Abstract
This review describes the pharmacokinetics, efficacy, and safety of gadopiclenol, a new macrocyclic gadolinium-based contrast agent (GBCA) recently approved by the Food and Drug Administration at the dose of 0.05 mmol/kg. Gadopiclenol is a high relaxivity contrast agent that shares similar pharmacokinetic characteristics with other macrocyclic GBCAs, including a predominant renal excretion. In pediatric patients aged 2–17 years, the pharmacokinetic parameters (assessed through a population pharmacokinetics model) were comparable to those observed in adults, indicating no need for age-based dose adjustment. For contrast-enhanced magnetic resonance imaging (MRI) of the central nervous system (CNS) and body indications, gadopiclenol at 0.05 mmol/kg was shown to be noninferior to gadobutrol at 0.1 mmol/kg in terms of 3 lesion visualization parameters (ie, lesion border delineation, internal morphology, and contrast enhancement). Moreover, for contrast-enhanced MRI of the CNS, compared with gadobenate dimeglumine at 0.1 mmol/kg, gadopiclenol exhibited superior contrast-to-noise ratio at 0.1 mmol/kg and comparable contrast-to-noise ratio at 0.05 mmol/kg. A pooled safety analysis of 1047 participants showed a favorable safety profile for gadopiclenol. Comparative studies showed that the incidence and nature of adverse drug reactions with gadopiclenol were comparable to those observed with other GBCAs. Importantly, no significant safety concerns were identified in pediatric and elderly patients, as well as in patients with renal impairment. Overall, these findings support the clinical utility and safety of gadopiclenol for MRI in adult and pediatric patients aged 2 years and older in CNS and body indications.
Key Words: gadopiclenol, gadolinium-based contrast agent (GBCA), pharmacokinetics, efficacy, safety, MRI, central nervous system (CNS), body, pediatric patients
Gadolinium-based contrast agents (GBCAs) have demonstrated their pivotal role in improving the diagnostic potential of magnetic resonance imaging (MRI), by providing enhanced visualization and facilitating lesion detection and characterization. Contrast-enhanced MRI is widely used to assess disease burden, progression, and treatment response.1–3 Based on their structure, GBCAs can be classified into macrocyclic and linear agents, and can be further classified depending on the charge into ionic and nonionic agents.4 Linear GBCAs are generally less stable than macrocyclic ones and have a higher likelihood of releasing gadolinium ions.5
Since their introduction in 1988, GBCAs have been administered to over 700 million patients worldwide, with an estimated annual utilization rate of approximately 50 million procedures.6 Several studies have shown an increased use of GBCAs, in correlation with the increasing number of MRI examinations performed.7 For instance, it has been estimated that GBCAs are administered in approximately 45% of MRI examinations conducted in the United States.8 Nonetheless, the use of GBCAs has recently prompted substantial scrutiny and concern within the medical community regarding the safety profile and associated risks of GBCAs.
Up to 2006, all approved GBCAs were considered safe, due to a low incidence of adverse events (AEs).9 Yet, in 2006, nephrogenic systemic fibrosis (NSF) was linked to GBCA administration for the first time.10 Nephrogenic systemic fibrosis is a potentially fatal fibrosing disorder that involves the skin and other internal organs. Nephrogenic systemic fibrosis develops exclusively in patients with renal impairment who have received GBCAs, and 98% of the reported NSF cases were associated with linear GBCAs (ie, gadodiamide, gadopentetate dimeglumine).11
Another concern regarding the use of GBCAs, especially the linear ones, was their tendency to accumulate within various tissues of the human body, a phenomenon occurring even in patients with normal renal function.12–14 Initial clinical investigations assessed gadolinium deposition by examining tissue samples. In one study, gadolinium was measurable in bone samples of patients who have received GBCAs, in contrast to those who did not receive such agents, and higher amounts were quantified when the linear GBCA gadodiamide was used in comparison to the macrocyclic agent gadoteridol.13 Another study performed on brain tumor biopsies from patients who previously underwent at least 1 contrast-enhanced MRI examination also highlighted the presence of gadolinium-containing deposits.12 Gadolinium deposition in the brain was confirmed by Kanda et al15 who have reported an increase in signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images on patients previously exposed to linear GBCAs. Following these reports, and contrary to the macrocyclic GBCAs, the linear GBCAs were suspended from the European market, except for the liver-specific agents (gadoxetate disodium and gadobenate dimeglumine) when used for liver scans, and gadopentetate dimeglumine when given intra-articularly for joint scans.16 On the other hand, the Food and Drug Administration requested performing preclinical and clinical studies to assess the potential long-term consequences of repeated administrations of GBCAs on motor and cognitive functions.17
Although there is currently no conclusive evidence from well-designed studies indicating adverse biological or neurological effects of gadolinium deposition, cautious decision-making is still advised when considering GBCA administration. This is especially true for children, patients with renal insufficiency, or those needing multiple lifetime doses.18 Gadolinium-based contrast agents should be administered only when clinically necessary and at the lowest effective dose.
In addition, there is growing concern about the environmental impact of GBCAs by contaminating the aquatic environment after excretion by the patients.19,20
To address these concerns, there is a need to develop new contrast agents with high relaxivity, allowing the administration of a lower dose (therefore reducing the total amount of gadolinium administered to patients) as well as enhanced image quality and improved diagnostic accuracy.21
Gadopiclenol (marketed as Elucirem by Guerbet, and Vueway by Bracco) is a novel nonspecific macrocyclic GBCA that exhibits a higher r1 relaxivity (ie, 12.8/11.6 mM−1 s−1 in human plasma at 37°C, at 1.5 and 3 T, respectively) when compared with currently available GBCAs such as gadobutrol (5.2/5 mM−1 s−1), gadoteridol (4.1/3.7 mM−1 s−1), or gadoterate meglumine (3.6/3.5 mM−1 s−1).22 The stability of GBCAs depends on the dissociation rates of gadolinium from its ligand, which is based on their kinetic (ie, the speed at which gadolinium ion is released) and thermodynamic stabilities (ie, the affinity of gadolinium for its ligand). Gadopiclenol has a standard thermodynamic stability (ie, Log KTherm) of 18.7, and a high kinetic inertness under acidic conditions (ie, pH 1.2), with a dissociation half-life of 20 ± 3 days. Gadopiclenol does not interact with plasma proteins.22
The purpose of this article is to review the diagnostic efficacy of the new GBCA, gadopiclenol, in patients undergoing diagnostic contrast-enhanced MRI of the central nervous system (CNS) or other body regions. In addition, the article aimed to evaluate the global safety of gadopiclenol based on all clinical studies performed so far and provide a concise overview of its pharmacokinetic profile.
PHARMACOKINETICS OF GADOPICLENOL
The pharmacokinetics of gadopiclenol was previously evaluated in 3 clinical studies including adult healthy volunteers,23,24 adults with renal impairment,23 and pediatric patients aged 2–17 years.25 In adult healthy volunteers, the pharmacokinetic of gadopiclenol is linear, with a terminal elimination half-life of 1.5 to 2 hours. Gadopiclenol is distributed within the extracellular water compartment and has a distribution volume consistent with this distribution characteristics (ie, 182–254 mL/kg). Gadopiclenol is mainly eliminated via renal excretion, with 98% of the administered dose recovered in urine under unchanged form within 48 hours.24 The systemic exposure of gadopiclenol, assessed by the area under the curve gradually increased with the degree of renal impairment (54%, 148%, and 769% higher in adults with mild [eGFR: 60–89 mL/min], moderate [eGFR: 30–59 mL/min], or severe [eGFR: 15–29 mL/min] renal impairment, respectively), and its terminal half-life was prolonged as well (3.3, 3.8, and 11.7 hours for patients with mild, moderate, and severe renal impairment, respectively).23 Gadopiclenol renal clearance in this population was delayed with the degree of renal impairment (96% [96 hours postinjection], 92% [120 hours postinjection], and 84% [168 hours postinjection] in adults with mild, moderate, or severe renal impairment, respectively).23
A population pharmacokinetics approach was used to assess the pharmacokinetics of gadopiclenol in pediatric patients.25 Only minor differences were reported between pediatric patients and adults with a median clearance ranging between 0.08 L/h/kg for the 12- to 17-year-old patients and 0.12 L/h/kg for the 2- to 6-year-old patients (vs 0.08 L/h/kg for adults). The median terminal half-life ranged between 1.29 hours for the 2- to 6-year-old patients and 1.77 hours for the 12- to 17-year-old patients (vs 1.82 hours for adults). It was judged that there is no need for age-based dose adaptation of gadopiclenol in pediatric patients aged between 2 and 17 years.25
EFFICACY OF GADOPICLENOL
Several studies have been conducted to assess the efficacy of gadopiclenol across various indications for MRI, including the CNS and other anatomical regions of the body such as head and neck, breast, abdomen, pelvis, and musculoskeletal system. Gadopiclenol, given at different doses, was compared with 2 other GBCAs, namely, gadobenate dimeglumine and gadobutrol, both used at the standard dose of 0.1 mmol/kg.
Gadopiclenol Efficacy in MRI of the Central Nervous System
An international, double-blind, randomized, controlled, cross-over phase IIb study was conducted on patients exhibiting highly suspicious CNS lesions.26 A total of 240 patients underwent 2 MRI scans separated by a time interval of 2–14 days. The patients were randomly administered gadopiclenol at a dose of 0.025, 0.05, 0.1, or 0.2 mmol/kg, for the first MRI and gadobenate dimeglumine at a dose of 0.1 mmol/kg for the second MRI, or vice versa.26 The majority of patients (72%) presented with brain tumors, 22% had brain or spine metastases, and 6% presented with other pathologies.
The primary evaluation criterion was contrast-to-noise ratio (CNR) assessed by 3 independent readers. Compared with gadobenate dimeglumine at 0.1 mmol/kg, CNR was 32% to 45% higher with gadopiclenol at 0.1 mmol/kg (P ≤ 0.0007 for all 3 readers), and at similar level with gadopiclenol at 0.05 mmol/kg (P ≥ 0.19 for all 3 readers).26 Likewise, the secondary evaluation criteria lesion-to-brain ratio (LBR), which quantify the visibility of a lesion relative to the surrounding normal tissue, and the percentage of contrast enhancement (increase in the lesion's signal intensity relative to its baseline value) were both significantly higher with gadopiclenol at 0.1 mmol/kg (P < 0.0002), and of similar magnitude with gadopiclenol at 0.05 mmol/kg (P ≥ 0.11 for all 3 readers) compared with gadobenate dimeglumine at 0.1 mmol/kg.26
As for the diagnostic preference, the 3 readers expressed in majority a preference for images with gadopiclenol when used at 0.1 mmol/kg (45.3% to 86.8% of images), and in majority no preference between images with gadopiclenol at 0.05 mmol/kg and gadobenate dimeglumine at 0.1 mmol/kg (46.6% to 77.6% of images).26
The pivotal phase III study, named PICTURE,27 was also performed to evaluate the efficacy of gadopiclenol in MRI of the CNS. It was an international randomized, double-blind, controlled, and cross-over study. Based on the same design as for the phase IIb study, patients with highly suspicious CNS lesions underwent a series of 2 MRIs: one with gadopiclenol at 0.05 mmol/kg and another with gadobutrol at 0.1 mmol/kg. A total of 242 patients were randomized and received the 2 GBCAs. Most patients (72%) presented with brain tumors, 20% had brain or spine metastases, and 8% presented with other pathologies.
The primary evaluation criteria were lesion visualization parameters (lesion border delineation, internal morphology, and contrast enhancement) assessed by 3 independent blinded readers, using a 4-point scoring system. The study showed that contrast-enhanced MRI with gadopiclenol at 0.05 mmol/kg was noninferior to that with gadobutrol at 0.1 mmol/kg, for all lesion visualization parameters and all 3 readers (lower limit of the 95% confidence interval [CI] of the difference ≥−0.06, above the noninferiority margin [−0.35], P < 0.0001).
No meaningful differences were noted in the total number of lesions detected with gadopiclenol as compared with gadobutrol.
The objective quantitative measurements in the PICTURE study demonstrated overall higher values with gadopiclenol at 0.05 mmol/kg as compared with gadobutrol at 0.1 mmol/kg. The LBR and the percentage of contrast enhancement were significantly higher with gadopiclenol for all 3 readers, and the CNR was significantly higher with gadopiclenol for 2 of the 3 readers. Images with gadopiclenol at 0.05 mmol/kg were also in majority preferred to images with gadobutrol at 0.1 mmol/kg (44.8% to 57.3% of images).
A post hoc analysis of the PICTURE study was performed on the subpopulation of patients with brain metastases (N = 46). The noninferiority of gadopiclenol 0.05 mmol/kg to gadobutrol 0.1 mmol/kg was also demonstrated for all readers and all visualization parameters in this subgroup, with a lower limit of the 95% CI between −0.04 and −0.30 depending on the reader (Fig. 1). For quantitative parameters, there was no significant difference between the 2 GBCAs in terms of CNR, whereas LBR was significantly higher with gadopiclenol for the 3 readers (P ≤ 0.0036) and percentage of contrast enhancement was significantly higher for 2 of 3 readers (P ≤ 0.0097). For this subgroup of patients, the overall image preference was in favor of gadopiclenol for 54% to 59% of evaluations, depending on the reader (data on file). Example MRI scans of a patient with a brain glioblastoma obtained after gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg) administration are provided in Figure 2.
FIGURE 1.
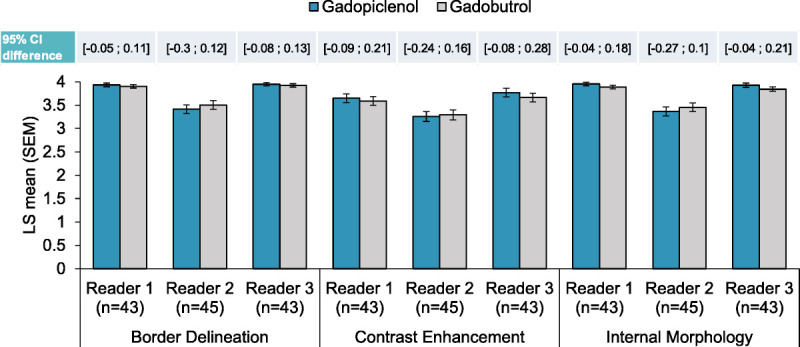
Noninferiority of gadopiclenol (0.05 mmol/kg) versus gadobutrol (0.1 mmol/kg) for the visualization of brain metastases (post hoc analysis of the PICTURE study). The noninferiority analysis between gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg) combined unenhanced and contrast-enhanced images was performed on the subpopulation of patients with brain metastases from the PICTURE study. Up to 3 most representative lesions in each patient were qualitatively scored (from 1 to 4) for each parameter by 3 offsite independent blinded readers, and a mean of scores was calculated. Data are presented as bar graphs showing the least squares (LS) mean (standard error of the mean [SEM]) of the scores, and the confidence intervals (CIs) of the difference between the scores are shown above the graph.
FIGURE 2.

Contrast-enhanced brain MRI with gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg). Two-dimensional T1-weighted spin-echo (SE) MRI scans (A, B, D, E) and 3D T1-weighted gradient-echo (GRE) MRI scans (C and F) were acquired for a 37-year-old male patient diagnosed with glioblastoma. The scans were obtained before (A) and after the administration of gadopiclenol at a dose of 0.05 mmol/kg (B and C), as well as before (D) and after gadobutrol administration at a dose of 0.1 mmol/kg (E and F).
Gadopiclenol Efficacy in Body MRI
The other pivotal phase III study, named PROMISE, was conducted to assess the efficacy of gadopiclenol at 0.05 mmol/kg in MRI of different body regions other than the CNS.28 This study had the same design and evaluation criteria as those of the PICTURE study. A total of 277 patients were randomized and received the 2 GBCAs (ie, gadopiclenol at 0.05 mmol/kg and gadobutrol at 0.1 mmol/kg). The body regions investigated in this study were the abdomen (including the liver, pancreas, and kidneys) for 35% of the patients, thorax (including mainly breast) for 28% of patients, pelvis (including the uterus, ovary, and prostate) for 22% of patients, head and neck for 8% of patients, and the musculoskeletal system for 8% of patients. This study showed that contrast-enhanced MRI with gadopiclenol at 0.05 mmol/kg was noninferior to that with gadobutrol at 0.1 mmol/kg for all lesion visualization parameters and all 3 readers (lower limit of the 95% CI of the difference ≥−0.10, above the noninferiority margin [−0.35], P < 0.0001).
No preference between images with gadopiclenol and those with gadobutrol was reported in most cases (75%–83%, depending on the reader). Example MRI scans of patients with breast cancer or liver metastasis from colon cancer, obtained after gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg) administration, are provided in Figure 3 and Figure 4, respectively.
FIGURE 3.
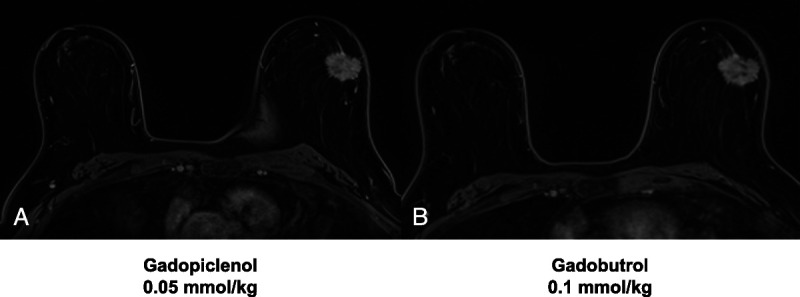
Contrast-enhanced breast MRI with gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg). Axial 3D T1-weighted dynamic contrast-enhanced (DCE) MRI for a 62-year-old female with breast cancer. Images were acquired after gadopiclenol administration at 0.05 mmol/kg (A) or gadobutrol administration at 0.1 mmol/kg (B).
FIGURE 4.

Contrast-enhanced liver MRI with gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg). Axial 3D T1-weighted dynamic contrast-enhanced (DCE) MRI during the arterial (A, D), portal venous (B and E) and delayed (C and F) phases for a 58-year-old female with liver metastasis from colon cancer; images were obtained after administration of gadopiclenol at 0.05 mmol/kg (A–C) or gadobutrol at 0.1 mmol/kg (D–F).
Regarding the quantitative measurements, the percentage of contrast enhancement was significantly higher with gadopiclenol for 2 of 3 readers, whereas no significant difference was observed between the 2 GBCAs for LBR.
Taking into account the 3 body regions including at least 20% of patients, namely, the abdomen, thorax, and pelvis, the noninferiority of gadopiclenol at 0.05 mmol/kg versus gadobutrol at 0.1 mmol/kg was also demonstrated in each of these regions.
No meaningful differences were noted in the total number of lesions detected with gadopiclenol as compared with gadobutrol.
OVERALL SAFETY OF GADOPICLENOL
Based on the first 8 clinical studies performed with gadopiclenol, comprising phase I to III studies23–29 (including 1 study not yet published), an overall analysis of the safety of gadopiclenol has been performed on the pooled data according to a statistical analysis plan.
Safety was assessed through reporting of AEs, vital signs, biochemistry and hematology parameters, and electrocardiography (ECG) in some studies.23–25,29 The cardiac safety of gadopiclenol was more specifically evaluated in a thorough QT study.29
Overall, a total of 1047 participants (ie, 92 healthy volunteers and 955 patients) were exposed to gadopiclenol at doses ranging from 0.025 to 0.3 mmol/kg. The mean (SD) age of participants exposed to gadopiclenol was 50.8 (18.4) years, 80 (7.6%) were aged between 2 and 17 years, 697 (66.6%) aged between 18 and 64 years, and 270 (25.8%) aged above 64 years. Overall, 5.4% of participants exposed to gadopiclenol had moderate renal impairment (30 ≤ eGFR < 60 mL/min/1.73 m2); 1.5% had severe renal impairment (eGFR < 30 mL/min/1.73 m2). Furthermore, 11.5% of participants had allergic diseases, 9.6% had cardiac diseases, and 6.4% had hepatic diseases.
Most participants (67.6%) received gadopiclenol at the dose of 0.05 mmol/kg. A single dose was administered except in the thorough QT study where participants received 2 doses (ie, 0.1 and 0.3 mmol/kg) as well as moxifloxacin (a universal positive control for thorough QT studies).29 In 3 clinical studies with a cross-over design,26–28 the same patients received in addition to gadopiclenol, another GBCA: gadobenate dimeglumine (0.1 mmol/kg) in 1 study,26 and gadobutrol (0.1 mmol/kg) in 2 studies.27,28
Magnetic resonance imaging of the CNS was performed for 515 adults and 60 pediatric patients, whereas body MRI was performed for 328 adults and 20 pediatric patients.
Overall, AEs were reported in 247 (23.6%) participants exposed to gadopiclenol, and AEs were considered by the investigators as related to gadopiclenol (ie, adverse drug reactions [ADRs]) for 89 (8.5%) participants (Table 1). Serious AEs were reported in 12 (1.1%) participants exposed to gadopiclenol, and only one was considered related to gadopiclenol. The latter consisted in an increase in blood creatinine level >25% within 24 hours in a participant administered with gadopiclenol at 0.1 mmol/kg (however, with a value remaining in the normal range). The patient did not experience any clinical symptom, and the serious AE resolved without treatment. No NSF cases were reported in any of the participants.
TABLE 1.
Most Frequent Adverse Drug Reactions Reported With Gadopiclenol
| All Doses (n = 1047) | 0.05 mmol/kg (n = 708) | |
|---|---|---|
| At least 1 adverse drug reaction | 89 (8.5%) | 33 (4.7%) |
| Injection site pain | 20 (1.9%) | 5 (0.7%) |
| Headache | 14 (1.3%) | 5 (0.7%) |
| Nausea | 7 (0.7%) | 3 (0.4%) |
| Injection site coldness | 6 (0.6%) | 2 (0.3%) |
| Fatigue | 4 (0.4%) | — |
| Diarrhea | 4 (0.4%) | 1 (0.1%) |
| Injection site edema | 3 (0.3%) | — |
| Feeling hot | 3 (0.3%) | 1 (0.1%) |
| Injection site warmth | 3 (0.3%) | 3 (0.4%) |
| Dizziness | 3 (0.3%) | 2 (0.3%) |
| Blood creatinine increased | 3 (0.3%) | 1 (0.1%) |
| Dysgeusia | 2 (0.2%) | 1 (0.1%) |
| Abdominal pain | 2 (0.2%) | — |
| Vomiting | 2 (0.2%) | 1 (0.1%) |
| Electrocardiogram QT prolonged | 2 (0.2%) | 1 (0.1%) |
| Pruritus | 2 (0.2%) | 1 (0.1%) |
| Back pain | 2 (0.2%) | — |
Adverse drug reactions reported for all gadopiclenol doses in more than 1 participant are presented.
The intensity of the AEs reported with gadopiclenol was graded as mild for the majority (325/390; 83.3%), moderate for 50 (12.8%), and severe for 14 (3.6%) (the intensity of 1 AE was not reported). Among the AEs of severe intensity, 3 were considered related to gadopiclenol: injection site pain in 2 patients and upper abdominal pain in 1 of these patients, all occurring within 1 hour after injection of 0.2 mmol/kg and all resolved within 1 day.
Among participants who were exposed to gadopiclenol at 0.05 mmol/kg, AEs were reported in 119 (16.8%) participants, and ADRs in 33 (4.7%) participants (Table 1). Serious AEs were reported in 7 (1.0%) participants, and none was related to gadopiclenol.
In comparative studies, the incidence of ADRs with gadopiclenol was comparable to that reported with other GBCAs: 11.7% (for all doses of gadopiclenol) versus 12.1% with gadobenate dimeglumine in the phase IIb study,26 4.2% and 4.9% with gadopiclenol at 0.05 mmol/kg versus 5.5% and 6.9% with gadobutrol 0.1 mmol/kg, in the PROMISE and PICTURE phase III studies, respectively.27,28 No differences were shown between gadopiclenol and the 2 other GBCAs (ie, gadobenate dimeglumine and gadobutrol) with regard to the nature, severity, and seriousness of the reported ADRs.
Overall, the most frequently reported ADRs with gadopiclenol (at all doses) were reactions at injection site (including pain, coldness, edema, and warmth); gastrointestinal disorders such as nausea, diarrhea, vomiting, and abdominal pain; and other nervous or general disorders such as headache, fatigue, and dizziness. With gadopiclenol at 0.05 mmol/kg, the most frequent ADRs were injection site reactions, headache, and nausea (Table 1).
Overall, both hematology and biochemistry values remained stable. Blood creatinine increase was the most frequent abnormal laboratory result reported as ADR, reported similarly for all GBCAs used in the clinical trials: in 3 patients (0.3%) with gadopiclenol, 2 patients (0.8%) with gadobenate dimeglumine, and 2 patients (0.4%) with gadobutrol. All cases were of mild intensity and resolved.
Vital signs including blood pressure and heart rate were stable overall. In 4 studies where ECG data were assessed,23–25,29 no consistent changes in median values for ECG intervals were observed. Two cases of abnormal QT interval of mild intensity were reported as ADRs in the phase IIb study,26 and one in the pediatric study.25 In the thorough QT/QTc study,29 the upper limit of the 90% CI of the largest time-matched placebo-corrected, mean change from baseline in QTcF was below 10 milliseconds for both the clinical (0.1 mmol/kg) and supraclinical (0.3 mmol/kg) doses, demonstrating no significant effect of gadopiclenol on QTc interval.29
In the pediatric population,25 clinical laboratory, ECG, and vital signs evaluation did not highlight any safety concerns. ADRs were reported for 2 participants: A mild QT interval prolongation in a 5-year-old patient, and a moderate maculopapular rash in a 9-year-old patient.
In participants ≥65 years old, ADRs were reported for 13 patients (4.8%), and the most frequent were injection site pain and headache. Clinical laboratory, ECG, and vital signs evaluation did not highlight any safety concerns.
As for renal function status, ADRs were reported in 5 participants (8.8%) with moderate renal impairment, and 1 participant (6.3%) with severe renal impairment. Among these ADRs, renal failure and blood creatinine increase were reported in 1 participant with moderate impairment each, and hyperkalemia in the participant with severe impairment. The incidence of ADRs in these participants was comparable to that reported in those with no or mild renal impairment (ie, 8.5%).
Globally for all 8 studies, there was no difference in the nature and incidence of ADRs between participants with and without cardiac, allergic, or hepatic diseases (Table 2).
TABLE 2.
Adverse Drug Reactions Incidence With Gadopiclenol According to Clinical Syndrome
| At Least 1 Adverse Drug Reaction, N (%) Patients | |
|---|---|
| Renal impairment | |
| No or mild (n = 974) | 83 (8.5%) |
| Moderate (n = 57) | 5 (8.8%) |
| Severe (n = 16) | 1 (6.3%) |
| Hepatic diseases | |
| With (n = 67) | 7 (10.4%) |
| Without (n = 980) | 82 (8.4%) |
| Cardiac diseases | |
| With (n = 100) | 4 (4.0%) |
| Without (n = 947) | 85 (9.0%) |
| Allergic diseases | |
| With (n = 120) | 11 (9.2%) |
| Without (n = 927) | 78 (8.4%) |
CONCLUSIONS AND PERSPECTIVES
The new GBCA, gadopiclenol, is distinguished by its high relaxivity and strong stability. It was developed in response to the medical need of reducing the administered dose of gadolinium, to minimize potential risks associated with gadolinium exposure, while maintaining a high level of diagnostic efficacy.
The 2 pivotal phase III studies performed with gadopiclenol for MRI of the CNS (PICTURE) and other body regions (PROMISE) showed that the diagnostic efficacy of gadopiclenol at half the standard dose used with other GBCAs (ie, 0.05 mmol/kg) can be at least as good as that of gadobutrol at 0.1 mmol/kg.27,28 The results of the pediatric study25 indicate that the pharmacokinetic profile of gadopiclenol in pediatric patients aged 2 to 17 years and adults is comparable. Therefore, extrapolation from adult efficacy data is appropriate, and no dose adjustment is needed in this patient population other than per body weight.
The results from the phase IIb study indicated that gadopiclenol at a dose of 0.1 mmol/kg provides significantly higher contrast enhancement of lesions compared with gadobenate dimeglumine at the same dosage. These findings suggest that gadopiclenol, when used at 0.1 mmol/kg, has the potential to detect lesions that may be overlooked with other GBCAs.26 Yet, further studies are needed to evaluate the potential clinical benefits of using gadopiclenol at this dose, particularly in the detection of brain metastases.
The safety analysis performed on 1047 participants showed that gadopiclenol has a favorable safety profile, comparable to that observed with other GBCAs. The most common ADRs with gadopiclenol were injection site reactions, headache, and nausea. Overall, no significant changes were observed in hematology and biochemistry values, vital signs, and ECG data. No significant safety concerns were identified in pediatric and elderly patients, as well as in patients with renal impairment. These finding will be confirmed in real-world clinical settings.
The excellent kinetic inertness of gadopiclenol combined with its good thermodynamic stability is considered as a good predictor for in vivo complex stability. However, the reduced propensity for retention in humans needs to be further investigated. Nevertheless, a preclinical study investigated the long-term gadolinium retention in the brain of healthy rats,30 who had received 5 injections of either gadopiclenol, gadobutrol, or gadodiamide at 2.4 mmol/kg over 5 weeks, and showed a 80% washout of gadolinium for gadopiclenol and gadobutrol compared with only 15% gadolinium washout observed with gadodiamide.30 Another study on healthy rats, who had received 20 injections of gadopiclenol or gadobenate dimeglumine at 0.6 mmol/kg over 5 weeks, showed significantly lower gadolinium concentrations in the cerebellum, cortical brain, subcortical brain, and muscle with gadopiclenol.31
The presence of gadolinium as an emerging aquatic microcontaminant and its detection in water systems have raised concerns.32 Anthropogenic gadolinium, originating from GBCAs, has been detected in various water sources globally.19,33 The concentration of anthropogenic gadolinium and GBCAs in water remains unregulated, posing potential risks to both human health and the environment.34 Remediation techniques for GBCAs in water are limited, with reverse osmosis membrane technology being the most efficient but costly option.19,35 One possible solution to address this issue is to reduce the administered dose of GBCAs to patients, thus a lower quantity of GBCA released in the environment after urinary excretion by the patient. In this regard, high relaxivity GBCAs such as gadopiclenol play a significant role as they can provide enhanced imaging with lower gadolinium dosage. By using high relaxivity GBCAs, it may be possible to mitigate the environmental impact of GBCAs while still maintaining the diagnostic efficacy of contrast-enhanced MRI examinations.
Based on the efficacy and safety data reported in this review, the Food and Drug Administration has granted gadopiclenol approval in 2022, and the product is currently under review by the regulatory authorities in Europe. In the United States, gadopiclenol is approved for use in adult and pediatric patients aged 2 years and older to detect and visualize lesions with abnormal vascularity in the CNS (brain, spine, and associated tissues) and the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system). The recommended dose of gadopiclenol for adult and pediatric patients aged 2 years and older is half the standard dose currently used for other GBCAs (ie, 0.05 mmol/kg, equivalent to 0.1 mL/kg) administered intravenously at approximately 2 mL/s.36,37
In summary, the new GBCAs gadopiclenol at half the standard dose (ie, 0.05 mmol/kg) of other GBCAs has demonstrated an efficacy comparable to other GBCAs to detect and visualize lesions with abnormal vascularity in the CNS and the body for patients aged 2 years and older. Gadopiclenol exhibited a favorable safety profile, similar to other macrocyclic GBCAs. Its high relaxivity offers the potential to reduce patient and environmental burden while maintaining diagnostic efficacy in contrast-enhanced MRI examinations. However, there is still much to discover about gadopiclenol, including its application in other indications, as well as evaluating its potential clinical benefits when used at the standard dose of 0.1 mmol/kg.
Footnotes
Conflicts of interest and sources of funding: All authors are employed by Guerbet. The clinical studies performed for the evaluation of the efficacy and safety of gadopiclenol were funded by Guerbet.
Contributor Information
Camille Pitrou, Email: camille.pitrou@guerbet.com.
Philippe Bourrinet, Email: philippe.bourrinet@guerbet.com.
REFERENCES
- 1.Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging. 2019;50:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang HY Chen J Xia CC, et al. Noninvasive imaging of hepatocellular carcinoma: from diagnosis to prognosis. World J Gastroenterol. 2018;24:2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derks SHAE, van der Veldt AAM, Smits M. Brain metastases: the role of clinical imaging. Br J Radiol. 2022;95:20210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellin MF, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol. 2008;66:160–167. [DOI] [PubMed] [Google Scholar]
- 5.Mathur M, Jones JR, Weinreb JC. Gadolinium deposition and nephrogenic systemic fibrosis: a radiologist's primer. Radiographics. 2020;40:153–162. [DOI] [PubMed] [Google Scholar]
- 6.Radiological Society of The Netherlands . Guideline Safe Use of Contrast Media Part 3. Available at: https://www.radiologen.nl/sites/default/files/Kwaliteit/guideline_safe_use_of_contrast_media_part_3_final_8nov2022_eng.pdf. Accessed May 24, 2023.
- 7.Chazot A Barrat JA Gaha M, et al. Brain MRIs make up the bulk of the gadolinium footprint in medical imaging. J Neuroradiol. 2020;47:259–265. [DOI] [PubMed] [Google Scholar]
- 8.Enterline DKJ, Malinzak M, Porter KK, Sancrant J, Soto JA. Why gadolinium matters today. Available at: https://appliedradiology.com/articles/why-gadolinium-matters-today. Accessed May 24, 2023.
- 9.Strickler SE, Clark KR. Gadolinium deposition: a study review. Radiol Technol. 2021;92:249–258. [PubMed] [Google Scholar]
- 10.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. [DOI] [PubMed] [Google Scholar]
- 11.Kanda T Oba H Toyoda K, et al. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol. 2016;34:3–9. [DOI] [PubMed] [Google Scholar]
- 12.Xia D Davis RL Crawford JA, et al. Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive x-ray spectroscopy. Acta Radiol. 2010;51:1126–1136. [DOI] [PubMed] [Google Scholar]
- 13.Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39:138–142. [DOI] [PubMed] [Google Scholar]
- 14.Kanda T Fukusato T Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–232. [DOI] [PubMed] [Google Scholar]
- 15.Kanda T Ishii K Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 16.Gadolinium-containing contrast agents . European Commission Decision, July 1, 2010. Available at: https://www.ema.europa.eu/en/medicines/human/referrals/gadolinium-containing-contrast-agents-0. Accessed June 16, 2022.
- 17.Lancelot E, Desche P. Gadolinium retention as a safety signal: experience of a manufacturer. Invest Radiol. 2020;55:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body. Accessed August 8, 2022.
- 19.Schmidt K Bau M Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401–1408. [DOI] [PubMed] [Google Scholar]
- 20.Laczovics A Csige I Szabo S, et al. Relationship between gadolinium-based MRI contrast agent consumption and anthropogenic gadolinium in the influent of a wastewater treatment plant. Sci Total Environ. 2023;877:162844. [DOI] [PubMed] [Google Scholar]
- 21.Runge VM, Heverhagen JT. Advocating the development of next-generation high-relaxivity gadolinium chelates for clinical magnetic resonance. Invest Radiol. 2018;53:381–389. [DOI] [PubMed] [Google Scholar]
- 22.Robic C Port M Rousseaux O, et al. Physicochemical and pharmacokinetic profiles of gadopiclenol: a new macrocyclic gadolinium chelate with high T1 relaxivity. Invest Radiol. 2019;54:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradu A Penescu M Pitrou C, et al. Pharmacokinetics, dialysability, and safety of gadopiclenol, a new gadolinium-based contrast agent, in patients with impaired renal function. Invest Radiol. 2021;56:486–493. [DOI] [PubMed] [Google Scholar]
- 24.Hao J, Bourrinet P, Desché P. Assessment of pharmacokinetic, pharmacodynamic profile, and tolerance of gadopiclenol, a new high relaxivity GBCA, in healthy subjects and patients with brain lesions (phase I/IIa study). Invest Radiol. 2019;54:396–402. [DOI] [PubMed] [Google Scholar]
- 25.Jurkiewicz E Tsvetkova S Grinberg A, et al. Pharmacokinetics, safety, and efficacy of gadopiclenol in pediatric patients aged 2 to 17 years. Invest Radiol. 2022;57:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendszus M Roberts D Kolumban B, et al. Dose finding study of gadopiclenol, a new macrocyclic contrast agent, in MRI of central nervous system. Invest Radiol. 2020;55:129–137. [DOI] [PubMed] [Google Scholar]
- 27.Loevner LA Kolumban B Hutoczki G, et al. Efficacy and safety of gadopiclenol for contrast-enhanced MRI of the central nervous system: the PICTURE randomized clinical trial. Invest Radiol. 2023;58:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhl C Csőszi T Piskorski W, et al. Efficacy and safety of half-dose gadopiclenol versus full-dose gadobutrol for contrast-enhanced body MRI. Radiology. 2023;308:e222612. [DOI] [PubMed] [Google Scholar]
- 29.Funck-Brentano C Felices M Le Fur N, et al. Randomized study of the effect of gadopiclenol, a new gadolinium-based contrast agent, on the QTc interval in healthy subjects. Br J Clin Pharmacol. 2020;86:2174–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funke SKI Factor C Rasschaert M, et al. Long-term gadolinium retention in the healthy rat brain: comparison between gadopiclenol, gadobutrol, and gadodiamide. Radiology. 2022;305:179–189. [DOI] [PubMed] [Google Scholar]
- 31.Violas X Rasschaert M Santus R, et al. Small brain lesion enhancement and gadolinium deposition in the rat brain: comparison between gadopiclenol and gadobenate dimeglumine. Invest Radiol. 2022;57:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence MG. Detection of anthropogenic gadolinium in the Brisbane River plume in Moreton Bay, Queensland, Australia. Mar Pollut Bull. 2010;60:1113–1116. [DOI] [PubMed] [Google Scholar]
- 34.Oluwasola IE Ahmad AL Shoparwe NF, et al. Gadolinium based contrast agents (GBCAs): uniqueness, aquatic toxicity concerns, and prospective remediation. J Contam Hydrol. 2022;250:104057. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence MG, Keller J, Poussade Y. Removal of magnetic resonance imaging contrast agents through advanced water treatment plants. Water Sci Technol. 2010;61:685–692. [DOI] [PubMed] [Google Scholar]
- 36.GlobeNewswire. Guerbet announces U.S. Food and Drug Administration (FDA) approval of Elucirem™ (Gadopiclenol). Available at: https://www.globenewswire.com/news-release/2022/09/21/2520478/0/en/Guerbet-announces-U-S-Food-and-Drug-Administration-FDA-approval-of-Elucirem-Gadopiclenol.html. Accessed January 25, 2023.
- 37.Highlights of prescribing information for Elucirem. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216986s000lbl.pdf. Accessed May 30, 2023.


