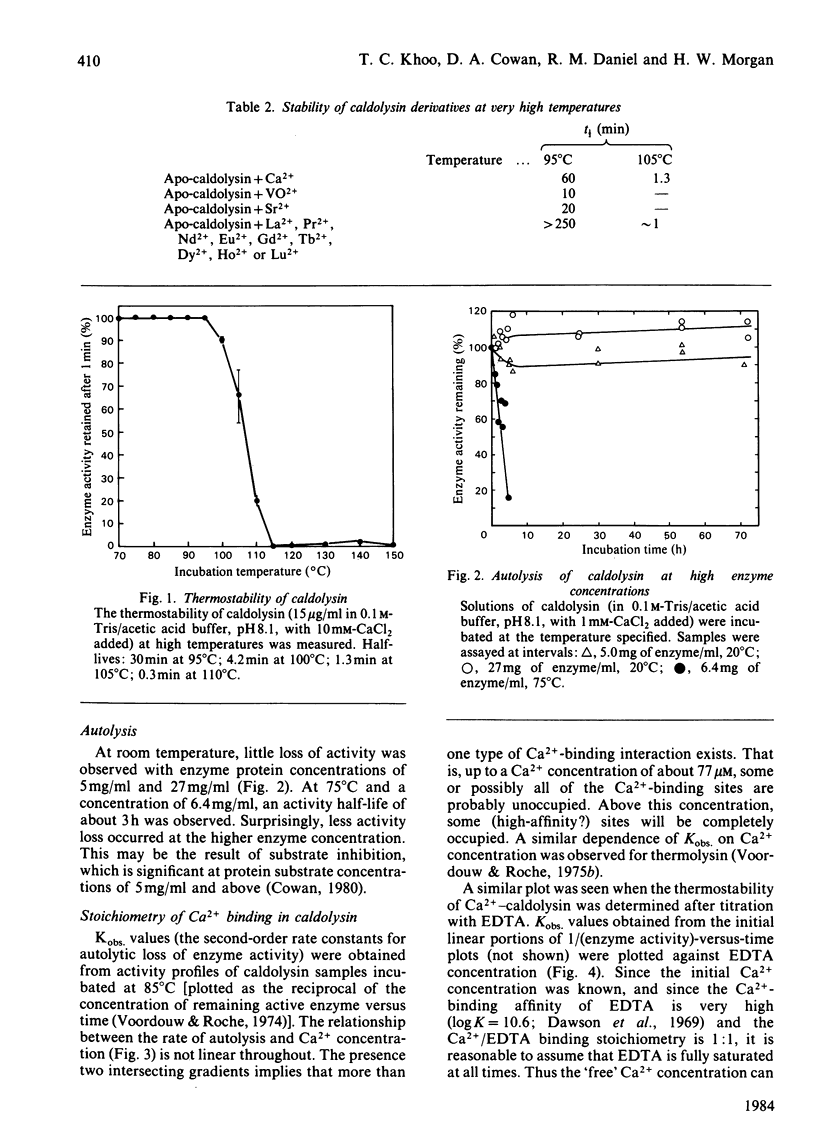
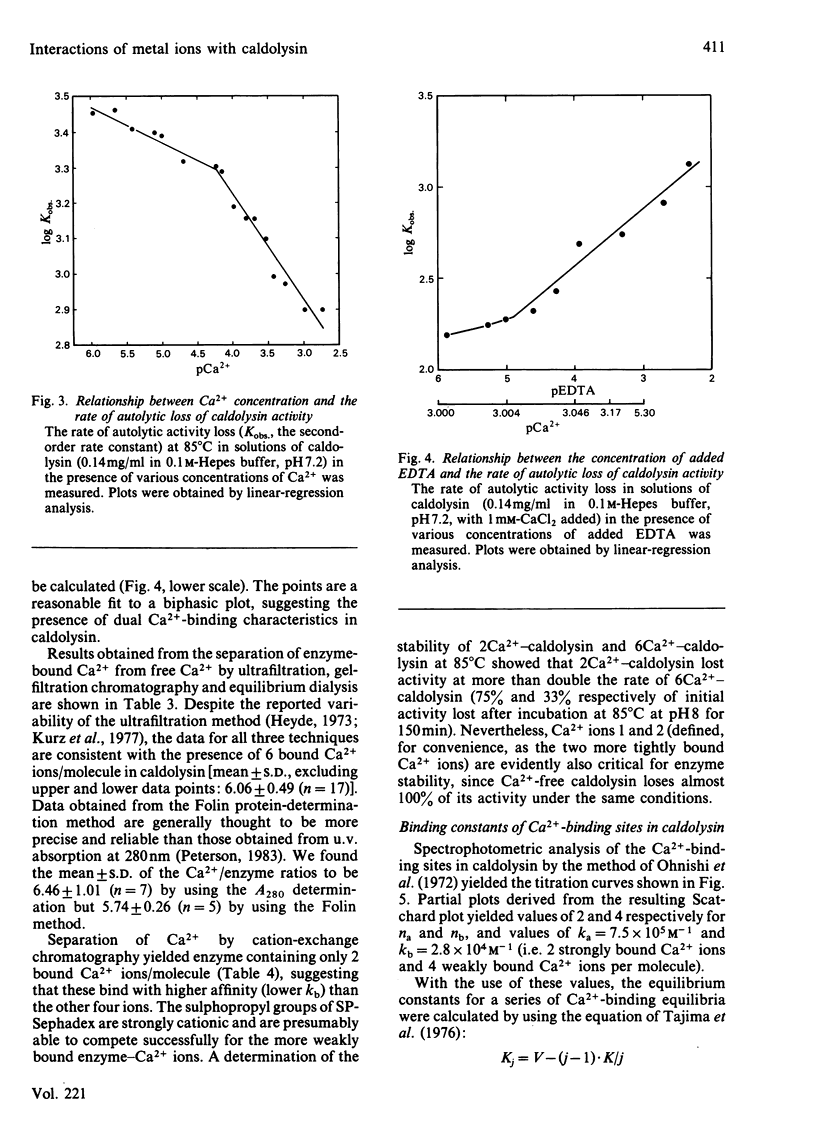
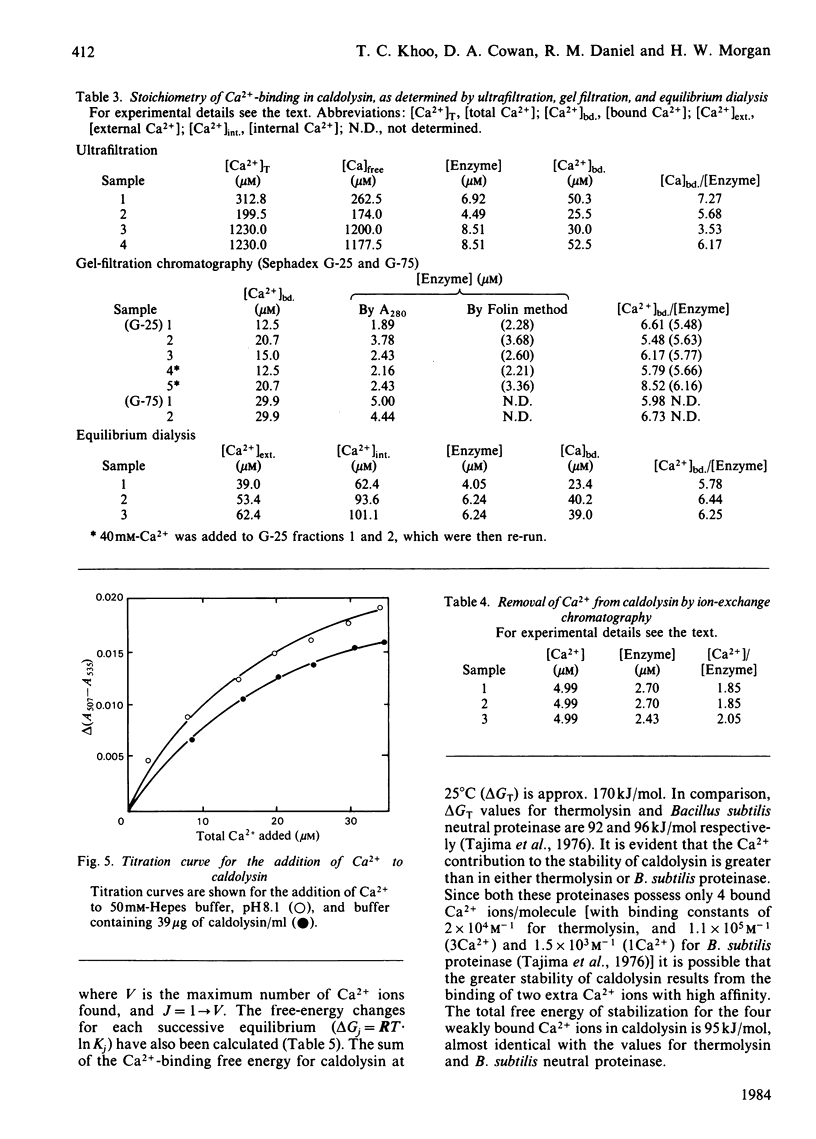
Caldolysin, the extracellular proteinase from the extreme thermophile Thermus aquaticus strain T351, is stabilized by Ca2+. A variety of metal ions were able to substitute for Ca2+. Most were unable to confer as much stability as Ca2+, with the exception of the lanthanide ions, which increased the half-life at 95 degrees C from 1 h to more than 4 h. Results from a variety of separation methods indicated that caldolysin binds 6 Ca2+ ions/molecule of enzyme. The presence of non-linear Ca2+ titration plots, and the removal of 4 Ca2+ ions/molecule by treatment with a cationic ion-exchange gel suggested that caldolysin possesses at least two different types of Ca2+-binding sites, with different affinities. Average binding constants of the two types of binding sites were 2.8 X 10(4)M-1 (for the low-affinity sites) and 7.5 X 10(5) M-1 (for the high-affinity sites). The total Ca2+-binding free energy for caldolysin was shown to be greater than for either thermolysin or Bacillus subtilis neutral proteinase. It appears that the higher thermostability of caldolysin is due to the presence of 6 Ca2+ ions rather than 4 Ca2+ ions/molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott F., Gomez J. E., Birnbaum E. R., Darnall D. W. The location of the calcium ion binding site in bovine alpha-trypsin and beta-trypsin using lanthanide ion probes. Biochemistry. 1975 Nov 4;14(22):4935–4943. doi: 10.1021/bi00693a024. [DOI] [PubMed] [Google Scholar]
- Barach J. T., Adams D. M. Thermostability at ultrahigh temperatures of thermolysin and a protease from a psychrotrophic Pseudomonas. Biochim Biophys Acta. 1977 Dec 8;485(2):417–423. doi: 10.1016/0005-2744(77)90177-2. [DOI] [PubMed] [Google Scholar]
- Cowan D. A., Daniel R. M. Purification and some properties of an extracellular protease (caldolysin) from an extreme thermophile. Biochim Biophys Acta. 1982 Aug 10;705(3):293–305. doi: 10.1016/0167-4838(82)90251-5. [DOI] [PubMed] [Google Scholar]
- Dorogi P. L., Neumann E. Spectrophotometric determination of reaction stoichiometry and equilibrium constants of metallochromic indicators. I. General calculational method. Biophys Chem. 1981 Apr;13(2):117–123. doi: 10.1016/0301-4622(81)80010-5. [DOI] [PubMed] [Google Scholar]
- Epstein M., Reuben J., Levitzki A. Calcium binding site of trypsin as probed by lanthanides. Biochemistry. 1977 May 31;16(11):2449–2457. doi: 10.1021/bi00630a021. [DOI] [PubMed] [Google Scholar]
- Feder J., Garrett L. R., Wildi B. S. Studies on the role of calcium in thermolysin. Biochemistry. 1971 Nov 23;10(24):4552–4556. doi: 10.1021/bi00800a032. [DOI] [PubMed] [Google Scholar]
- Frömmel C., Höhne W. E. Influence of calcium binding on the thermal stability of 'thermitase', a serine protease from Thermoactinomyces vulgaris. Biochim Biophys Acta. 1981 Aug 28;670(1):25–31. doi: 10.1016/0005-2795(81)90044-1. [DOI] [PubMed] [Google Scholar]
- HARNACH F., COOLIDGE T. B. DETERMINATION OF IONIZED CALCIUM IN SERUM WITH MUREXIDE. Anal Biochem. 1963 Dec;6:477–485. doi: 10.1016/0003-2697(63)90140-4. [DOI] [PubMed] [Google Scholar]
- HSIU J., FISCHER E. H., STEIN E. A. ALPHA-AMYLASES AS CALCIUM-METALLOENZYMES. II. CALCIUM AND THE CATALYTIC ACTIVITY. Biochemistry. 1964 Jan;3:61–66. doi: 10.1021/bi00889a011. [DOI] [PubMed] [Google Scholar]
- HUGHES T. R., KLOTZ I. M. Analysis of metal-protein complexes. Methods Biochem Anal. 1956;3:265–299. doi: 10.1002/9780470110195.ch9. [DOI] [PubMed] [Google Scholar]
- Heinen W., Lauwers A. M. Amylase activity and stability at high and low temperature depending on calcium and other divalent cations. Experientia Suppl. 1976;26:77–89. doi: 10.1007/978-3-0348-7675-9_7. [DOI] [PubMed] [Google Scholar]
- Heyde E. Difficulties encountered with an ultrafiltration method for measuring the binding of ligands to protein. Anal Biochem. 1973 Jan;51(1):61–66. doi: 10.1016/0003-2697(73)90452-1. [DOI] [PubMed] [Google Scholar]
- Kurz H., Trunk H., Weitz B. Evaluation of methods to determine protein-binding of drugs. Equilibrium dialysis, ultrafiltration, ultracentrifugation, gel filtration. Arzneimittelforschung. 1977 Jul;27(7):1373–1380. [PubMed] [Google Scholar]
- Kwak J. C., Joshi Y. M. The binding of divalent metal ions to polyelectrolytes in mixed counterion systems. I. The dye spectrophotometric method. Biophys Chem. 1981 Feb;13(1):55–64. doi: 10.1016/0301-4622(81)80025-7. [DOI] [PubMed] [Google Scholar]
- Onishi T., Masoro E. J., Bertrand H. A., Yu B. P. Analysis of a calcium ion binding system composed of two different sites. Biophys J. 1972 Oct;12(10):1251–1265. doi: 10.1016/s0006-3495(72)86160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Roche R. S., Voordouw G. The structural and functional roles of metal ions in thermolysin. CRC Crit Rev Biochem. 1978;5(1):1–23. doi: 10.3109/10409237809177138. [DOI] [PubMed] [Google Scholar]
- Russin D. J., Floyd B. F., Toomey T. P., Brady A. H., Awad W. M., Jr The proteolytic enzymes of the K-1 strain of Steptomyces griseus obtained from a commerical preparation (pronase). Stabilization of the trypsin component by calcium and guanidine. J Biol Chem. 1974 Oct 10;249(19):6144–6148. [PubMed] [Google Scholar]
- Stauffer C. E. A linear standard curve for the Folin Lowry determination of protein. Anal Biochem. 1975 Dec;69(2):646–648. doi: 10.1016/0003-2697(75)90172-4. [DOI] [PubMed] [Google Scholar]
- Stepaniak L., Fox P. F., Daly C. Isolation and general characterization of a heat-stable proteinase from Pseudomonas fluorescens aft 36. Biochim Biophys Acta. 1982 Aug 6;717(2):376–383. doi: 10.1016/0304-4165(82)90192-1. [DOI] [PubMed] [Google Scholar]
- Switzer M. E. The lanthanide ions as probes of calcium ion binding sites in biological systems. Sci Prog. 1978 Spring;65(257):19–30. [PubMed] [Google Scholar]
- Tajima M., Urabe I., Yutani K., Okada H. Role of calcium ions in the thermostability of thermolysin and Bacillus subtilis var. amylosacchariticus neutral protease. Eur J Biochem. 1976 Apr 15;64(1):243–247. doi: 10.1111/j.1432-1033.1976.tb10293.x. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L., STEIN E. A., SUMERWELL W. N., FISCHER E. H. Metal content of alpha-amylases of various origins. J Biol Chem. 1959 Nov;234:2901–2905. [PubMed] [Google Scholar]
- Voordouw G., Milo C., Roche R. S. Role of bound calcium ions in thermostable, proteolytic enzymes. Separation of intrinsic and calcium ion contributions to the kinetic thermal stability. Biochemistry. 1976 Aug 24;15(17):3716–3724. doi: 10.1021/bi00662a012. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Milo C., Roche R. S. The determination of the binding constant of metalloenzymes for their active site metal ion from ligand inhibition data. Theoretical analysis and application to the inhibition of thermolysin by 1,10-phenanthroline. Anal Biochem. 1976 Feb;70(2):313–326. doi: 10.1016/0003-2697(76)90452-8. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Roche R. S. The cooperative binding of two calcium ions to the double site of apothermolysin. Biochemistry. 1974 Nov 19;13(24):5017–5021. doi: 10.1021/bi00721a024. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Roche R. S. The role of bound calcium ions in thermostable, proteolytic enzymes. I. Studies on thermomycolase, the thermostable protease fron the fungus Malbranchea pulchella. Biochemistry. 1975 Oct 21;14(21):4659–4666. doi: 10.1021/bi00692a015. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Roche R. S. The role of bound calcium ions in thermostable, proteolytic enzymes. II. Studies on thermolysin, the thermostable protease from Bacillus thermoproteolyticus. Biochemistry. 1975 Oct 21;14(21):4667–4673. doi: 10.1021/bi00692a016. [DOI] [PubMed] [Google Scholar]


