Abstract
Coronavirus disease 2019 (COVID-19) is a worldwide pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has affected millions of lives. Individuals who survive severe COVID-19 can experience sustained respiratory symptoms that persist for months after initial infection. In other airway diseases, abnormal airway mucus contributes to sustained airway symptoms. However, the impact of SARS-CoV-2 on airway mucus has received limited attention. In the current review, we assess literature describing the impact of SARS-CoV-2 on airway pathophysiology with specific emphasis on mucus production. Accumulating evidence suggests that the 2 major secreted airway mucin glycoproteins, MUC5AC and MUC5B, are abnormal in some patients with COVID-19. Aberrations in MUC5AC or MUC5B in response to SARS-CoV-2 infection are likely due to inflammation, though the responsible mechanisms have yet to be determined. Thus, we also provide a proposed model highlighting mechanisms that can contribute to acute and sustained mucus abnormalities in SARS-CoV-2, with an emphasis on inflammatory cells and mediators, including mast cells and histamine. Last, we bring to light the challenges of studying abnormal mucus production in SARS-CoV-2 infections and discuss the strengths and limitations of model systems commonly used to study COVID-19. The evidence to date suggests that ferrets, nonhuman primates, and cats may have advantages over other models to investigate mucus in COVID-19.
Keywords: mucus, MUC5AC, MUC5B, airway, obstruction, histamine, SARS-CoV-2, COVID-19
Coronavirus disease 2019 (COVID-19) is a worldwide pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-sense RNA virus. Following viral exposure, onset of clinical disease occurs in 2 to 14 days (median ~4–5 days).41 While most individuals experience mild to moderate clinical disease, approximately 10% to 15% of clinically affected individuals will develop severe illness characterized by dyspnea leading to hospitalization, intensive care treatment, and/or death. Severe COVID-19 cases are often diagnosed with acute respiratory distress syndrome (ARDS), and consistent with this condition, diffuse alveolar disease is detectable in many autopsy cases.5 Age, sex, preexisting health conditions, socioeconomic background, and race/ethnicity are among factors that influence disease outcomes.76,84,106
Changes in composition and amount of airway mucus are common to multiple airway diseases, such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease (COPD). Abnormal mucus is fundamental in the pathogenesis of these conditions.7,17,112,113,124 Autopsy studies indicate that accumulation of airway mucus is associated with COVID-19-related lung disease. This mucus could be a consequence of airway intubation, which disrupts mucociliary clearance and necessitates regular mucus treatments.6 However, there is growing clinical evidence that COVID-19 causes sustained airway dysfunction in some individuals.61 These individuals exhibit persistent symptoms, including cough and shortness of breath.55 Given that mucus is a common trigger for cough and can obstruct conduction of air to make it difficult to breathe, long-term alterations in mucus production and/or its biophysical properties may contribute to sustained COVID-19 airway symptoms. Here, we review evidence that mucus is abnormal in COVID-19 and propose potential mechanisms. We will highlight 2 major secreted airway mucins, MUC5AC and MUC5B, and the potential contribution of mast cells and histamine in their regulation. We focus this review on mucins in the lung, a site of significant disease in individuals with severe COVID-19, but we recognize that mucins are also important in the sinonasal cavity where viral infection initially occurs. Although other proteins, such as defensins, trefoil proteins, and immunoglobin A, are secreted and part of mucus, mucins provide the major viscoelastic properties of mucus and are thus the focus of this review.2,25
Evidence for Abnormal Mucus in COVID-19
Mucus is a protective substance that normally forms a thin cover over airway epithelial surfaces and consists mostly of glycoproteins and water. Mucins, which are heterogeneous glycoproteins, make up a large portion of mucus.29,35,116 MUC5AC and MUC5B represent the major secreted gelforming mucins in the human airway and drive the biophysical properties of mucus in airway health and disease.22
Many patients with COVID-19 are mechanically ventilated. It is speculated that changes in mucins can leave the airway susceptible to injury and impact ventilation efforts for alveolar gas exchange. Support for this concept is provided largely by animal models of ventilator-induced injury. In mice, for example, ventilator-induced injury decreases MUC5B expression but increases MUC5AC expression.50 In that study, changes in MUC5B and MUC5AC abundances were associated with airway obstruction and pulmonary edema. MUC5AC was highlighted as a driver of ventilator-induced injury since pulmonary edema and inflammation were less severe in mice lacking MUC5AC.50 Furthermore, mice lacking MUC5AC had improved gas exchange in response to ventilator-induced injury, indicating a detrimental role for MUC5AC in airway injury. An additional study in lambs found that ventilator injury increased airway MUC5B expression approximately 10 hours after injury.16 The increase in MUC5B was preceded by an increase in pro-inflammatory mediators, including IL-1β. The authors did not report on MUC5AC levels.16 In humans 50 years of age or older, a MUC5B variant associated with increased MUC5B promoter activity increases the risk of developing ARDS.93 Combined, these studies highlight how mucin expression affects lung injury and inflammation during mechanical ventilation, and thus is hypothesized to influence COVID-19 pathogenesis and recovery.
Support for this hypothesis is provided by several COVID-19 studies. For example, a multicenter study of COVID-19 patient autopsies identified diffuse alveolar damage and neutrophilic inflammation as common lung lesions. Interestingly, dense mucoid material was often observed in airway lumens of bronchi and bronchioles.10 In another autopsy study of COVID-19 cases, mucus plugging was observed in small airways that extended into alveoli.118 While intubation can cause mucus accumulation, one patient in that study received oxygen intranasally without intubation, but still displayed increased airway mucus, indicating that intubation was not the cause. Additionally, samples from individuals with severe acute respiratory distress syndrome (SARS) were also studied but lacked evidence of mucus plugs. Therefore, the authors concluded that excess mucus was specific to COVID-19 and not necessarily a consequence of intubation and ventilator-induced lung injury.118 In another study consisting of 21 individuals, postmortem analysis revealed inflammation of the tracheal mucosa with evidence of increased mucus production in one third of the samples.67 Sputum, which consists in part of mucus, was found in another study to be “stickier” in patients that were critically ill with COVID-19.121
Although elevations in MUC5AC and/or MUC5B expression are associated with abnormal mucus composition (eg, increased viscosity) in airway diseases such as cystic fibrosis, chronic obstructive pulmonary disease, and asthma,13,24,38,49,78 limited studies have specifically examined MUC5AC or MUC5B in COVID-19. One small cohort study of 16 COVID-19 patients found mucus retention in the airways.60 In this study, bronchoscopic aspiration was required to remove the highly viscous mucus. The recovered material exhibited elevated amounts of MUC5AC. Although ~79% of the patients in that study required ventilation, all 16 patients recovered and were later discharged. The authors postulated that the high recovery rate was due to removal of the sticky mucus, resulting in better ventilation and reduced hypoxia. An additional study using single-cell RNA sequencing of airway epithelia obtained from patients with COVID-19 demonstrated increased transcript abundance for MUC5AC and MUC5B in club cells.36 The authors concluded that club cells were hyper-secreting mucin and speculated that increased mucin secretion could amplify inflammation and lead to secondary infection. Our own unpublished observations suggest that the SARS-CoV-2 spike protein per se can also augment MUC5AC production, independent of inflammation, in cultured airway epithelial cells. Additionally, a small study found that individuals with higher SARS-CoV-2 infectivity rates and disease severity had increased representation of a MUC5B promoter polymorphism known to also increase susceptibility to severe asthma.43 The authors concluded that polymorphisms in the MUC5B promoter influence infectivity rates and disease progression in humans. Since alterations in the abundance of mucins can change the biophysical properties (eg, viscosity) of mucus and impair mucociliary transport,2,39 it is also important to consider that impaired mucociliary transport could additionally make COVID-19 patients susceptible to secondary bacterial infection.114 Although lower airway mucociliary transport has not been measured in humans with COVID-19, nasal mucociliary clearance is impaired in COVID-19 patients.51
A large portion of COVID-19 patients develop pulmonary fibrosis and several mechanisms of viral immunopathology have been proposed.58,119,128 One consideration not often discussed is the potential influence and interaction of MUC5B in the context of COVID-19-related lung infection. For example, Seibold and colleagues reported that a polymorphism in the MUC5B promoter was associated with pulmonary fibrosis in humans.99 Interestingly, the MUC5B promoter polymorphism resulted in up to 14× greater MUC5B expression in the airways compared to wildtype.99 An additional study found that the same polymorphism was associated with interstitial lung disease, which has been proposed by some to represent early or mild fibrosis.83 Taken together, these observations suggest that in addition to obstructing the airways, altered MUC5B expression could contribute to pulmonary fibrosis.
Last, though we have highlighted excess mucus as a contributor to COVID-19, it is equally important to consider the protective role of mucus in the airway. For example, Roy and colleagues found that loss of MUC5B in mice impaired mucociliary clearance, leading to accumulation of materials in the airways.96 Consequently, mice lacking MUC5B also exhibited airway bacterial infections. Similar findings have been reported in pigs lacking submucosal glands and concomitant MUC5B originating from submucosal glands.79 Therefore, MUC5B is essential for proper mucociliary clearance. Though excess MUC5AC is typically associated with airway diseases, a study in mice demonstrated that excess MUC5AC increased protection against pathogens.18 Thus, it is possible that excess mucus in COVID-19 is a response meant to protect the airway.
Potential Mechanisms of Abnormal Mucus and Mucins in COVID-19
Typically, COVID-19 is associated with a significant type I immune response and subsequent release of pro-inflammatory cytokines. For example, multiple reports indicate that humans infected with SARS-CoV-2 had elevated IL-1β,11,64 where it can serve as a primary driver of inflammation in ARDS.19,32 Consistent with this, a small study revealed that blocking IL-1β signaling in COVID-19 patients improved patient outcome.12 In airway epithelium, IL-1β is a potent inducer of MUC5AC.27,33,34,46,105 One mechanism of IL-1β mediated induction of MUC5AC is through cAMP response element-binding protein (CREB)-dependent NF-kB-based transcriptional regulation.27,105 IL-1β was associated with increased abundance of MUC5B in patients with cystic fibrosis.13 Thus, these data indicate that MUC5AC and/or MUC5B are likely to be increased in COVID-19 through IL-1β signaling.
TNF-α is likely to play a critical role in COVID-19 disease and specifically mucus production. For example, Del Valle and colleagues found that TNF-α serum levels were a strong predictor of patient survival.15 In another study, use of a TNF-α inhibitor was associated with decreased risk of hospitalization in COVID-19.30 TNF-a induces numerous downstream cytokines and regulates both MUC5AC103 and MUC5B.104 In mice, for example, a 2-week administration of TNF-α increased MUC5AC expression in the airway.8 In intestinal cancer cells, TNF-α increased MUC5B, but not MUC5AC. Therefore, it is plausible that TNF-α directly or indirectly regulates mucin and contributes to abnormal mucus in COVID-19.20
Increasing evidence indicates that hyperinflammatory responses in some COVID-19 patients likely involve dysfunctional mast cells.1 Mast cells secrete large amounts of cytokines and chemokines and are generally thought of in the context of inducing allergic reactions. However, mast cells are a unique cell type that can both store presynthesized TNF-α granules for rapid release and also synthesize this cytokine de novo.31,91 Motta Junior and colleagues reported elevated mast cell density in a small cohort of airway samples from postmortem COVID-19 lung samples.72 An additional autopsy case report of an 87-year-old patient found high numbers of alveolar mast cells in the lung.90 Activation of mast cells is speculated to occur in COVID-19 through binding of SARS-CoV-2 to toll-like receptors located on mast cells98 or via cytokine activation.1
Histamine is a key mediator of tissue changes following mast cell degranulation. Histamine directly interacts with tissues expressing histamine receptors (H1-4) and can promote a variety of changes that could contribute to COVID-19 pathogenesis such as inflammation, platelet aggregation, broncho-constriction, vasodilation, edema, and mucus secretion.111 Given that mast cells, via release of histamine, are key regulators of mucins,48,120,125 it is tenable that sustained or abnormal mast cell responses contribute to airway disease in COVID-19 through multiple mechanisms, including inflammation and abnormal mucus (Fig. 1). If mast cells functionally contribute to COVID-19 disease, one could speculate that antihistamines have a therapeutic effect, in part through blocking the H1 receptor. Consistent with this speculation, coincident usage of antihistamines such as diphenhydramine, hydroxyzine, and azelastine was associated with reduced incidence of SARS-CoV-2 infection in elderly subjects (>61 years).89 Similarly, in one study early in the pandemic, nursing home residents (N = 84, mean age 85 years) with suspected clinical COVID-19 were treated with antihistamines + azithromycin. All antihistamine-treated residents were later confirmed as seropositive for SARS-CoV-2, indicative of prior SARS-CoV2 infection. All antihistamine-treated residents in this study survived without hospitalization or death, whereas patient hospitalizations and deaths were present in the same facility immediately prior to the start of the study. This observation was also in stark contrast to the average 28% COVID-19 mortality observed at comparable nursing homes without an antihistamine treatment protocol over the same time period, suggesting that antihistamine therapy may have limited progression to severe disease.71 While it is interesting to speculate that antihistamines reduce clinical progression of COVID-19 through antagonism of histamine binding with H1 receptor, other histamine receptors and mechanisms could be involved. For example, angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS-CoV-2 and antihistamines directly affect the interaction of the virus with ACE2.77,89
Figure 1.
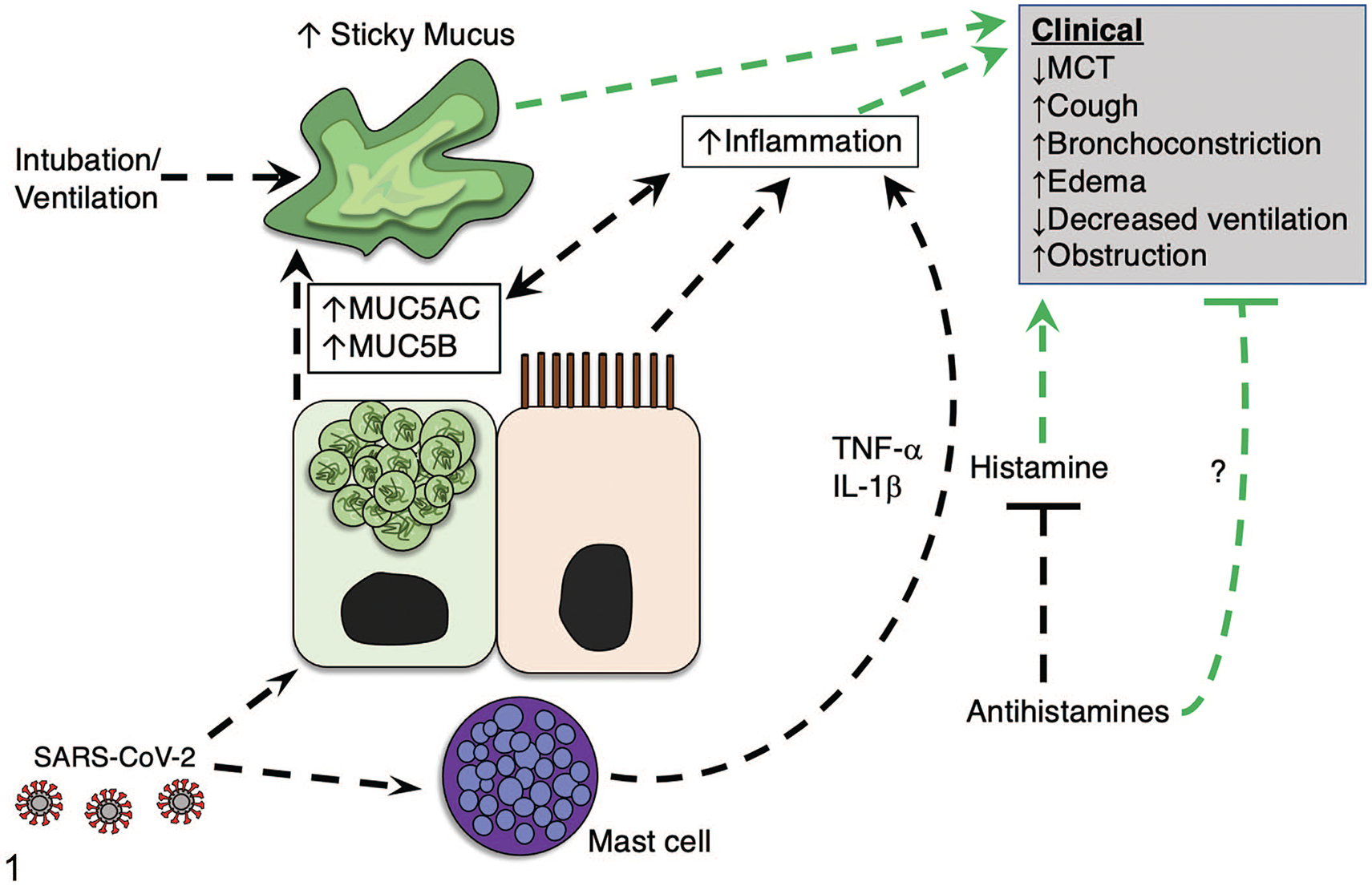
Proposed model indicating the influence of mast cells and histamine on mucus production in COVID-19. Mounting evidence suggests that SARS-CoV-2 infection increases mucus production. We propose that SARS-CoV-2 may influence mucus through activating mast cells directly or via adjacent epithelia, leading to mast cell degranulation. As a result, inflammatory mediators (eg, IL-1β and TNF-α), and histamine released from mast cells contribute to the elevated inflammatory state and exacerbate abnormal mucus production, aggravating clinical respiratory disease. Additionally, histamine released from mast cells induces clinical parameters such as cough, edema, and airway broncho-constriction. An increase in mucus production can result in abnormally sticky mucus that impedes mucociliary transport to promote airway obstruction and limit subsequent airway ventilation. Antihistamines may be useful agents to block histamine signaling (and possibly other mechanisms) and mitigate clinical severity of COVID-19. Green lines indicate modifiers of clinical disease. Abbreviations: MCT, mucociliary transport; MUC5AC, mucin5AC; MUC5B, mucin5B; IL-1β, interleukin 1-β; TNF-α, tumor necrosis factor-α.
Though inflammatory mediators can drive mucin expression by directly increasing mucin expression at the transcriptional level, these mediators can induce mucous cell metaplasia and hyperplasia resulting in increased airway mucus. For example, in asthma, mucous cell metaplasia is driven by type II inflammatory mediators, including IL-13 and IL-4. Respiratory syncytial virus may also induce airway goblet cell metaplasia through type II inflammatory mediators.75,107 While there is no peer-reviewed literature to indicate that SARS-CoV-2 causes mucous cell metaplasia, chronic TNF-α treatment in mice has been reported to induce mucous cell metaplasia.8 Similarly, overexpression of IL-1β in mouse airways also induces mucous cell metaplasia,54 whereas mucous cell hyperplasia in mice with experimental allergic asthma has been shown to partly depend on mast cells.126 These data highlight that in addition to transcriptional regulation, inflammatory mediators associated with SARS-CoV-2 also have the capacity to increase mucin production through eliciting mucous cell metaplasia and hyperplasia.
In intubated and ventilated individuals with COVID-19, it is important to consider how other nonviral factors such as secondary bacterial infection and/or mechanical inflation pressures can contribute to increased mucus production. High incidence of secondary infections has been reported in people hospitalized with COVID-19, including infection with Pseudomonas aeruginosa and Staphylococcus aureus.92,100 Since bacterial cell wall components regulate mucin production,56,57 increased mucus could also be a consequence of bacterial infection. It is also possible that epithelial stress due to mechanical ventilation increases mucus production. For example, ATP is released in response to mechanical stress,9 and studies suggest that ATP stimulates mucin release.52 Thus, these factors in addition to (or independent of) inflammatory mediators may promote excess mucus.
Challenges for Modeling SARS-CoV-2 and Airway Mucus
Emergence of the COVID-19 pandemic has highlighted an important role of animal models to study SARS-CoV-2 infection.73 Selection of an animal model for SARS-CoV-2 requires many considerations, including infrastructure for animal care, permissiveness to viral replication or transmission, evaluation of vaccines or therapies, clinical disease, and pathology.53 While several animal models have been successfully studied to investigate SARS-CoV-2 infection28,66,97,102,108 or airway mucus,21,23,26,44,68,78,96 consideration for the strengths and limitations of each animal model as it relates to the human condition should be considered when studying mucus in the context of COVID-19.
In humans, cartilaginous airways extend from the trachea into the intrapulmonary bronchi and are associated with the presence of submucosal glands, a rich source of airway secretions including mucus.68 In contrast, bronchioles are small conducting airways identified by a lack cartilage or submucosal glands. Goblet cells are preferentially found in the surface epithelium in large airways and serve as another source of airway mucus that can function with submucosal gland mucus to facilitate mucociliary transport.78 Of the 2 common mucins in airways, MUC5AC is restricted to goblet cells of the surface epithelium whereas MUC5B is present in mucous cells of the submucosal glands and in goblet cells.69 These 2 secreted mucins have distinct mucus morphology and interaction in their respective roles for normal mucociliary transport.78 Regional expression of genes and local environment influence secreted mucus properties. For instance, ATP12A is a nongastric H+/K+ transporter that can acidify secreted airway surface liquid of trachea and bronchi in disease conditions such as cystic fibrosis. This acidification can cause mucus to become more viscous and tenacious,88,101,110 and enhance airway reactivity leading to exaggerated airway contraction and mucus secretion.
Considerations to study airway mucus in animal models should include airway structural anatomy, cellularity, and gene expression, as differences in these parameters influence translatability to humans. In addition, for COVID-19 studies, evaluation of mucus should include a model that has a reasonable ability to be infected by SARS-CoV-2. Mouse models are a convenient tool to study SARS-CoV-2 infection given variety of approaches and strains available for use.73,108,129 While mouse models have successfully been used to study respiratory mucus in various diseases,23,50,96,130 they have some inherent limitations for translatability of mucus studies to human disease. Within the mouse lung, the conducting airways are exclusively bronchioles.68,95 Murine bronchioles lack mucus-secreting submucosal glands and goblet cells, but when stimulated, the bronchiolar club cells can be transformed into mucus producing cells.86 These and other features indicate the mouse trachea and lungs are most similar in structure, cellularity, and function to the distal small airways and pulmonary acinus in humans (Table 1). While mice are useful to test SARS-CoV-2 infection, these and other small rodent models are limited to study the interaction of mucus derived primarily from submucosal glands and goblet cells of surface epithelia. Hamsters are another rodent that is amendable to SARS-CoV-2 infection,102 but these rodents also have submucosal glands in restricted distribution to the upper trachea and primary extrapulmonary bronchus, similar to mice and rats.123
Table 1.
Comparison of select structural, cellular, and expression parameters between human and mouse airways related to airway mucus.
| Airway parameter | Human trachea |
Human distal airways |
Mouse trachea |
|---|---|---|---|
| Caliber (mm)74,80,85 | 15–20 | 0.5–1.0 | ~1.5 |
| Submucosal glands (μL cm−2)14,70 | 25–40 | Zero | Zero to rare |
| Surface epithelium height (μm)87,94 | 50–100 | 15 | 11–14 |
| Club cells (% of surface epithelium)81 | 0 | 11–41 | >50 |
| Goblet cells (% of surface epithelium)81,87 | 9 | 0–2 | <1 |
| ATP12A expression101 | High | Nominal to absent | Nominal to absent |
Large and medium-sized animal models can help fill the limitations of small rodents as candidates for study of mucus in SARS-CoV-2 infection. For example, ferrets have intrapulmonary bronchi with submucosal glands and are susceptible to SARS-CoV-2 infection.37,45,47,63 Similarly, cats have intrapulmonary bronchi with submucosal glands, are a well-known veterinary model of asthma, and are naturally susceptible to SARS-CoV-2.40,115 Pigs have airway structure, cell lineages, and composition similar to humans and have submucosal glands in intrapulmonary bronchi.95 However, the use of adult pigs is limited because of their apparent lack of susceptibility to SARS-CoV-2 infection.65,117 Nonhuman primates are an additional model of SARS-CoV-2 infection.59 Like humans, nonhuman primates have a dichotomous pattern of airway branching and intrapulmonary bronchi with submucosal glands.82 Nonhuman primates (like humans and pigs) also have mucous cells in the surface epithelium of airways under basal conditions, which contrasts with mice. Therefore, these anatomical and physiological differences among animal models should be carefully considered when interpreting lung pathology in response to SARS-CoV-2, especially as it relates to airway mucus.
Based on current evidence, the cat, nonhuman primate, and ferret appear to be useful animal models to study mucus in SARS-CoV-2 infection. All exhibit natural SARS-CoV-2 infection and have ACE2 structure similar to humans.59,122,127 However, the ferret has several advantages over cats and non-human primates, which are likely to appeal to investigators. First, the ferret is increasingly used as a model to understand human diseases, and particularly to study both infectious and noninfectious airway diseases.4,109 It was used to study the initial SARS coronavirus nearly 2 decades ago.62 Thus, there is significant information available regarding mucus abnormalities in ferrets, as well as detailed information on conducting coronavirus studies in ferrets. Furthermore, ferrets are cost-effective, easily handled, and housed conventionally, which is an advantage compared with cats and nonhuman primates.42 Additionally, due to their increasing use in biomedical research, reagents specific for ferret studies are now available. Furthermore, though nonhuman primates have been used to study human airway diseases, there are reportedly species-specific differences in their ability to reproduce some features of COVID-19.59 Last, the societal and cultural perceptions surrounding animal research, especially those involving domestic cats and nonhuman primates, may limit their use.3
Conclusions
Mucus is an important pathological feature in multiple airway diseases. Mast cells can regulate mucus composition and secretion via histamine and other secreted mediators. Abnormal mucus in SARS-CoV-2 infection can obstruct the airways to interfere with ventilation strategies or promote secondary infections due to inhibition of mucociliary clearance. Many of the cytokines responsible for severe COVID-19 are known regulators of mucins, the major constituents of airway mucus. Therefore, it is anticipated that mucus is abnormal in SARS-CoV-2 infections. Consistent with this, multiple studies indicate that the amount, composition, and/or biophysical properties of mucus in COVID-19 patients is abnormal. Such information could have a significant impact on clinical treatment and care now and extending into the future.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the National Institutes of Health (OD026582, HL152101, HL091842, HL051670, DK054759) and the Cystic Fibrosis Foundation.
Footnotes
Declarations of Conflicting Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Afrin LB, Weinstock LB, Molderings GJ. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atanasova KR, Reznikov LR. Strategies for measuring airway mucus and mucins. Respir Res. 2019;20(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck AP, Meyerholz DK. Evolving challenges to model human diseases for translational research. Cell Tissue Res. 2020;380(2):305–311. [DOI] [PubMed] [Google Scholar]
- 4.Belser JA, Katz JM, Tumpey TM. The ferret as a model organism to study influenza A virus infection. Dis Model Mech. 2011;4(5):575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branson RD. Secretion management in the mechanically ventilated patient. Respir Care. 2007;52(10):1328–1342. [PubMed] [Google Scholar]
- 7.Burgel PR, Montani D, Danel C, et al. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse PJ, Zhang TF, Srivastava K, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116(6):1256–1263. [DOI] [PubMed] [Google Scholar]
- 9.Button BM, Button B. Structure and function of the mucus clearance system of the lung. Cold Spring Harb Perspect Med. 2013;3(8):a009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauchois R, Koubi M, Delarbre D, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117(32):18951–18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020; 2(6):e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Sun L, Kato T, et al. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest. 2019;129(10):4433–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat. 2000;197(pt 3):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deptula N, Royse E, Kemp MW, et al. Brief mechanical ventilation causes differential epithelial repair along the airways of fetal, preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2016;311(2):L412–L420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128(3):997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehre C, Worthington EN, Liesman RM, et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci U S A. 2012;109(41):16528–16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019;4(2):e124061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enss ML, Cornberg M, Wagner S, et al. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res. 2000;49(4):162–169. [DOI] [PubMed] [Google Scholar]
- 21.Erickson NA, Gruber AD, Mundhenk L. The family of chloride channel regulator, calcium-activated proteins in the feline respiratory tract: a comparative perspective on airway diseases in man and animal models. J Comp Pathol. 2020;174:39–53. [DOI] [PubMed] [Google Scholar]
- 22.Evans CM, Kim K, Tuvim MJ, et al. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans CM, Raclawska DS, Ttofali F, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122(6 suppl):320S–326S. [DOI] [PubMed] [Google Scholar]
- 25.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Petty CM, Hughes GW, Bowers HL, et al. A glycopolymer improves vascoelasticity and mucociliary transport of abnormal cystic fibrosis mucus. JCI Insight. 2019;4(8):e125954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujisawa T, Velichko S, Thai P, et al. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009; 183(10):6236–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaudreault NN, Trujillo JD, Carossino M, et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect. 2020;9(1):2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. [DOI] [PubMed] [Google Scholar]
- 30.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990;11(12):458–464. [DOI] [PubMed] [Google Scholar]
- 32.Grailer JJ, Canning BA, Kalbitz M, et al. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192(12):5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray T, Coakley R, Hirsh A, et al. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L320–L330. [DOI] [PubMed] [Google Scholar]
- 34.Gray T, Nettesheim P, Loftin C, et al. Interleukin-1beta-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase a signaling. Mol Pharmacol. 2004;66(2):337–346. [DOI] [PubMed] [Google Scholar]
- 35.Gum JR Jr. Mucin genes and the proteins they encode: structure, diversity, and regulation. Am J Respir Cell Mol Biol. 1992;7(6):557–564. [DOI] [PubMed] [Google Scholar]
- 36.He J, Cai S, Feng H, et al. Single-cell analysis reveals bronchoalveolar epithelial dysfunction in COVID-19 patients. Protein Cell. 2020;11(9):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helke KL, Meyerholz DK, Beck AP, et al. Research relevant background lesions and conditions: ferrets, dogs, swine, sheep, and goats. ILAR J. Published online March 13, 2021. doi: 10.1093/ilar/ilab005 [DOI] [PubMed] [Google Scholar]
- 38.Henke MO, John G, Germann M, et al. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175(8):816–821. [DOI] [PubMed] [Google Scholar]
- 39.Hoegger MJ, Fischer AJ, McMenimen JD, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345(6198):818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosie MJ, Hofmann-Lehmann R, Hartmann K, et al. Anthropogenic infection of cats during the 2020 COVID-19 pandemic. Viruses. 2021;13(2):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2020;19(3):141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Institute of Medicine, National Research Council. International Animal Research Regulations: Impact on Neuroscience Research. National Academies Press; 2012. [PubMed] [Google Scholar]
- 43.Iyer GR, Samajder S, Zubeda S, et al. Infectivity and progression of COVID-19 based on selected host candidate gene variants. Front Genet. 2020;11:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jamil S, Christensen TG. Phenotypic variation in hamster bronchial mucous cells induced by different airway irritants. Int J Exp Pathol. 1997;78(3):163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson-Delaney CA, Orosz SE. Ferret respiratory system: clinical anatomy, physiology, and disease. Vet Clin North Am Exot Anim Pract. 2011;14(2):357–367. [DOI] [PubMed] [Google Scholar]
- 46.Kim YD, Kwon EJ, Park DW, et al. Interleukin-1beta induces MUC2 and MUC5AC synthesis through cyclooxygenase-2 in NCI-H292 cells. Mol Pharmacol. 2002;62(5):1112–1118. [DOI] [PubMed] [Google Scholar]
- 47.Kim YI, Kim SG, Kim SM, et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YM, Won TB, Kim SW, et al. Histamine induces MUC5AC expression via a hCLCA1 pathway. Pharmacology. 2007;80(4):219–226. [DOI] [PubMed] [Google Scholar]
- 49.Kirkham S, Kolsum U, Rousseau K, et al. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(10):1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koeppen M, McNamee EN, Brodsky KS, et al. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 2013;6(4):762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koparal M, Kurt E, Altuntas EE, et al. Assessment of mucociliary clearance as an indicator of nasal function in patients with COVID-19: a cross-sectional study. Eur Arch Otorhinolaryngol. 2021;278(6):1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreda SM, Okada SF, van Heusden CA, et al. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584(pt 1):245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S, Yadav PK, Srinivasan R, et al. Selection of animal models for COVID-19 research. Virusdisease. 2020;31(4):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lappalainen U, Whitsett JA, Wert SE, et al. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–318. [DOI] [PubMed] [Google Scholar]
- 55.Leviner S. Recognizing the clinical sequelae of COVID-19 in adults: COVID-19 long-haulers. J Nurse Pract. 2021;17(8):946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li JD, Dohrman AF, Gallup M, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997;94(3):967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Yan F, Zhou H, et al. P. aeruginosa lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly through Duox1 in vitro and in vivo. PLoS One. 2013;8(5):e63945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Shen C, Wang L, et al. Pulmonary fibrosis and its related factors in discharged patients with new corona virus pneumonia: a cohort study. Respir Res. 2021;22(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu S, Zhao Y, Yu W, et al. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct Target Ther. 2020;5(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu W, Liu X, Wang T, et al. Elevated MUC1 and MUC5AC mucin protein levels in airway mucus of critical ill COVID-19 patients. J Med Virol. 2021;93(2):582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manckoundia P, Franon E. Is persistent thick copious mucus a long-term symptom of COVID-19? Eur J Case Rep Intern Med. 2020;7(12):002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martina BE, Haagmans BL, Kuiken T, et al. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425(6961):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarron A, Donnelley M, Parsons D. Airway disease phenotypes in animal models of cystic fibrosis. Respir Res. 2018;19(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202(6):812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meekins DA, Morozov I, Trujillo JD, et al. Susceptibility of swine cells and domestic pigs to SARS-CoV-2. bioRxiv. 2020. doi: 10.1101/2020.08.15.252395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meekins DA, Morozov I, Trujillo JD, et al. Susceptibility of swine cells and domestic pigs to SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyerholz DK, Beck AP, Goeken JA, et al. Glycogen depletion can increase the specificity of mucin detection in airway tissues. BMC Res Notes. 2018;11(1):763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyerholz DK, Lambertz AM, Reznikov LR, et al. Immunohistochemical detection of markers for translational studies of lung disease in pigs and humans. Toxicol Pathol. 2016;44(3):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyerholz DK, Suarez CJ, Dintzis SM, et al. Comparative Anatomy and Histology: A Mouse, Rat and Human Atlas. 2nd ed. Academic Press; 2018. [Google Scholar]
- 71.Moran Blanco JI, Alvarenga Bonilla JA, Homma S, et al. Antihistamines and azithromycin as a treatment for COVID-19 on primary health care—a retrospective observational study in elderly patients. Pulm Pharmacol Ther. 2021;67:101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Motta Junior JDS, Miggiolaro AFR, Nagashima SD, et al. Mast cells in alveolar septa of COVID-19 patients: a pathogenic pathway that may link interstitial edema to immunothrombosis. Front Immunol. 2020;11:574862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munoz-Fontela C, Dowling WE, Funnell SGP, et al. Animal models for COVID-19. Nature. 2020;586(7830):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Navarro M, Ruberte J, Carretero A.. Respiratory apparatus. In: Ruberte J, Carretero A, Navarro M, eds. Morphological Mouse Phenotyping: Anatomy, Histology and Imaging. Academic Press; 2017. [Google Scholar]
- 75.Norlander AE, Peebles RS Jr. Innate type 2 responses to respiratory syncytial virus infection. Viruses. 2020;12(5):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York city. JAMA Netw Open. 2020;3(12):e2026881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ortiz ME, Thurman A, Pezzulo AA, et al. Heterogeneous expression of the SARS-coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine. 2020;60:102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ostedgaard LS, Moninger TO, McMenimen JD, et al. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci U S A. 2017;114(26):6842–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ostedgaard LS, Price MP, Whitworth KM, et al. Lack of airway submucosal glands impairs respiratory host defenses. Elife. 2020;9:e59653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinkerton KE, Van Winkle LS, Plopper CG, et al. Architecture of the tracheo-bronchial tree. In: Parent RA, ed. Comparative Biology of the Normal Lung. 2nd ed. Academic Press; 2015. [Google Scholar]
- 81.Plopper CG, Hyde DM. Epithelial cells of the bronchiole. In: Parent RA, ed. Comparative Biology of the Normal Lung. 2nd ed. Academic Press; 2015. [Google Scholar]
- 82.Plopper CG, Hyde DM. The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther. 2008;21(5):755–766. [DOI] [PubMed] [Google Scholar]
- 83.Putman RK, Gudmundsson G, Araki T, et al. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J. 2017;50(3):1700537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quan D, Luna Wong L, Shallal A, et al. Impact of race and socioeconomic status on outcomes in patients hospitalized with COVID-19. J Gen Intern Med. 2021;36(5):1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ravaglia C, Poletti V. Bronchiolitis and bronchiolar disorders. Semin Respir Crit Care Med. 2020;41(2):311–332. [DOI] [PubMed] [Google Scholar]
- 86.Reader JR, Tepper JS, Schelegle ES, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol. 2003;162(6):2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynolds SD, Pinkerton KE, Mariassy AT. Epithelial cells of the trachea and bronchi. In: Parent RA, ed. Comparative Biology of the Normal Lung. 2nd ed. Academic Press; 2015. [Google Scholar]
- 88.Reznikov LR, Meyerholz DK, Adam RJ, et al. Acid-sensing ion channel 1a contributes to airway hyperreactivity in mice. PLoS One. 2016;11(11):e0166089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reznikov LR, Norris MH, Vashisht R, et al. Identification of antiviral antihistamines for COVID-19 repurposing. Biochem Biophys Res Commun. 2021;538:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ribeiro Dos Santos Miggiolaro AF, da Silva Motta Junior J, Busatta Vaz de Paula C, et al. Covid-19 cytokine storm in pulmonary tissue: anatomopathological and immunohistochemical findings. Respir Med Case Rep. 2020;31:101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rijnierse A, Koster AS, Nijkamp FP, et al. TNF-alpha is crucial for the development of mast cell-dependent colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G969–G976. [DOI] [PubMed] [Google Scholar]
- 92.Ripa M, Galli L, Poli A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27(3):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogers AJ, Solus JF, Hunninghake GM, et al. MUC5B promoter polymorphism and development of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;198(10):1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rogers AV, Dewar A, Corrin B, et al. Identification of serous-like cells in the surface epithelium of human bronchioles. Eur Respir J. 1993;6(4):498–504. [PubMed] [Google Scholar]
- 95.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295(2):L240–L263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roy MG, Livraghi-Butrico A, Fletcher AA, et al. Muc5b is required for airway defence. Nature. 2014;505(7483):412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryan KA, Bewley KR, Fotheringham SA, et al. Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity. Nat Commun. 2021;12(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012;3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shafran N, Shafran I, Ben-Zvi H, et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11:12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah VS, Meyerholz DK, Tang XX, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smirnova MG, Birchall JP, Pearson JP. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine. 2000;12(11):1732–1736. [DOI] [PubMed] [Google Scholar]
- 104.Smirnova MG, Kiselev SL, Birchall JP, et al. Up-regulation of mucin secretion in HT29-MTX cells by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-6. Eur Cytokine Netw. 2001;12(1):119–125. [PubMed] [Google Scholar]
- 105.Song KS, Lee WJ, Chung KC, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003;278(26):23243–23250. [DOI] [PubMed] [Google Scholar]
- 106.Ssentongo P, Ssentongo AE, Heilbrunn ES, et al. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One. 2020;15(8):e0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stokes KL, Currier MG, Sakamoto K, et al. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol. 2013;87(18):10070–10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun J, Zhuang Z, Zheng J, et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020;182(3):734–743.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun X, Yan Z, Yi Y, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118(4):1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang XX, Ostedgaard LS, Hoegger MJ, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126(3):879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thangam EB, Jemima EA, Singh H, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. 2018;9:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thornton DJ, Sheehan JK, Carlstedt I. Heterogeneity of mucus glycoproteins from cystic fibrotic sputum. Are there different families of mucins? Biochem J. 1991;276(pt 3):677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turkovic L, Caudri D, Rosenow T, et al. Presence of mucus plugging is predictive of long term lung function in children with cystic fibrosis. Eur Resp J. 2017;50:OA4401. [Google Scholar]
- 114.Vaillancourt M, Jorth P. The unrecognized threat of secondary bacterial infections with COVID-19. mBio. 2020;11(4):e01806–e01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venema CM, Williams KJ, Gershwin LJ, et al. Histopathologic and morphometric evaluation of the nasal and pulmonary airways of cats with experimentally induced asthma. Int Arch Allergy Immunol. 2013;160(4):365–376. [DOI] [PubMed] [Google Scholar]
- 116.Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med. 2012;2(11):a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vergara-Alert J, Rodon J, Carrillo J, et al. Pigs are not susceptible to SARS-CoV-2 infection but are a model for viral immunogenicity studies. Transbound Emerg Dis. 2020;68(4):1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang C, Xie J, Zhao L, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57:102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang J, Wang BJ, Yang JC, et al. Research advances in the mechanism of pulmonary fibrosis induced by coronavirus disease 2019 and the corresponding therapeutic measures [in Chinese]. Zhonghua Shao Shang Za Zhi. 2020;36(8):691–697. [DOI] [PubMed] [Google Scholar]
- 120.Wang W, Shao S, Wang S. The role for human nasal epithelial nuclear factor kappa B activation in histamine-induced mucin 5 subtype B overproduction. Int Forum Allergy Rhinol. 2016;6(3):264–270. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Zhang M, Yu Y, et al. Sputum characteristics and airway clearance methods in patients with severe COVID-19. Medicine (Baltimore). 2020;99(46):e23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei Y, Aris P, Farookhi H, et al. Predicting mammalian species at risk of being infected by SARS-CoV-2 from an ACE2 perspective. Sci Rep. 2021;11(1):1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Widdicombe JH, Chen LL, Sporer H, et al. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 2001;198(pt 2):207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams OW, Sharafkhaneh A, Kim V, et al. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol. 2006;34(5):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamauchi K, Ogasawara M. The role of histamine in the pathophysiology of asthma and the clinical efficacy of antihistamines in asthma therapy. Int J Mol Sci. 2019;20(7):1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu M, Tsai M, Tam SY, et al. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116(6):1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zamoto A, Taguchi F, Fukushi S, et al. Identification of ferret ACE2 and its receptor function for SARS-coronavirus. Adv Exp Med Biol. 2006;581:519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang C, Wu Z, Li JW, et al. Discharge may not be the end of treatment: pay attention to pulmonary fibrosis caused by severe COVID-19. J Med Virol. 2021;93(3):1378–1386. [DOI] [PubMed] [Google Scholar]
- 129.Zheng J, Wong LYR, Li K, et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589(7843):603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou-Suckow Z, Duerr J, Hagner M, et al. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367(3):537–550. [DOI] [PubMed] [Google Scholar]


