Abstract
Adeno-associated virus serotype 4 (AAV4) and AAV5 have different tropisms compared to AAV2 and to each other. We recently reported that α2-3 sialic acid is required for AAV5 binding and transduction. In this study, we characterized AAV4 binding and transduction and found it also binds sialic acid, but the specificity is significantly different from AAV5. AAV4 can hemagglutinate red blood cells from several species, whereas AAV5 hemagglutinates only rhesus monkey red blood cells. Treatment of red blood cells with trypsin inhibited hemagglutination for both AAV4 and AAV5, suggesting that the agglutinin is a protein. Treatment of Cos and red blood cells with neuraminidases also indicated that AAV4 bound α2-3 sialic acid. However, resialylation experiments with neuraminidase-treated red blood cells demonstrated that AAV4 binding required α2–3 O-linked sialic acid, whereas AAV5 required N-linked sialic acid. Similarly, resialylation of sialic acid-deficient CHO cells supported this same conclusion. The difference in linkage specificity for AAV4 and AAV5 was confirmed by binding and transduction experiments with cells incubated with either N-linked or O-linked inhibitors of glycosylation. Furthermore, AAV4 transduction was only blocked with soluble α2-3 sialic acid, whereas AAV5 could be blocked with either α2–3 or α2-6 sialic acid. These results suggest that AAV4 and AAV5 require different sialic acid-containing glycoproteins for binding and transduction of target cells and they further explain the different tropism of AAV4 and AAV5.
The first step in viral infection is attachment of the virus to the cell surface. While inhibition of infection can occur at other stages such as entry, uncoating, expression, or assembly, for a number of viruses the initial binding step is thought to determine the tissue tropism of the virus. For many viruses this initial interaction is through charged carbohydrates, such as heparan sulfate or sialic acid (5).
Both JC virus and mouse polyomavirus bind sialic acid, but they have different specificities. JC virus binds α2-6-linked sialic acid while mouse polyomavirus binds α2-3-linked sialic acid (8, 22). Heparan sulfate (HS) serves as an initial receptor for the binding of both herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) to cell surfaces. However, each virus recognizes different structural features of the HS and as a result has a different epidemiology and cell tropism (18). HSV-1 binds human synaptosomes and glial cells, while HSV-2 binds HeLa cells more efficiently (32, 33). HS also serves as a receptor for dengue virus. In this case, the degree of sulfanation of HS in the liver is thought to be a determinant of viral tropism for the liver (9, 19).
Like HSV and dengue virus, adeno associated virus type 2 (AAV2) has been shown to bind heparan sulfate proteoglycans (HSPs) on the cell surface (28). This interaction with HSPs has been shown to have a role in viral infection. Competition experiments have demonstrated that soluble heparin can block virus binding and transduction. Furthermore, differentiated airway lung epithelial cells, which express very little HSP on their apical surface, are poorly transduced (15).
AAV2 is a member of the Dependovirus genus, a small group of viruses that were classified based on a similar size and structure and dependence upon a helper virus for replication. The cloning of five other members of this genus and their initial characterization indicate that each has unique binding characteristics (2, 10, 11, 23, 26, 37). Comparison of the capsid proteins indicates that AAV4 and AAV5 are the most divergent of the six cloned AAV isolates, exhibiting only 60% homology to AAV2 or to each other. Serological data indicated that AAV4 is naturally found in African green monkeys while AAV5 was originally isolated from a human sample (3, 6). Both AAV4 and AAV5 are insensitive to heparin competition, whereas AAV2 transduction is blocked by soluble heparin (11, 14). Competition cotransduction experiments indicate distinct mechanisms of uptake and tropism for these isolates (10, 11). In vitro and in vivo experiments with AAV5 demonstrate improved binding and transduction of airway lung epithelia compared to that of AAV2 (38). Injection of AAV5 into the striatum results in transduction of both ependymal cells and neuronal cells throughout the injected hemisphere. AAV4, like AAV2, does not efficiently transduce airway epithelia via the apical surface, but direct injection of AAV4 into the striatum demonstrates a strong tropism of this virus for ependymal cells (14). AAV2 transduces primarily neuronal cells at the site of injection.
Little is known about the interactions necessary for AAV4 and AAV5 binding and transduction. It was recently reported that AAV5 is able to agglutinate red blood cells (RBCs) from rhesus monkeys and that α2-3 sialic acid is required for cell binding and transduction (35). Initial reports indicate that AAV4 is able to hemagglutinate RBCs from several species (21), suggesting a possible interaction with sialic acid. Hemagglutination (HA) through interaction with sialic acid residues has been reported in a number of autonomous parvoviruses. Both canine parvovirus (CPV) and the related feline panleukopenia virus bind sialic acid, although the functional significance of this interaction is not clear (4, 30). Another autonomous parvovirus, minute virus of mice (MVM), also binds sialic acid. In this case the interaction is important for transduction, since treatment of target cells with neuraminidase blocks replication (13).
In this study we have examined the binding and transduction requirements of AAV4. We show that sialic acid binding is important for both AAV4 HA and infectivity, but the specific carbohydrate linkage required by AAV4 and AAV5 appears to be distinct.
MATERIALS AND METHODS
Cell culture.
Cos and 293T cells were maintained as monolayer cultures in D10 medium (Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 100 mg of penicillin/ml, 100 U of streptomycin/ml, and amphotericin B [Fungizone]) as recommended by the manufacturer (Biofluids, Rockville, Md.). 293 cells were cultured in Eagle's minimal essential medium (EMEM) supplemented with 10% fetal calf serum, 100 mg of penicillin/ml, 100 U of streptomycin/ml, and 13 U of amphotericin B (Fungizone)/ml. A parental CHO cell line (Pro5) and a Pro5 mutant (Lec2) were obtained from the American Type Culture Collection. The Lec2 mutant is deficient in transport of CMP-sialic acid into the Golgi compartment and does not process sialic acid onto its cell surface. Cells were cultured in alpha minimal essential medium (Biofluids) supplemented with 10% fetal calf serum, 100 mg of penicillin/ml, and 100 U of streptomycin/ml.
Production of rAAV.
Recombinant AAV2 (rAAV2), rAAV4, and rAAV5 were produced using a three-plasmid procedure previously described (1). Briefly, semiconfluent 293T cells were transfected by calcium phosphate with three plasmids: an adenovirus helper plasmid (pAd12) containing the VA RNA, E2, and E4; an AAV helper plasmid containing the Rep and Cap genes for the serotype to be packaged; and a vector plasmid containing the inverted terminal repeats corresponding to the serotypes flanking a reporter β-galactosidase (β-Gal) gene with a Rous sarcoma virus promoter. Forty-eight hours posttransduction the cells were harvested by scraping in TD buffer (140 mM NaCl, 5 mM KCl, 0.7 mM K2HPO4, 25 mM Tris-HCl [pH 7.4]) and the cell pellet was concentrated by low-speed centrifugation. The virus was then purified using CsCl gradients.
HA assays.
HA assays were carried out using a microtiter method (20-μl volumes) in V-bottomed plates. Twofold serial dilutions of virus were prepared in dextrose gelatin albumin buffer (DGA; 5 g of dextrose/liter, 0.3 g of gelatin/liter, 0.2% bovine serum albumin [BSA], and fraction V, in 0.05 M phosphate-buffered saline [pH 6.5], unless otherwise stated). A further volume of DGA was added to each well before a 0.5% dilution (vol/vol) of indicator cells in saline (rhesus erythrocytes unless otherwise indicated) was added and the plates were incubated for 2 h at 4°C, room temperature, or 37°C. The end point was 50% HA (1 HA50 unit) of the RBCs.
Treatment of erythrocytes with trypsin or neuraminidase.
Erythrocytes were treated by a variety of different enzymes to remove cell surface proteins. Rhesus monkey erythrocytes (1% [vol/vol] in saline) were treated with enzyme at 37°C for 1 h, following which the cells were washed, resuspended in buffer, and tested in the HA assay. Untreated erythrocytes were used as controls. Trypsin was obtained from Difco Ltd., and neuraminidases were obtained from New England BioLabs, Roche Boehringer Mannheim, and Glyko. There was no significant effect (greater-than-fourfold change in HA titer) on HA with neuraminidases isolated from Salmonella enterica serovar Typhimurium, Streptococcus pneumoniae, or Vibrio cholerae under the tested conditions (data not shown).
Resialylation of erythrocytes.
Resialylation was performed using a modification of the method described by Paulson and Rogers (24). Briefly, rhesus monkey erythrocytes (1% [vol/vol] in DGA) were treated with neuraminidase for 3 h at 37°C to remove sialic acid and washed twice in saline. The cells were then resuspended in DGA (1% [vol/vol] in saline) and treated with sialyltransferases [α2-3 (O)- and α2-3 (N)-sialyltransferases; Calbiochem] in the presence of CMP-α-d-sialic acid (1 mM; Roche Boehringer Mannheim) for 3 h at 37°C. The cells were diluted to 0.5% in DGA and assayed directly in the HA test. Transferase activity was confirmed by restoring HA activity with α2-3-specific lectin (MAA; Vector Labs).
Neuraminidase treatment.
Cos cells were plated at 75% confluency 4 to 18 h prior to infection. The cells were then infected with wild-type adenovirus (multiplicity of infection [MOI], 10) for 1 h, the medium was removed, and the cells were treated with the indicated neuraminidase at the indicated concentration. After 1 h at 37°C the neuraminidase-containing medium was removed, and the cells were washed and infected with serial dilutions of rAAV2, rAAV4, or rAAV5, with highest MOI, 2 × 105 particles/cell. This amount of virus was sufficient to transduce greater than 80% of the cells. The cells were infected for 1 h, the medium was removed, and the cells were washed and incubated for an additional 24 to 36 h before staining for β-Gal activity. Transduced cells were visually scored (blue cells) using a light microscope. For quantitation, Cos cells were infected in duplicate in 10-fold serial dilutions with rAAV and stained 24 h postinfection. The titer was determined by identifying the linear endpoint dilution with less than 10 positive cells/well. Newcastle disease virus (NDV), Clostridium perfringens, Salmonella serovar Typhimurium, and Arthrobacter ureafaciens neuraminidases were purchased from Glyko. V. cholerae neuraminidase was purchased from Sigma, and S. pneumoniae neuraminidase was obtained from New England BioLabs.
Sialic acid competition.
Competition binding experiments were performed by preincubating serial dilutions of virus in media supplemented with the indicated conjugate for 1 h at room temperature. The highest MOI used was 2 × 105 particles/cell. This amount of virus was sufficient to transduce greater than 80% of the cells. Cells were plated at 75% confluency 18 h prior to infection. The cells were then infected with wild-type adenovirus (MOI, 10) for 1 h, the medium was removed, and the cells were washed with medium. The virus-conjugate medium was then added to the cells. After 1 h of incubation at 37°C, the cells were washed three times and then stained for β-Gal activity 24 to 36 h posttransduction, and transduction was quantified as described above.
Resialylation of CHO cells for transduction.
Sialic acid was restored to the surface of Lec2 cells in defined linkages using purified sialyltransferases. Lec2 cells were incubated with either ST-2,3 (O)-sialyltransferase (Calbiochem), ST-2,3 (N)-sialyltransferase (Calbiochem), or ST-2,6 sialyltransferase (Wako, Inc.) in the presence of 1 mM CMP-sialic acid (Boehringer Mannheim). Resialylation was carried out with 150 mU of sialyltransferase/ml in EMEM for 2 h at 37°C. The resialylation was confirmed by adsorption with specific lectins (Vector Labs). Following resialylation, Lec2 cells were transduced (MOI, 1) with either AAV5 or AAV4 in EMEM. β-Gal activity was measured 24 h posttransduction using a chemiluminescent assay (38). The resialylation experiments had n values of 4 and 3 for AAV5 and AAV4, respectively.
Glycosylation inhibitor studies.
Cos cells were plated at 20% confluency in 96-well plates 18 h before the addition of the inhibitors tunicamycin (Oxford) or N-benzyl-GalNAc (Calbiochem) at the indicated concentrations. The cells were then cultured with the inhibitors for 24 h and transduced with serial dilutions of rAAV2, rAAV4, or rAAV5 carrying the gene for β-Gal as described above. After a 1-h infection, the medium was removed and the cells were washed and incubated for 60 h before staining for β-Gal activity. Transduction was quantified as described above.
Binding assays.
Virus bound to the cell surface was quantitated by chilling the cells on ice for 30 min prior to virus addition. Cells were plated in a 96-well format. Virus was added to cells (MOI, 1,000 particles/cell) and allowed to bind for 30 min at 4°C. Cells were then washed twice with cold medium. Plates were allowed to warm to room temperature and 10 μl of PCRnGo (Pierce) buffer was added to each well and incubated for 10 min prior to freezing. The cells were then thawed, 40 μl of water was added, and the cell lysate was mixed. One microliter of this material was added to a PCR mixture (1× SYBR Green master mix; Applied Biosystems [ABI], Foster City, Calif.) with 0.25 pmol of forward and reverse primers per μl, and amplification was detected using an ABI 7700 sequence detector. Primers specific for the RSV promoter were designed by using the Primer Express program (ABI): forward 5′GATGAGTTAGCAACAT-GCCTTACAA, and reverse 5′TCGTACCACCTTACTTCCACCAA. Following a 96°C, 10-min denaturing step, the two-step cycling conditions were 96°C for 15 s, and 60°C for 1 min for 40 cycles. The viral DNA in each sample was then quantified by comparing the fluorescence profiles for the different samples with those of a set of DNA standards. The amount of bound virus was then compared in the treated and untreated samples and the difference was plotted.
RESULTS
HA by AAV4 and AAV5.
Previous experiments with AAV4 had detected HA activity with erythrocytes from a number of species (21). HA experiments with RBCs demonstrated that AAV4 could agglutinate rhesus monkey, human, mouse, rat, and rabbit erythrocytes when DGA buffer diluent (pH 6.5) at 4°C was used (Table 1). There was no HA detected at 37°C. Optimization of pH for HA of rhesus monkey erythrocytes demonstrated greater HA activity at a more acidic pH than at basic pH, and a pH 6.5 buffer was found to be optimum for the assay (data not shown). In contrast, AAV5 only hemagglutinated rhesus monkey cells. While the HA activity of rhesus monkey cells was similar for AAV4 and AAV5, AAV5 HA activity was not detected with human, mouse, rat, or rabbit cells that were hemagglutinated by AAV4 (Table 1). This difference in HA activity for the two viruses suggests that the agglutinin for AAV4 and AAV5 may be distinct. To test this hypothesis, we conducted the following experiments to characterize the agglutinin for AAV4 and AAV5.
TABLE 1.
Ability of AAV4 or AAV5 to hemagglutinate erythrocytes from different speciesa
| Species | Dilution producing HA50 in:
|
|
|---|---|---|
| AAV4 | AAV5 | |
| Rhesus monkey (Macaca sp.) | 256 | 128 |
| Human | 128 | <2 |
| Mouse | 64 | <2 |
| Rat | 64 | <2 |
| Rabbit | 16 | <2 |
| Tamarin (Saguinus sp.) | <2 | <2 |
| Horse | <2 | <2 |
| Cow | <2 | <2 |
| Sheep | <2 | <2 |
| Cat | <2 | <2 |
| Chicken | <2 | <2 |
Doubling dilutions of stock solutions of AAV4 and AAV5 containing 128 HA50 units (with rhesus RBC) were tested in the hemagglutination assay using red cells from different species, and the dilution giving 50% agglutination was measured.
Enzymatic treatment of erythrocytes.
To identify the AAV4 and AAV5 agglutinin, rhesus monkey erythrocytes were treated with trypsin to determine if the agglutinin had a protein component. Treatment with trypsin inhibited HA for both viruses, suggesting the involvement of a protein in HA (Table 2). Like trypsin, treatment with neuraminidase also had a significant effect on HA activity. Neuraminidase isolated from A. ureafaciens significantly reduced the HA activity for both AAV4 and AAV5 in a dose-dependent manner (Table 2). Similarly, neuraminidase isolated from C. perfringens also inhibited AAV4 and AAV5 HA activity in a dose-dependent manner. These results indicate that glycoproteins and sialic acid are involved in AAV4 and AAV5 hemagglutination.
TABLE 2.
Effects of trypsin and different neuraminidases on the agglutination of rhesus erythrocytes by AAV4 and AAV5a
| Enzyme | Concn | Decrease in HA titer (fold change) for:
|
|
|---|---|---|---|
| AAV4 | AAV5 | ||
| Trypsin | 0.2% | 256 | 128 |
| A. ureafaciens neuraminidaseb | 100 mU/ml | >500 | >1,000 |
| 10 mU/ml | >500 | >1,000 | |
| 1 mU/ml | 250 | 32 | |
| C. perfringens neuraminidaseb | 5 mU/ml | 64 | 250 |
| 1 mU/ml | 2 | 16 | |
Red cells were treated with neuraminidases isolated from different bacterial sources for 1 h at 37°C, washed, and tested in the HA assay in comparison with untreated red cells. A change of greater than fourfold is considered a significant result. Values presented are fold changes in HA titer required for 50% agglutination of cells compared to HA titer with untreated red cells.
Obtained from Roche Boehringer Mannheim.
Resialylation of erythrocytes.
To identify the specific sialic acid required for agglutination, sialic acid was removed from red cells with neuraminidase and then added back using specific sialyltransferases in the presence of a CMP-sialic acid substrate. The change in HA titers for the resialylated red cells was then compared to that of the neuraminidase-only-treated red cells (Table 3). Neuraminidase from A. ureafaciens was used in this experiment because it had the strongest effect on both AAV4 and AAV5 HA under the indicated buffer conditions. The ability to hemagglutinate rhesus monkey cells with AAV4 was restored after treatment with α2-3(O) sialyltransferase. However, this treatment did not affect AAV5 HA (Table 3). HA with AAV5 could be restored with 50 mU of α2-3 (N) sialyltransferase/ml, but this treatment did not affect AAV4 HA (Table 3). Experiments with higher concentrations of sialyltransferase led to significant cell lysis (data not shown). Thus, the agglutinin for both AAV4 and AAV5 is α2-3 sialic acid, but the two viruses bind different sialyloligosaccharides; AAV4 binds O-linked α2-3 sialic acid and AAV5 only binds the N-linked form.
TABLE 3.
Effects of different sialyltransferases on rhesus erythrocytes pretreated with neuraminidasea
| Sialyltransferase specificity | Concn (mU/ml) | Fold change in HA titer
|
|
|---|---|---|---|
| AAV4 | AAV5 | ||
| No treatment | <2 | <2 | |
| α2,3 (O) | 2 | 4 | <2 |
| 20 | 64 | <2 | |
| α2,3 (N) | 20 | <2 | <2 |
| 50 | <2 | 16 | |
Rhesus cells were treated with 2 mU of neuraminidase/ml from A. ureafaciens to remove sialic acid and then incubated with different concentrations of specific sialyltransferases for 3 h. The red cells were washed and tested for an increase in the HA titer. A change of greater than fourfold was considered significant. Values presented are fold changes in HA titer compared to red cells treated with only neuraminidase.
Effect of neuraminidase treatment on rAAV transduction.
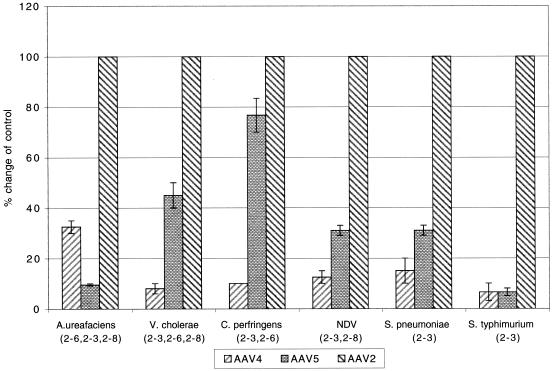
While HA has been reported for other parvoviruses, the role of this activity in the life cycle of the virus is not always clear. Thus, the importance of sialic acid binding of AAV4 in transduction was examined. Treatment of Cos cells with the different neuraminidases inhibited AAV4 and AAV5 transduction in a dose-dependent manner (data not shown). The amounts that gave the largest difference in transduction efficiency for AAV4 and AAV5 are presented in Fig. 1. Treatment of Cos cells with broad-specificity neuraminidase isolated from A. ureafaciens or V. cholerae inhibited both AAV4 and AAV5 transduction but had no effect on AAV2 (Fig. 1). These neuraminidases have only a slight preference for the cleavage of either α2-3, α2-6, or α2-8 sialic acid (Table 4). Neuraminidase isolated from C. perfringens or NDV is specific for either α2-3, α2-6 or α2-3, α2-8 sialic acid but not α2-8 or α2-6, respectively. Treatment with these enzymes also inhibited transduction, suggesting a role for α2-3 sialic acid in transduction (Fig. 1). Neuraminidase isolated from Streptococcus pneumoniae only cleaves α2-3 sialic acid, and neuraminidase isolated from Salmonella serovar Typhimurium is 260-fold more active against α2-3 sialic acid than against α2-6, 8, 9 (Table 4). Treatment of Cos cells with either of these neuraminidases inhibited transduction of both AAV4 and AAV5 (Fig. 1). No change in AAV2 transduction was observed (Fig. 1). This result is in agreement with the HA data and suggests that α2-3 sialic acid is involved in both HA and transduction with AAV4.
FIG. 1.
Neuraminidase effect on transduction. The effects of pretreatment of Cos cells with neuraminidases isolated from different sources were compared. Cos cells were incubated with different recombinant neuraminidases as indicated and transduced with the different serotypes of AAV in serial dilution. Each neuraminidase was tested at several doses and the amount that gave the largest difference in transduction efficiency for AAV4 and AAV5 is presented. Data are presented as the percent change in transduction compared to untreated cells for AAV4, AAV5, or AAV2, respectively (n = 3). Broad-spectrum neuraminidases (Glyko) were as follows: C. perfringens, 16.6 U/ml and A. ureafaciens, 1.6 U/ml. Intermediate specificity neuraminidases were V. cholerae (Sigma), 0.025 U/ml, and NDV (Glyko), 16.6 U/ml. Neuraminidases with high specificity for α2-3 sialyl linkages were S. pneumoniae (New England BioLabs), 8.3 U/ml, and Salmonella serovar Typhimurium (Glyko), 10 U/ml.
TABLE 4.
Neuraminidase specificity
| Neuraminidase source (supplier) | Rel. rate of hydrolysisa
|
||
|---|---|---|---|
| α2-3 | α2-6 | α2-8 | |
| A. ureafaciens (Glyko) | 1.0 | 2.0 | 1.0 |
| V. cholerae (Sigma) | 1.0 | 0.3 | 0.3 |
| C. perfringens (NEB)b | 1.0 | 0.5-.03 | ND |
| NDV (Glyko) | 1.0 | NDc | 1.0 |
| S. pneumoniae (Glyko) | 1.0 | ND | ND |
| Salmonella serovar Typhimurium (NEB) | 1.0 | <0.01 | <0.01 |
The relative rate of hydrolysis for each neuraminidase was determined by the manufacturer. Neuraminidases from A. urefaciens and V. cholerae have broad activity and will cleave sialic acid with all linkages. Neuraminidases from C. perfringens and NDV will only cleave α2-3 and α2-6 or α2-3 and α2-8, respectively. Neuraminidases from S. pneumoniae and Salmonella serovar Typhimurium are highly specific for cleavage of only α2-3 linked sialic acid.
NEB, New England BioLabs.
ND, activity not detected.
Blocking of rAAV transduction with sialic acid derivatives.
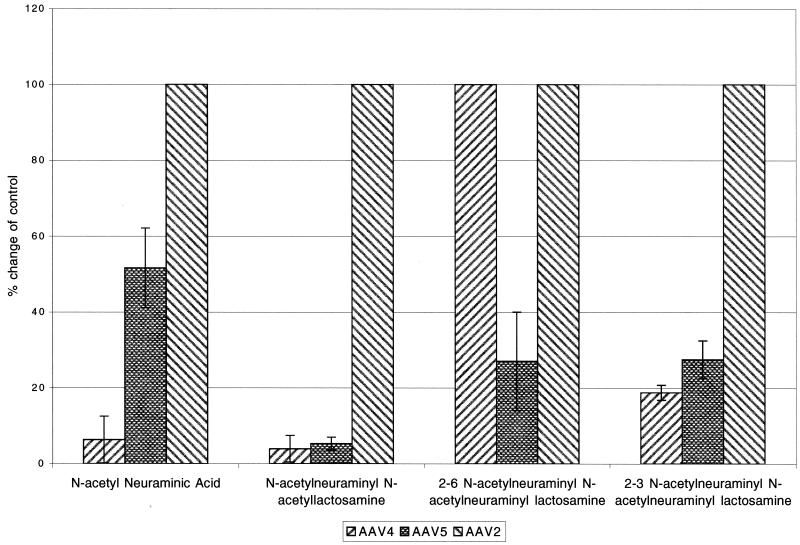
For AAV2, the affinity for HSPs is stable enough for competition binding experiments using soluble heparin (28). Preincubation of AAV2, AAV4, or AAV5 with crude unconjugated N-acetyl neuraminic acid or more purified conjugated N-acetyl-neuraminyl-lactosamine inhibited the transduction of both AAV4 and AAV5 but had no effect on AAV2 transduction (Fig. 2). The N-acetyl-neuraminyl-lactosamine is a mixture of both α2-3- and α2-6-linked forms. Competition experiments with the purified forms demonstrated a difference in sialic acid binding properties for each of the viruses. AAV4 transduction was only inhibited by α2-3-linked N-acetyl-neuramyl-lactosamine, while both the α2-3 and α2-6 forms inhibited AAV5 transduction (Fig. 2). As a control, AAV2 transduction was not inhibited by sialic acid conjugate competition. These data indicate that while AAV4 will only bind α2-3 sialic acid, AAV5 binds either soluble α2-3 or α2-6 sialic acid conjugated to neuramyl-lactosamine.
FIG. 2.
Competition with soluble sialic acid and sialyl sugar conjugates. Competition transduction experiments were carried out by incubating the different serotypes of AAV with soluble forms of sialic acid or sialyl sugar conjugates prior to transduction. Data are presented as the percent change in transduction compared to transduction in the absence of competitors for AAV4, AAV5, or AAV2, respectively (n = 4). The following competitors (and concentrations) were used: N-acetyl neuraminic acid (5 mM), N-acetylneuraminyl-N-acetyl-lactosamine (1 mM), α2-6 N-acetylneuraminyl-N-acetyl-lactosamine (0.2 mM), and α2-3 N-acetylneuraminyl-N-acetyl-lactosamine (0.2 mM).
Inhibition of cellular glycosylation on virus binding and rAAV transduction.
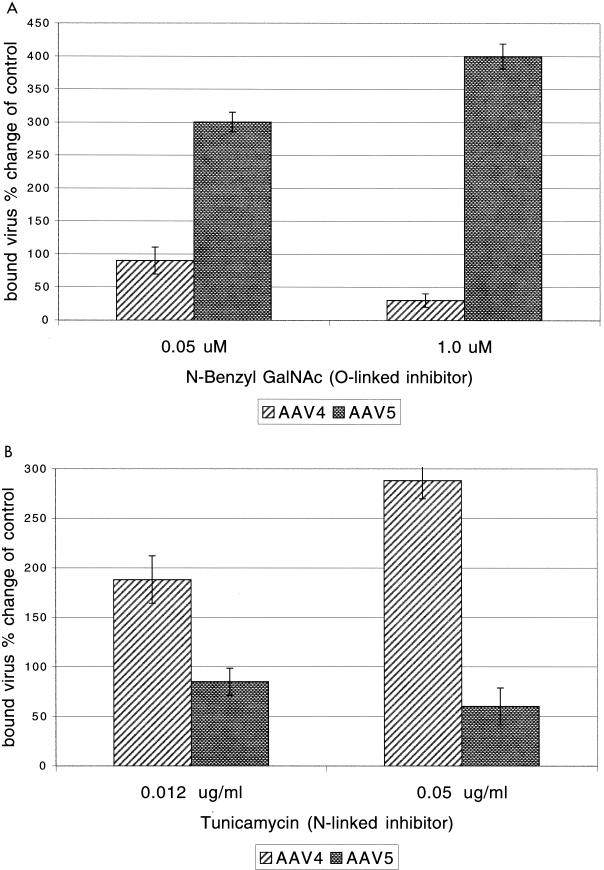
Resialylation experiments with rhesus monkey erythrocytes indicated that while AAV4 and AAV5 bind α2-3 sialic acid, there is a difference in the linkage of the agglutinin for the two viruses (Table 3). The specificity of linkage used in transduction was determined by culturing cells with inhibitors of N-linked (tunicamycin) or O-linked (N-benzyl GalNAc) glycosylation. The effect of these inhibitors for the cell surface step in transduction was determined by quantifying the amount of virus bound to chilled cells cultured with the different inhibitors (Fig. 3). AAV4 virus binding decreased threefold in a dose-dependent manner when target cells were cultured with the O-linked inhibitor. In contrast, AAV5 binding increased when target cells were cultured with N-benzyl-GalNAc (Fig. 3A). Binding experiments on cells cultured with the N-linked inhibitor tunicamycin showed reduced binding of AAV5 but increased binding of AAV4 (Fig. 3B). These data suggest that the agglutinin recognized by AAV4 or AAV5 also has the same linkage specificity that is necessary for efficient transduction of target cells. The increase in virus binding as a result of culturing the target cells with these agents may be the result of increased exposure of the binding sites compared to that in control cells.
FIG. 3.
Effects of N-linked or O-linked inhibitors on AAV binding. Amount of virus bound to treated or untreated target cells was determined using quantitative PCR. Following treatment with the inhibitor at the indicated concentration, target cells were cooled on ice and then incubated with virus at an MOI of 1,000 particles/cell. Unbound virus was washed off and the amount of bound virus was quantified using PCR primers specific for the recombinant vector. The amount of bound virus was then compared for the treated and untreated samples (n = 3). (A) N-Benzyl-GalNAc dose response; (B) tunicamycin dose response.
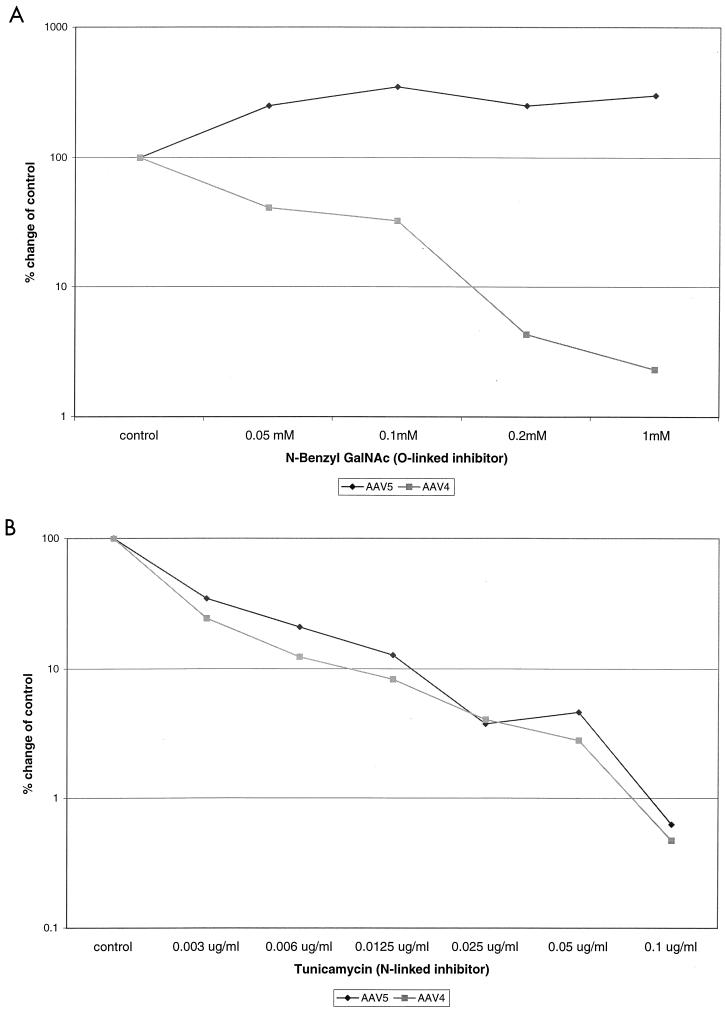
To determine if this change in binding affects transduction, Cos cells were cultured and transduced with rAAV4 and rAAV5 in the presence of the glycosylation inhibitors. Transduction was measured by staining for β-Gal activity. Treatment of Cos cells with N-benzyl-GalNAc, an inhibitor of O-linked glycosylation, prior to transduction inhibited AAV4 transduction 50-fold. In contrast, treatment with N-benzyl GalNAc increased AAV5 transduction twofold (Fig. 4A). This result paralleled the change in virus binding and the results from the HA experiments. Treatment with the N-linked inhibitor tunicamycin inhibited transduction with both viruses greater than 50-fold (Fig. 4B). Unlike the binding competition studies with sialylactose derivatives or the neuraminidase treatments, addition of glycosylation inhibitors can have a broad effect on intracellular activity, ranging from protein folding and secretion to signal transduction and transcription activation (16, 17, 36). Therefore, treatment with tunicamycin could also result in N-linked sugars playing a role in both AAV4 and AAV5 transduction.
FIG. 4.
Effects of N-linked or O-linked inhibitors on AAV transduction. Cos cells were treated with the indicated doses of tunicamycin or N-benzyl-GalNAc for 24 h prior to transduction and then stained 60 h posttransduction (n = 4). The relative transduction efficiency was determined by comparison with control untreated cells and is presented in log scale. (A) N-Benzyl-GalNAc dose response; (B) tunicamycin dose response.
Resialylation of sialic acid-deficient cells suggests AAV4 and AAV5 interact with α2-3 sialic acid on O-linked and N-linked carbohydrates, respectively.
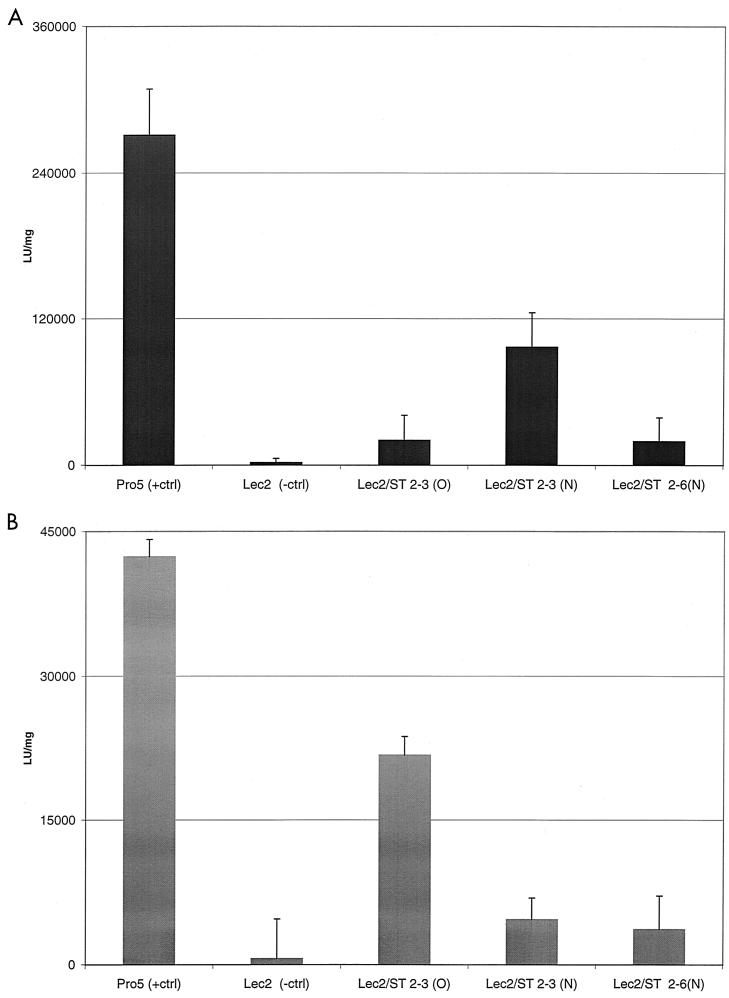
To further investigate the role of α2-3 and α2-6 sialic acids and their linkage specificities in AAV5 transduction and to separate the role of intracellular glycoproteins from that of cell surface proteins, we studied AAV5 transduction in resialylated cells naturally defective in the addition of sialic acid to carbohydrate chains. Lec2 cells are from a mutant clone derived from the parental Pro5 cell line that are deficient in the transport of CMP-sialic acid into the Golgi compartment (27). AAV5 transduced the Pro5 cells over 100-fold more efficiently than it transduced the Lec2 cells (Fig. 5). Resialylation with α2-3 (O) or α2-6 (N) sialyltransferase did not affect AAV5 transduction. However, resialylation with α2-3 N-linked sialyltransferase did result in a significant increase in transduction. AAV4 also transduced the Pro 5 cells but at a much lower efficiency than that of AAV5. In contrast to the results with AAV5, AAV4 transduction was only restored by resialylation with the α2-3 (O) sialyltransferase. No significant increase in transduction was detected by resialylation with α2-3 (N) or α2-6 (N) sialyltransferase. The activities of all three sialyltransferases were confirmed by incubating the cells with fluorescently labeled lectins specific for α2-3 or α2-6 sialic acid (data not shown). Therefore, while AAV5 was able to bind either α2-3 or α2-6 sialic acid conjugated to N-acetyl-neuraminyl-lactosamine, AAV5 transduction of sialic acid-deficient cells was restored only by resialylation with a α2-3(N) sialyltransferase, suggesting that α2-3 (N) but not α2-6 is the form of sialic acid required for AAV5 transduction. In contrast, AAV4 will only bind α2-3 sialic acid conjugates, and transduction requires α2-3 (O) sialic acid.
FIG. 5.
AAV5 transduction of resialylated sialic acid-deficient Lec2 and parental Pro5 cells. Lec 2 cells are a mutant clone derived from the parental Pro5 cell line that is deficient in the transport of CMP-sialic acid into the Golgi compartment. The following sialyltransferases were used to add specific sialic acids to the surface of the Lec2 cells: ST-2,3 (O) sialyltransferase, ST-2,3 (N) sialyltransferase, or ST-2,6 (N) sialyltransferase. The resialylation was confirmed by specific lectin adsorption (data not shown). Resialylated Lec2 cells were then transduced (MOI, 1) with either recombinant AAV5 or AAV4 and β-Gal activity was measured 24 h posttransduction using a chemiluminescent assay. (A) Transduction with AAV5 (n = 4); (B) transduction with AAV4 (n = 3).
DISCUSSION
AAV4 and AAV5 are distinct members of the Dependovirus genus. While the Rep proteins of AAV4 are highly homologous to those of the other dependoviruses, the capsids of both AAV4 and AAV5 are the most divergent from each other and the rest of the genus. Both AAV4 and AAV5 lack heparin-binding activity, an activity reported for other members of the genus. But the lack of cross-competition in cotransduction experiments and differences in in vivo transduction specificities indicate distinct mechanisms of uptake and tropism for these isolates.
Sialic acid binding is a property of several different virus families. Previous research had described this interaction in several members of the parvovirus family, but the significance of this interaction in the life cycle of the virus was not always clear. For parvovirus B19, HA activity and infectivity are linked. The receptor for B19 and the cellular antigen necessary for the HA activity is globoside, also known as the human blood group P antigen (7). MVM has been reported to bind sialic acid, and replication can be inhibited by pretreating target cells with neuraminidase (13). For CPV, mutations within the capsid of the virus can separate HA activity from infectivity, suggesting that sialic acid may not be a primary determinant of infection (4). Similar mutants of MVM have not been described. For CPV and MVM, the specificities of the interaction with the different types of sialic acid have not been investigated. The only dependoviruses with HA activity are AAV4 and AAV5.
Comparison of HA activity using RBCs from a number of species indicates that the agglutinins for AAV4 and AAV5 are distinct. Enzymatic treatment of RBCs determined the agglutinin for AAV4 is α2-3 O-linked sialic acid and is present on a variety of species. AAV5 HA requires α2-3 N-linked sialic acid and the agglutinin is restricted to rhesus monkeys. Both viruses exhibit similar pH dependence for HA activity, and protease sensitivity indicates that the agglutinin is a glycoprotein.
HA is a useful assay for studying virus-cell surface interactions; however, the role of this activity in the virus life cycle is not always clear. Alterations in virus-cell surface interactions as measured by a change in virus transduction are more relevant for virus tropism. Transduction of both AAV4 and AAV5 was inhibited by pretreatment of the target cells with neuraminidase and could be blocked by competition with soluble sialic acid or by treating the cells with inhibitors of glycosylation. All of these in vitro experiments demonstrated that while sialic acid binding is common to AAV4 and AAV5, the specificities of the interaction are distinct. Their different specificities for sialic acid agree with the different cell tropism of the two viruses (14, 38). While AAV4 transduction required α2-3 O-linked sialic acid, AAV5 could be blocked by either soluble α2-3 or α2-6 sialic acid. However, resialylation experiments with sialic acid-deficient CHO cells and glycosylation inhibitor studies with Cos cells determined that only α2-3 N-linked sialic acid was required for AAV5 transduction of Cos and CHO cells. It is possible that for other cell types an interaction with α2-6 sialic acid is important for AAV5 transduction and may account for the broad cell tropism of AAV5 compared to that of AAV4.
The observed differences in the abilities of some neuraminidases to affect both HA and transduction are due to differences in the activities of these enzymes in the two assays. The neuraminidases used in these experiments are active on a variety of conjugates, but their activity is directly dependent on the linkage (N- versus O-linked), the structure of the glycoconjugate, and the packing and organization of the oligosaccharides (for review, see references 12, 29, and 34). Moreover, enzyme activity is dependent on pH and ion concentration, which are different for the two assays (12, 20, 25, 31). The observed inconsistencies between the two assays can be explained by the differences in cell surface glycoprotein presentation between the erythrocytes and Cos cells and by the pH and ion concentrations of the buffers in the two assays. It should be remembered that between the two assays, only transduction has a direct relevance to virus tropism. While HA is a useful tool for studying intact-cell molecular interactions, its physiological relevance is not always clear. Sialic acid has a role in AAV4 and AAV5 transduction and HA and can therefore be listed as a receptor for these serotypes, but the context in which the sialic acid is presented on the cell surface is important in determining its recognition by AAV4 or AAV5.
Modification of proteins through glycosylation is thought to be directly responsible for their correct folding and secretion (17), nuclear transport, assembly into multimeric complexes including the preinitiation complex, or regulation of phosphorylation (16, 36). Therefore, the observed inconsistencies in binding and inhibition of transduction of both AAV4 and AAV5 with the N-linked inhibitor tunicamycin may be due to the broad effect of this inhibitor on cellular activity (for review, see references 16, 17, 36).
AAV4 and AAV5 have shown promise as vectors for gene transfer with distinct cell tropism compared to AAV2. Identification of sialic acid binding and its role in transduction for AAV4 and AAV5 is an important first step in understanding the tropism of these viruses at the molecular level and will ultimately lead to a better understanding of the utility of these viruses for gene therapy applications.
ACKNOWLEDGMENTS
We thank Beverly Handelman for excellent assistance, Ioannis Bossis for help with the HA assays, and John Cisar and Bruce Baum for helpful discussion.
REFERENCES
- 1.Alisky J M, Hughes S M, Sauter S L, Jolly D, Dubensky T W, Jr, Staber P D, Chiorini J A, Davidson B L. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport. 2000;11:2669–2673. doi: 10.1097/00001756-200008210-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bantel-Schaal U, Delius H, Schmidt R, zur Hausen H. Human adeno-associated virus type 5 is only distantly related to other known primate helper-dependent parvoviruses. J Virol. 1999;73:939–947. doi: 10.1128/jvi.73.2.939-947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bantel-Schaal U, zur Hausen H. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology. 1984;134:52–63. doi: 10.1016/0042-6822(84)90271-x. [DOI] [PubMed] [Google Scholar]
- 4.Barbis D P, Chang S F, Parrish C R. Mutations adjacent to the dimple of the canine parvovirus capsid structure affect sialic acid binding. Virology. 1992;191:301–308. doi: 10.1016/0042-6822(92)90192-r. [DOI] [PubMed] [Google Scholar]
- 5.Berns K I. Fundamental virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. [Google Scholar]
- 6.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adeno-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 7.Brown K E, Anderson S M, Young N S. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 8.Cahan L D, Paulson J C. Polyoma virus adsorbs to specific sialyloligosaccharide receptors on erythrocytes. Virology. 1980;103:505–509. doi: 10.1016/0042-6822(80)90208-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 10.Chiorini J A, Kim F, Yang L, Kotin R M. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiorini J A, Yang L, Liu Y, Safer B, Kotin R M. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corfield A P, Veh R W, Wember M, Michalski J C, Schauer R. The release of N-acetyl- and N-glycolloyl-neuraminic acid from soluble complex carbohydrates and erythrocytes by bacterial, viral and mammalian sialidases. Biochem J. 1981;197:293–299. doi: 10.1042/bj1970293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotmore S F, Tattersall P. A genome-linked copy of the NS-1 polypeptide is located on the outside of infectious parvovirus particles. J Virol. 1989;63:3902–3911. doi: 10.1128/jvi.63.9.3902-3911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson B L, Stein C S, Heth J A, Martins I, Kotin R M, Derksen T A, Zabner J, Ghodsi A, Chiorini J A. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan D, Yue Y, Yan Z, McCray P B, Jr, Engelhardt J F. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 16.Hart G W. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 17.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 18.Herold B C, Gerber S I, Belval B J, Siston A M, Shulman N. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J Virol. 1996;70:3461–3469. doi: 10.1128/jvi.70.6.3461-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilgard P, Stockert R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology. 2000;32:1069–1077. doi: 10.1053/jhep.2000.18713. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer L L, Roggentin P, Schauer R, Vimr E R. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl alpha 2–3 linkages. J Biochem (Tokyo) 1991;110:462–467. doi: 10.1093/oxfordjournals.jbchem.a123603. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Mayor H D. Hemagglutinin of type 4 adeno-associated satellite virus. J Immunol. 1968;100:61–68. [PubMed] [Google Scholar]
- 22.Liu C K, Wei G, Atwood W J. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal alpha(2-6)-linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muramatsu S, Mizukami H, Young N S, Brown K E. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 24.Paulson J C, Rogers G N. Resialylated erythrocytes for assessment of the specificity of sialyloligosaccharide binding proteins. Methods Enzymol. 1987;138:162–168. doi: 10.1016/0076-6879(87)38013-9. [DOI] [PubMed] [Google Scholar]
- 25.Paulson J C, Weinstein J, Dorland L, van Halbeek H, Vliegenthart J F. Newcastle disease virus contains a linkage-specific glycoprotein sialidase. Application to the localization of sialic acid residues in N-linked oligosaccharides of alpha 1-acid glycoprotein. J Biol Chem. 1982;257:12734–12738. [PubMed] [Google Scholar]
- 26.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley P, Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977;3:391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- 28.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor G. Sialidases: structures, biological significance and therapeutic potential. Curr Opin Struct Biol. 1996;6:830–837. doi: 10.1016/s0959-440x(96)80014-5. [DOI] [PubMed] [Google Scholar]
- 30.Tresnan D B, Southard L, Weichert W, Sgro J Y, Parrish C R. Analysis of the cell and erythrocyte binding activities of the dimple and canyon regions of the canine parvovirus capsid. Virology. 1995;211:123–132. doi: 10.1006/viro.1995.1385. [DOI] [PubMed] [Google Scholar]
- 31.Uchida Y, Tsukada Y, Sugimori T. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J Biochem (Tokyo) 1979;86:1573–1585. doi: 10.1093/oxfordjournals.jbchem.a132675. [DOI] [PubMed] [Google Scholar]
- 32.Vahlne A, Svennerholm B, Lycke E. Evidence for herpes simplex virus type-selective receptors on cellular plasma membranes. J Gen Virol. 1979;44:217–225. doi: 10.1099/0022-1317-44-1-217. [DOI] [PubMed] [Google Scholar]
- 33.Vahlne A, Svennerholm B, Sandberg M, Hamberger A, Lycke E. Differences in attachment between herpes simplex type 1 and type 2 viruses to neurons and glial cells. Infect Immun. 1980;28:675–680. doi: 10.1128/iai.28.3.675-680.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters, R. W., S. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem., in press. [DOI] [PubMed]
- 36.Wells L, Vosseller K, Hart G W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 37.Xiao W, Chirmule N, Berta S C, McCullough B, Gao G, Wilson J M. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zabner J, Seiler M, Walters R, Kotin R M, Fulgeras W, Davidson B L, Chiorini J A. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]