Abstract
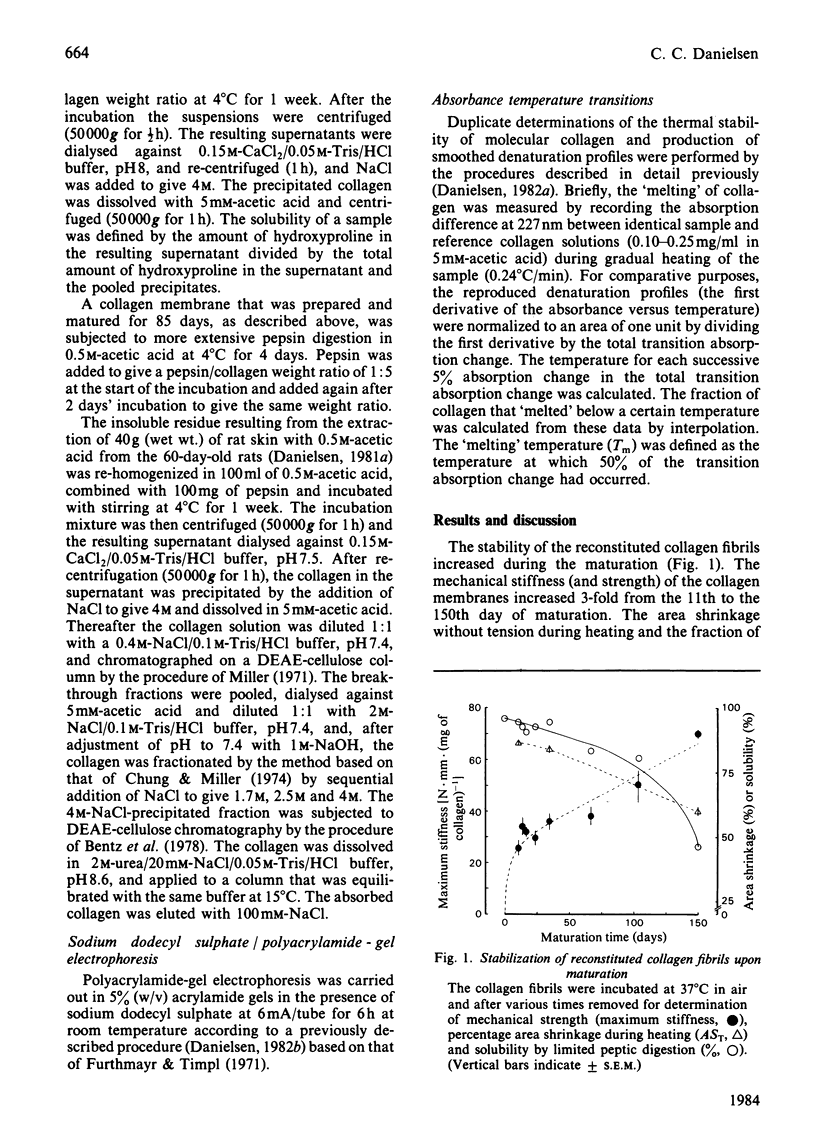
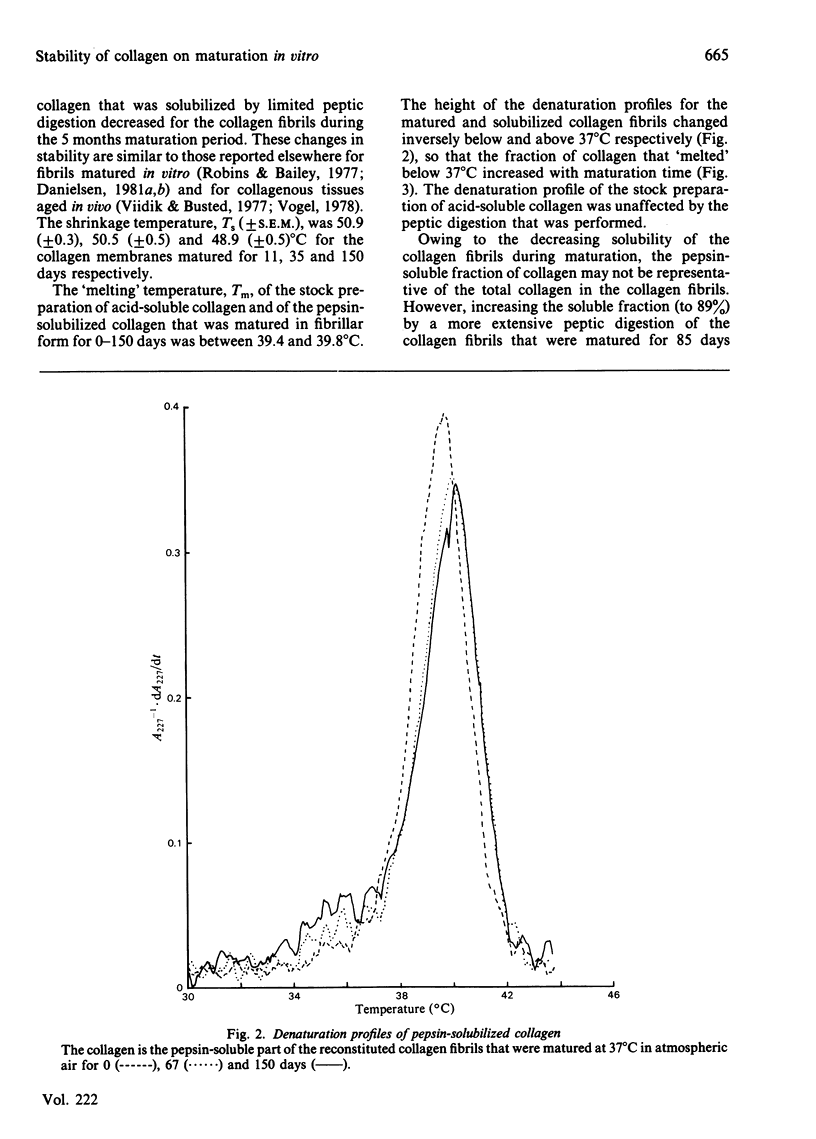
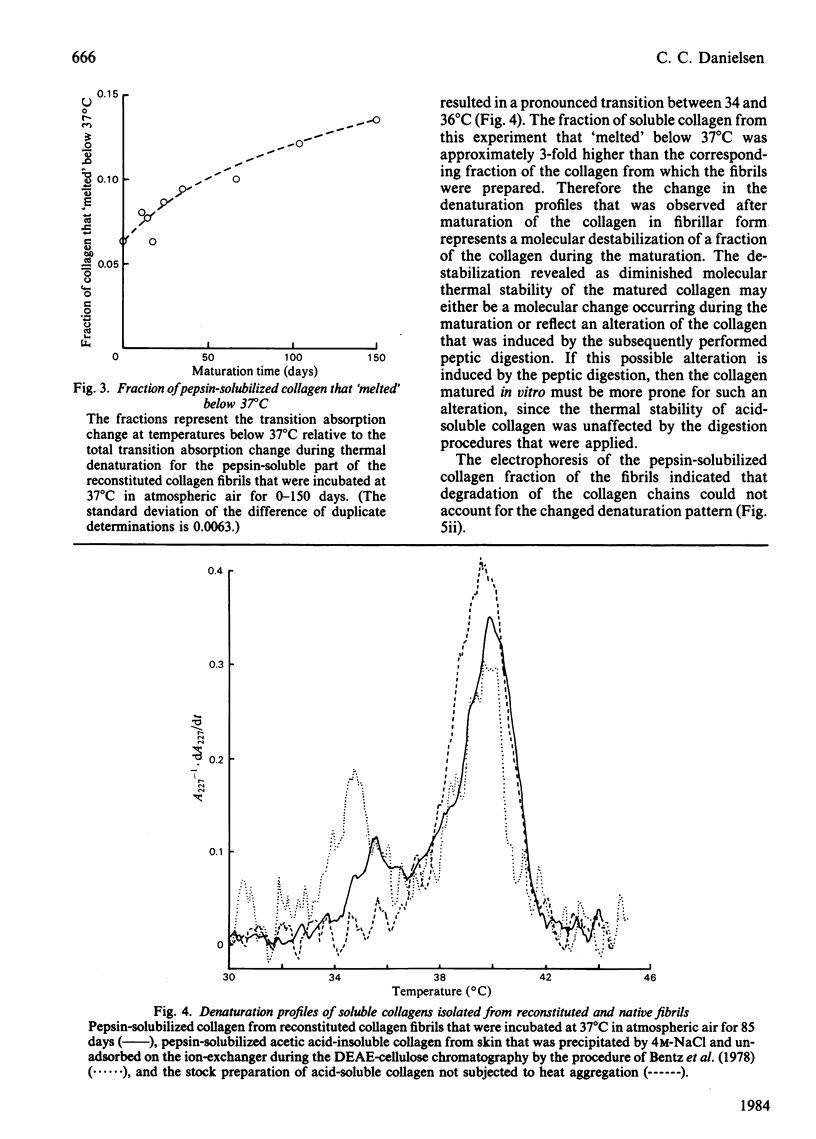
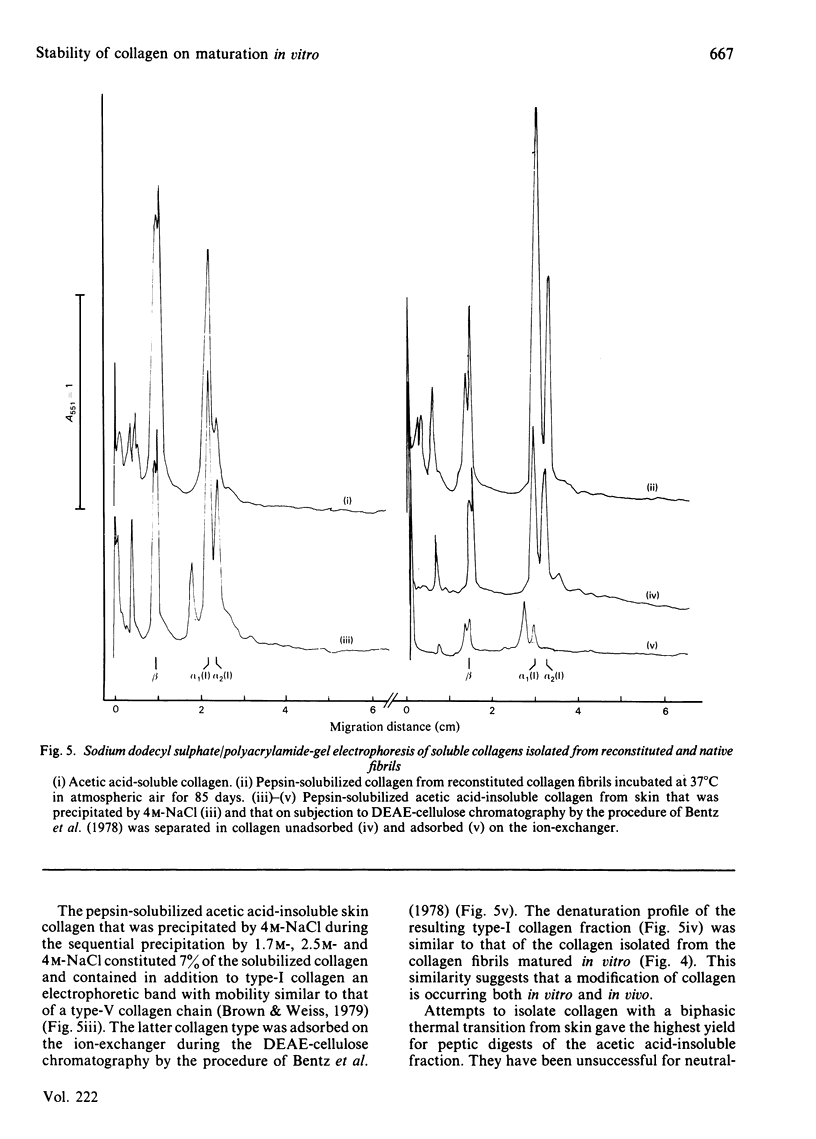
During the maturation in vitro of reconstituted collagen fibrils prepared from rat skin, the mechanical and thermal stability of collagen increased and the pepsin-solubility decreased. At the same time a larger fraction of the pepsin-soluble collagen attained a lower molecular thermal stability that resulted in a biphasic thermal transition of the soluble collagen. Type-I collagen, with a similar biphasic thermal transition, was isolated from acid-insoluble rat skin collagen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentz H., Bächinger H. P., Glanville R., Kühn K. Physical evidence for the assembly of A and B chains of human placental collagen in a single triple helix. Eur J Biochem. 1978 Dec;92(2):563–567. doi: 10.1111/j.1432-1033.1978.tb12778.x. [DOI] [PubMed] [Google Scholar]
- Brown R. A., Weiss J. B. Type V collagen: possible shared identity of alpha A, alpha B and alpha C chains. FEBS Lett. 1979 Oct 1;106(1):71–75. doi: 10.1016/0014-5793(79)80697-3. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C. Difference in thermal stability of type-I and type-II collagen from rat skin. Biochem J. 1982 Apr 1;203(1):323–326. doi: 10.1042/bj2030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen C. C. Mechanical properties of reconstituted collagen fibrils. A study on reconstitution methodology and influence of in vitro maturation. Connect Tissue Res. 1981;9(1):51–57. doi: 10.3109/03008208109160239. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C. Precision method to determine denaturation temperature of collagen using ultraviolet difference spectroscopy. Coll Relat Res. 1982 Mar;2(2):143–150. doi: 10.1016/s0174-173x(82)80030-7. [DOI] [PubMed] [Google Scholar]
- Danielsen C. C. Thermal stability of reconstituted collagen fibrils. Shrinkage characteristics upon in vitro maturation. Mech Ageing Dev. 1981 Mar;15(3):269–278. doi: 10.1016/0047-6374(81)90135-4. [DOI] [PubMed] [Google Scholar]
- Dick Y. P., Nordwig A. Effect of pH on the stability of the collagen fold. Arch Biochem Biophys. 1966 Nov;117(2):466–468. doi: 10.1016/0003-9861(66)90436-x. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Rauterberg J., Kühn K. The renaturation behaviour of modified collagen molecules. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):611–622. doi: 10.1515/bchm2.1968.349.1.611. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Some observations on the ageing in vitro of reprecipitated collagen fibres. Biochim Biophys Acta. 1977 Jun 24;492(2):408–414. doi: 10.1016/0005-2795(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Ryhänen L., Zaragoza E. J., Uitto J. Conformational stability of type I collagen triple helix: evidence for temporary and local relaxation of the protein conformation using a proteolytic probe. Arch Biochem Biophys. 1983 Jun;223(2):562–571. doi: 10.1016/0003-9861(83)90621-5. [DOI] [PubMed] [Google Scholar]
- Vogel H. G. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res. 1978;6(3):161–166. doi: 10.3109/03008207809152626. [DOI] [PubMed] [Google Scholar]


