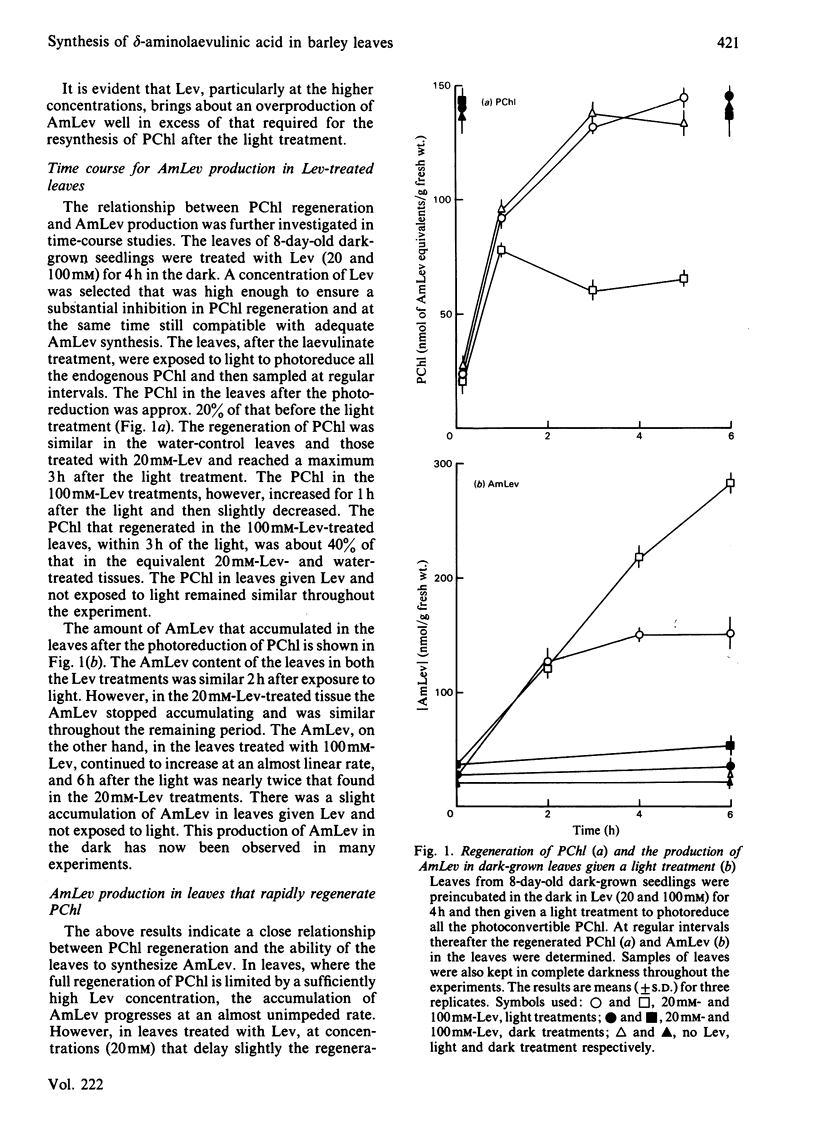
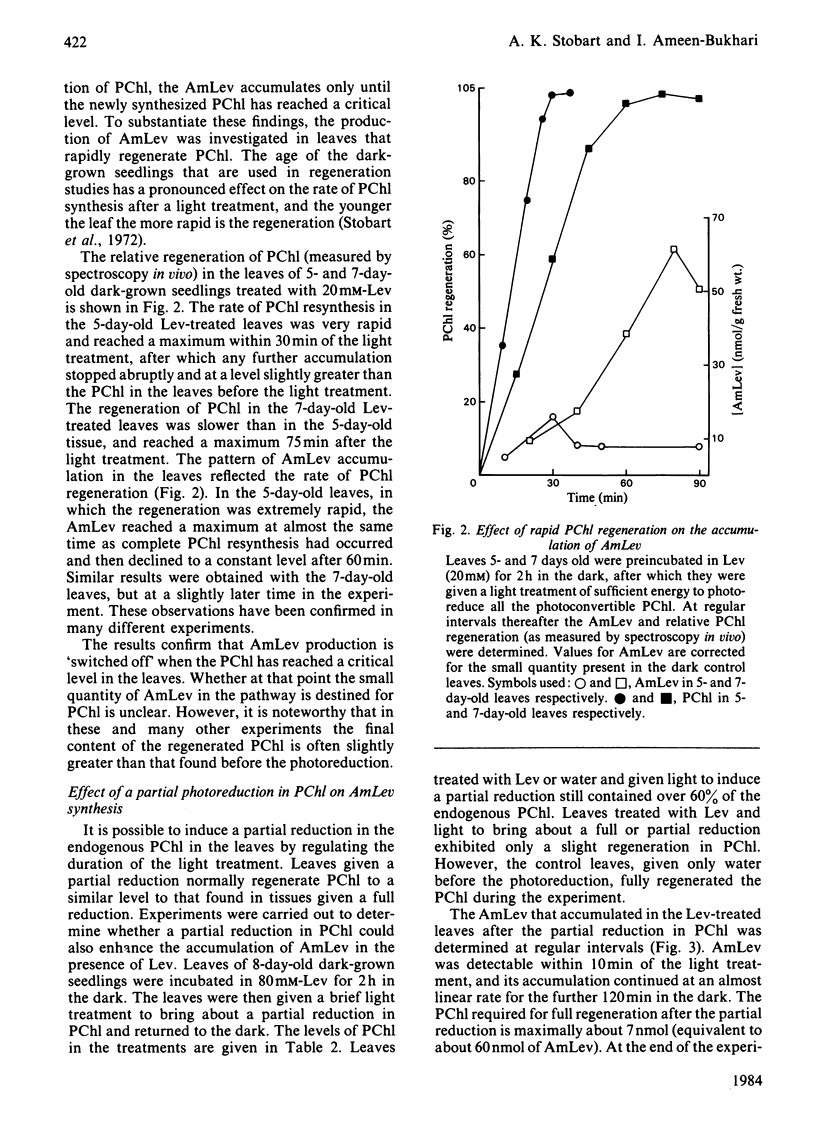
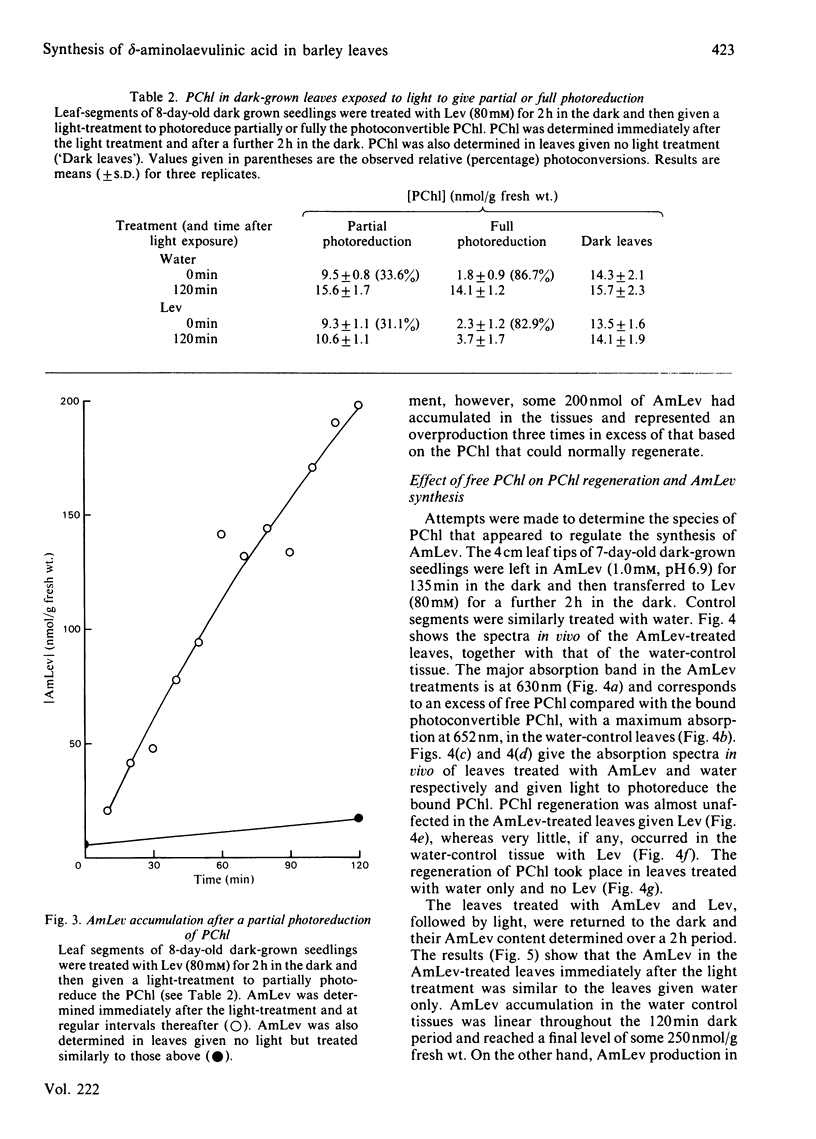
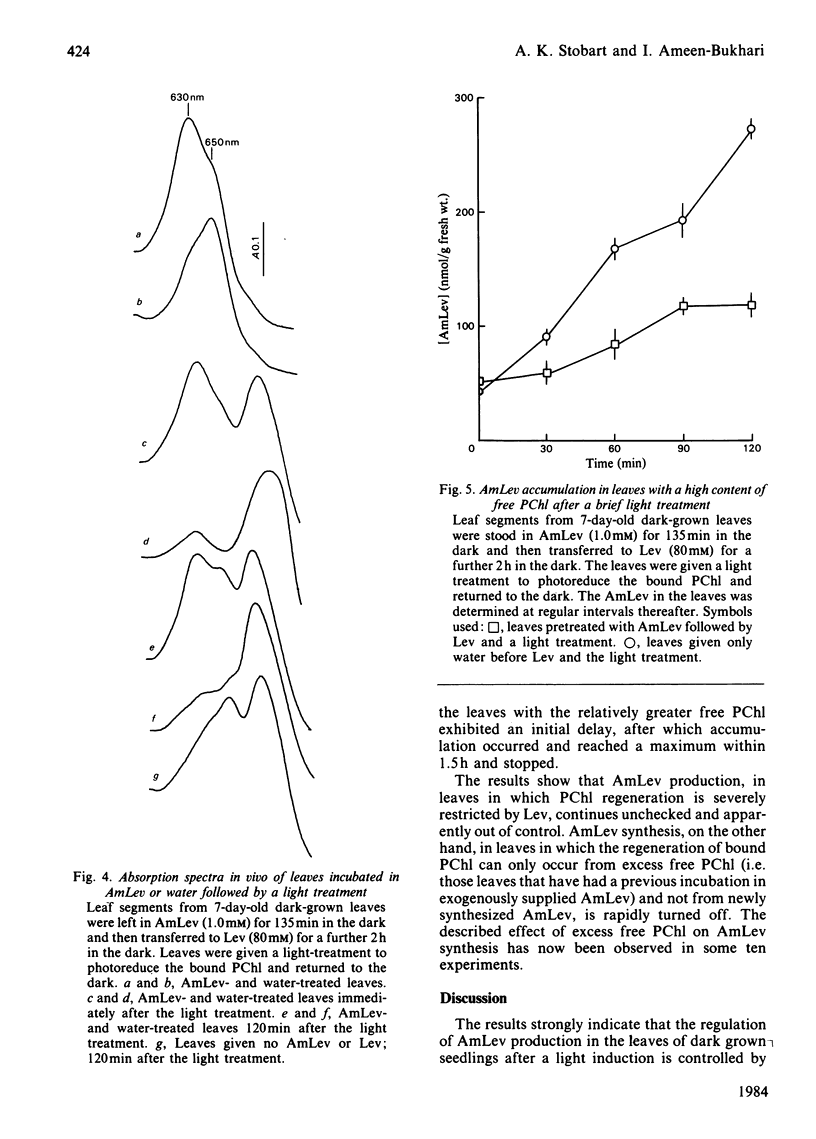
Abstract
Laevulinic acid (Lev) was used to control the rate of protocholorophyllide (PChl) regeneration in the leaves of dark-grown seedlings of barley (Hordeum vulgare) after a brief light treatment. In the leaves given Lev, at concentrations that severely block the resynthesis of protochlorophyllide, there was a massive overproduction of delta-aminolaevulinic acid (AmLev) that was well in excess of that required for the regeneration of PChl observed in the control leaves. Lev, at low concentrations, slightly delayed regeneration and held up, rather than inhibited, the utilization of the AmLev, which accumulated in the tissues. The overproduction and uncontrolled formation of AmLev also occurred in dark-grown leaves treated with a high concentration of Lev and given a light treatment of just sufficient energy to photoreduce only small quantities of the endogenous PChl. Experiments in which a high level of free PChl was induced by incubating the leaves in AmLev indicated that the active species of PChl was that associated with, and bound to, the PChl reductase protein. The results strongly demonstrate a close relationship between the PChl-protein complex and the ability of the leaves to synthesize AmLev.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castelfranco P. A., Jones O. T. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975 Mar;55(3):485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J. X., Meller E., Gassman M. L. Catabolism of 5-Aminolevulinic Acid to CO(2) by Etiolated Barley Leaves. Plant Physiol. 1982 Jan;69(1):19–22. doi: 10.1104/pp.69.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Harel E., Klein S., Meller E. Control of delta-Aminolevulinic Acid and Chlorophyll Accumulation in Greening Maize Leaves upon Light-Dark Transitions. Plant Physiol. 1975 Oct;56(4):497–501. doi: 10.1104/pp.56.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. T. Characterization of the terminal stages of chlorophyll (ide) synthesis in etioplast membrane preparations. Biochem J. 1975 Dec;152(3):623–635. doi: 10.1042/bj1520623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E., Klein S. Light dependent formation of -aminolevulinic acid in etiolated leaves of higher plants. Biochem Biophys Res Commun. 1972 Oct 17;49(2):364–370. doi: 10.1016/0006-291x(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Kay S. A., Griffiths W. T. Light-Induced Breakdown of NADPH-Protochlorophyllide Oxidoreductase In Vitro. Plant Physiol. 1983 May;72(1):229–236. doi: 10.1104/pp.72.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Katz E., Neeman E. Induction of delta-Aminolevulinic Acid Formation in Etiolated Maize Leaves Controlled by Two Light Systems. Plant Physiol. 1977 Sep;60(3):335–338. doi: 10.1104/pp.60.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]


