Abstract
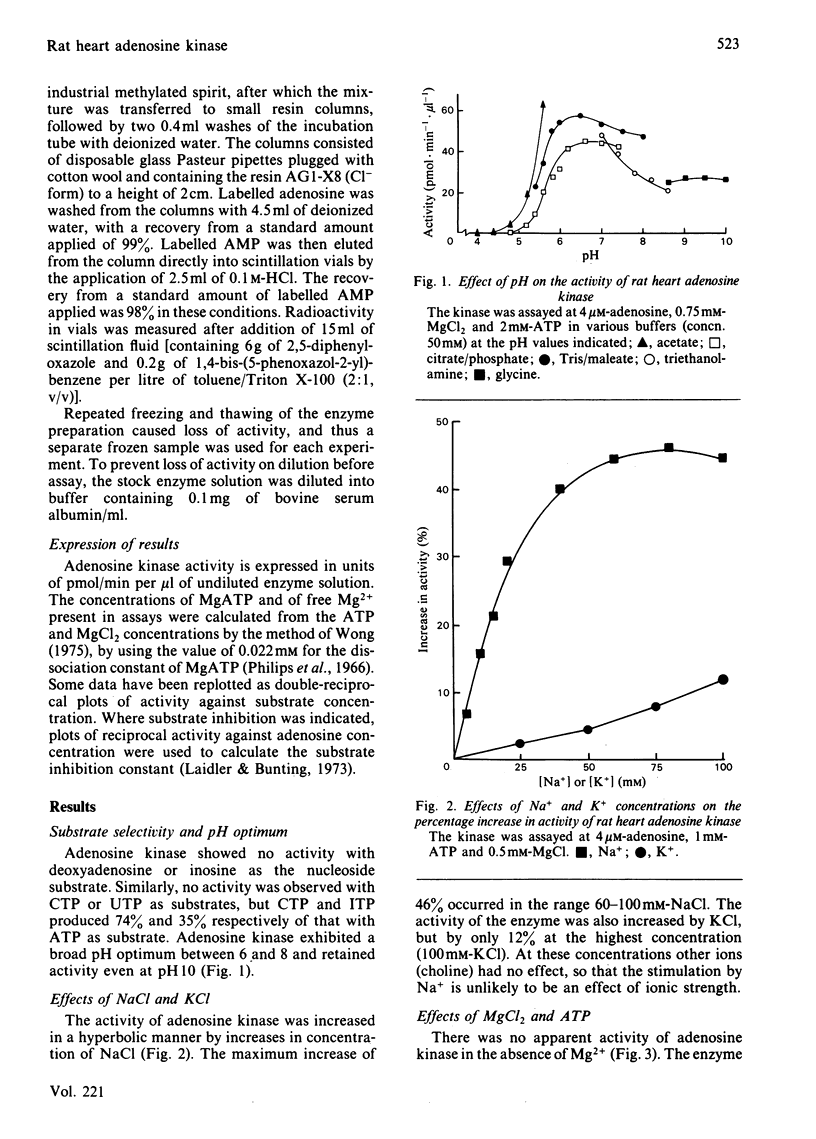
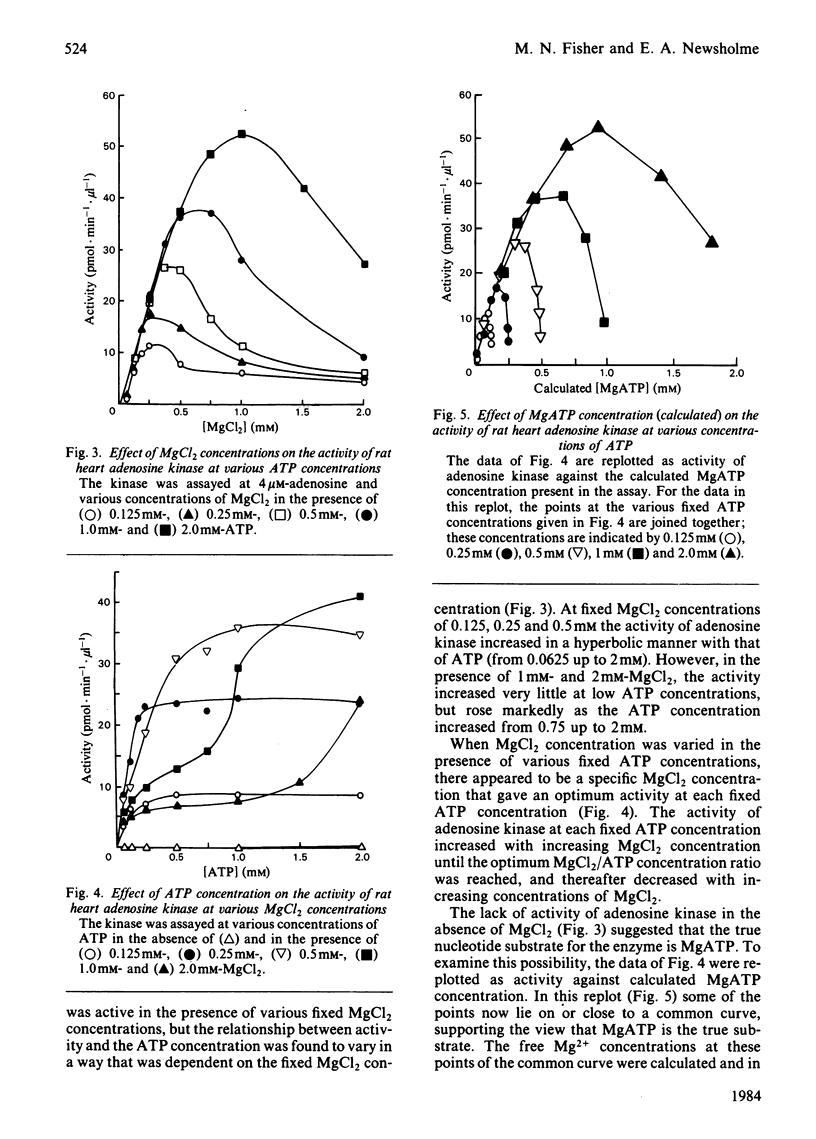
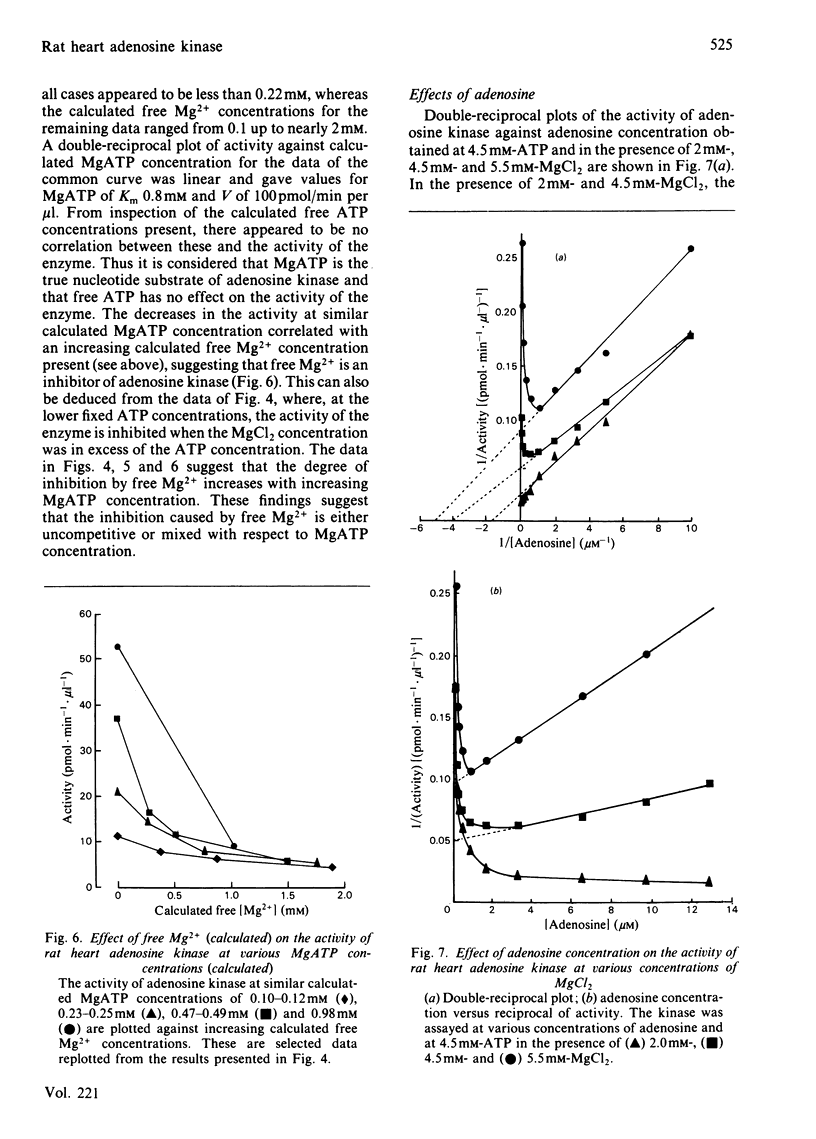
Adenosine kinase was purified 870-fold from rat heart by a combination of gel filtration and affinity chromatography. The preparation was free of purine-metabolizing enzymes that could interfere in the assay of the kinase. A study of the properties of the purified enzyme showed that it is activated by Na+ and K+, it possesses a broad pH optimum between 6 and 8, MgATP is the nucleotide substrate, free Mg2+ is an inhibitor with respect to both MgATP and adenosine, and the enzyme is subject to substrate inhibition by adenosine. The severity of this inhibition increases as the concentration of free Mg2+ increase. The Km for MgATP was calculated to be 0.8 mM and that for adenosine, at likely physiological concentrations of MgATP and free MgCl2, was about 0.2 microM. In vivo the enzyme is likely to be saturated with both MgATP and adenosine. Indeed, the adenosine concentration in rat heart in vivo is probably sufficient to cause substrate inhibition, and this would be increased by an increase in free Mg2+ concentration. Changes in the concentrations of adenosine and free Mg2+ may play a role in modifying the activity of the enzyme in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Baer H. P., Drummond G. I., Duncan E. L. Formation and deamination of adenosine by cardiac muscle enzymes. Mol Pharmacol. 1966 Jan;2(1):67–76. [PubMed] [Google Scholar]
- Berne R. M., Rubio R. Adenine nucleotide metabolism in the heart. Circ Res. 1974 Sep;35 (Suppl 3):109–120. [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Chang C. H., Brockman R. W., Bennett L. L., Jr Adenosine kinase from L1210 cells. Purification and some properties of the enzyme. J Biol Chem. 1980 Mar 25;255(6):2366–2371. [PubMed] [Google Scholar]
- De Jong J. W. Partial purification and properties of rat-heart adenosine kinase. Arch Int Physiol Biochim. 1977 Aug;85(3):557–569. doi: 10.3109/13813457709069872. [DOI] [PubMed] [Google Scholar]
- Foley D. H., Herlihy J. T., Thompson C. I., Rubio R., Berne R. M. Increased adenosine formation by rat myocardium with acute aortic constriction. J Mol Cell Cardiol. 1978 Mar;10(3):293–300. doi: 10.1016/0022-2828(78)90352-8. [DOI] [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Studies of 5'-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976 Oct 25;251(20):6372–6378. [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Kyd J. M., Bagnara A. S. Adenosine kinase from human erythrocytes: determination of the conditions required for assay in crude hemolystates. Clin Chim Acta. 1980 Apr 25;103(2):145–153. doi: 10.1016/0009-8981(80)90206-5. [DOI] [PubMed] [Google Scholar]
- Lindberg B., Klenow H., Hansen K. Some properties of partially purified mammalian adenosine kinase. J Biol Chem. 1967 Feb 10;242(3):350–356. [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H. Adenosine kinase from rabbit liver. I. Purification by affinity chromatography and properties. J Biol Chem. 1979 Apr 10;254(7):2339–2345. [PubMed] [Google Scholar]
- Namm D. H., Leader J. P. A sensitive analytical method for the detection and quantitation of adenosine in biological samples. Anal Biochem. 1974 Apr;58(2):511–524. doi: 10.1016/0003-2697(74)90219-x. [DOI] [PubMed] [Google Scholar]
- Namm D. H. Myocardial nucleotide synthesis from purine bases and nucleosides. Comparison of the rates of formation of purine nucleotides from various precursors and identification of the enzymatic routes for nucleotide formation in the isolated rat heart. Circ Res. 1973 Dec;33(6):686–695. doi: 10.1161/01.res.33.6.686. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Olsson R. A., Snow J. A., Gentry M. K., Frick G. P. Adenosine uptake by canine heart. Circ Res. 1972 Nov;31(5):767–778. doi: 10.1161/01.res.31.5.767. [DOI] [PubMed] [Google Scholar]
- Palella T. D., Andres C. M., Fox I. H. Human placental adenosine kinase. Kinetic mechanism and inhibition. J Biol Chem. 1980 Jun 10;255(11):5264–5269. [PubMed] [Google Scholar]
- Rubio R., Wiedmeier V. T., Berne R. M. Relationship between coronary flow and adenosine production and release. J Mol Cell Cardiol. 1974 Dec;6(6):561–566. doi: 10.1016/0022-2828(74)90036-4. [DOI] [PubMed] [Google Scholar]
- Schrader J., Haddy F. J., Gerlach E. Release of adenosine, inosine and hypoxanthine from the isolated guinea pig heart during hypoxia, flow-autoregulation and reactive hyperemia. Pflugers Arch. 1977 May 6;369(1):1–6. doi: 10.1007/BF00580802. [DOI] [PubMed] [Google Scholar]
- Sullivan J. M., Alpers J. B. In vitro regulation of rat heart 5'-nucleotidase by adenine nucleotides and magnesium. J Biol Chem. 1971 May 10;246(9):3057–3063. [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]
- Wiedmeier V. T., Rubio R., Berne R. M. Incorporation and turnover of adenosine-U- 14 C in perfused guinea pig myocardium. Am J Physiol. 1972 Jul;223(1):51–54. doi: 10.1152/ajplegacy.1972.223.1.51. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]
- Yamada Y., Goto H., Ogasawara N. Purification and properties of adenosine kinase from rat brain. Biochim Biophys Acta. 1980 Dec 4;616(2):199–207. doi: 10.1016/0005-2744(80)90138-2. [DOI] [PubMed] [Google Scholar]
- de Jong J. W., Kalkman C. Myocardial adenosine kinase: activity and localization determined with rapid, radiometric assay. Biochim Biophys Acta. 1973 Sep 14;320(2):388–396. doi: 10.1016/0304-4165(73)90320-6. [DOI] [PubMed] [Google Scholar]
- de Jong J. W., Keijzer E. Dipyridamole affects myocardial adenosine kinase activity. Arch Int Physiol Biochim. 1979 Aug;87(3):525–532. doi: 10.3109/13813457909070516. [DOI] [PubMed] [Google Scholar]
- de Jong J. W., Keijzer E., Uitendaal M. P., Harmsen E. Further purification of adenosine kinase from rat heart using affinity and ion-exchange chromatography. Anal Biochem. 1980 Jan 15;101(2):407–412. doi: 10.1016/0003-2697(80)90206-7. [DOI] [PubMed] [Google Scholar]


