Abstract
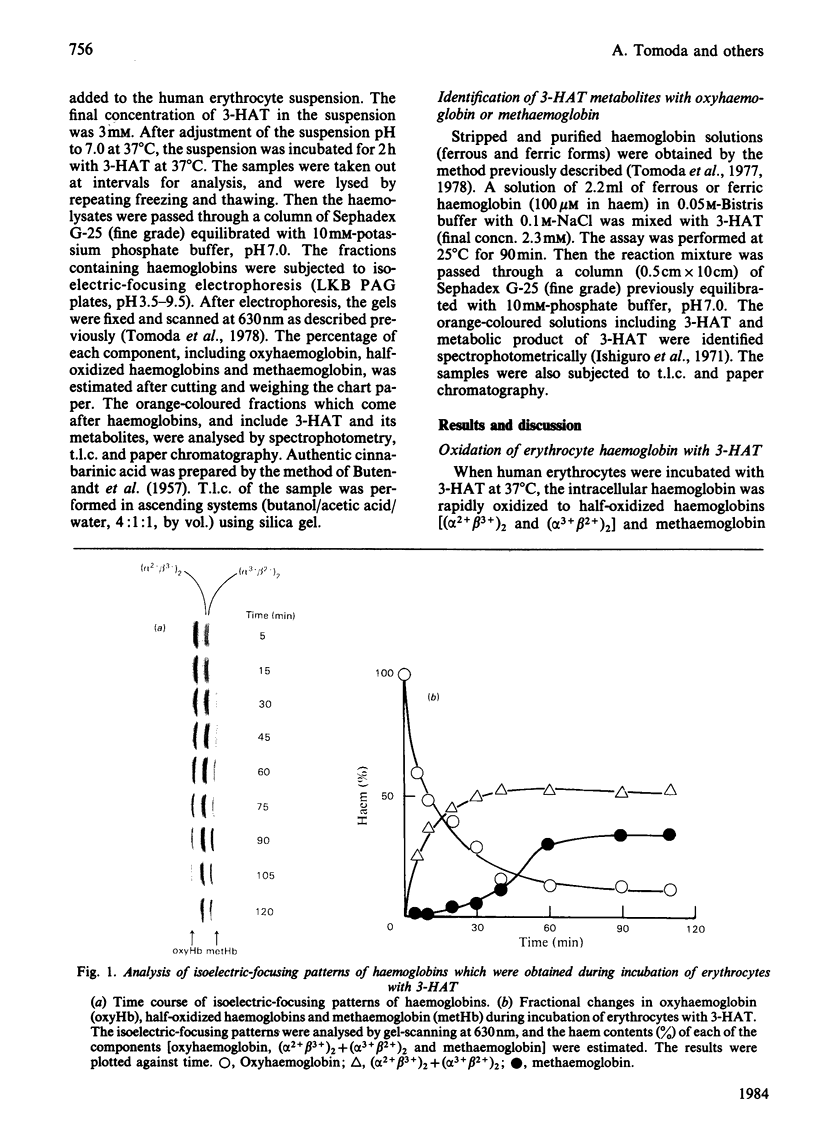
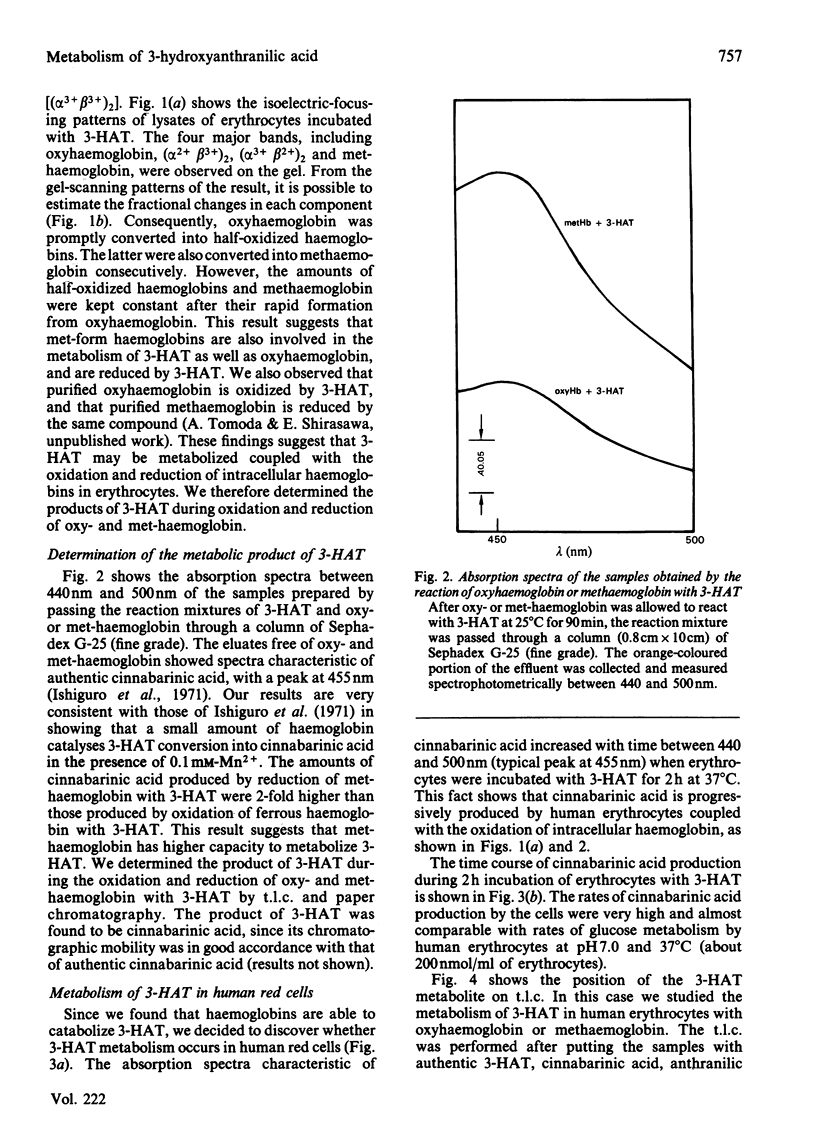
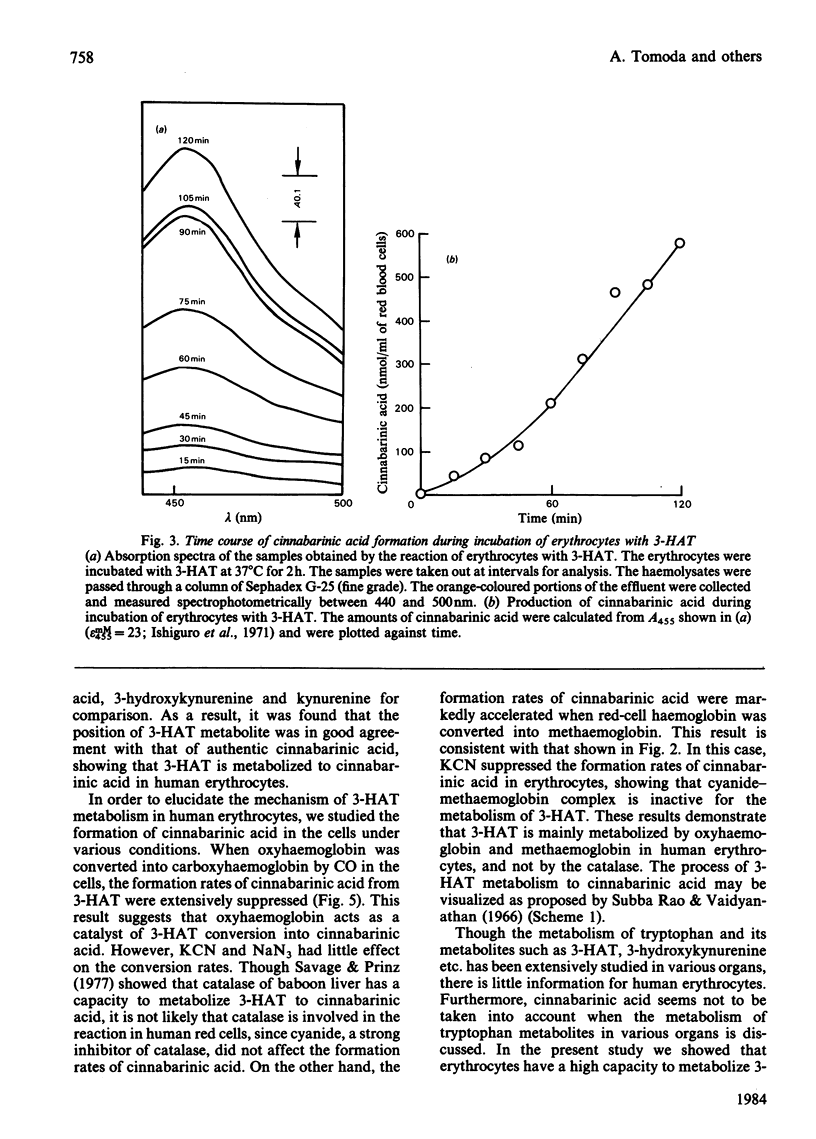
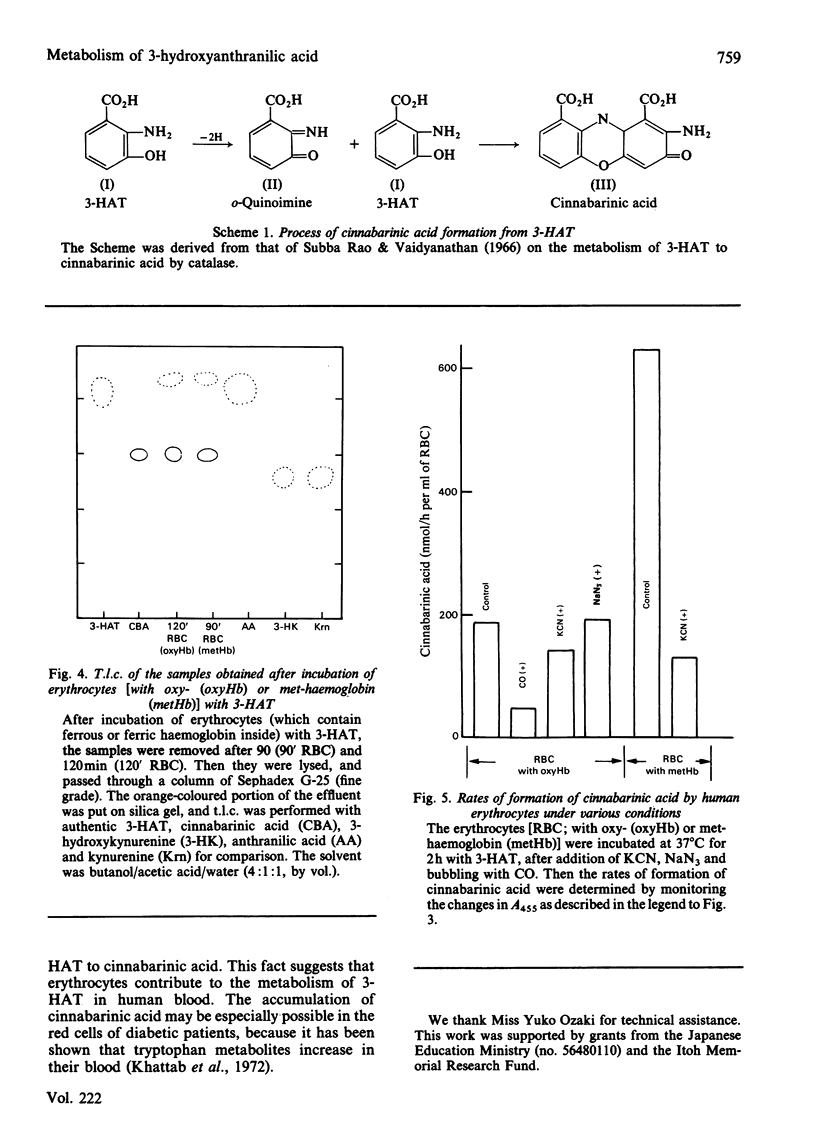
3-Hydroxyanthranilic acid, a metabolite of tryptophan, was rapidly metabolized by human erythrocytes. The final product was determined to be cinnabarinic acid as detected by spectrophotometry, paper chromatography and t.l.c. The formation of cinnabarinic acid from 3-hydroxyanthranilic acid in the cells was markedly inhibited by CO when intracellular haemoglobin was in a ferrous state, and by cyanide when it was in a ferric state. Ferrous haemoglobin in erythrocytes was oxidized to (alpha 3+ beta 2+)2, (alpha 2+ beta 3+)2 and (alpha 3+ beta 3+)2 by 3-hydroxyanthranilic acid, and the oxidation rates were very high, like those of cinnabarinic acid formation, suggesting that the metabolism of 3-hydroxyanthranilic acid is coupled with oxidoreductive reactions of intracellular haemoglobin. This view was further confirmed by the findings that 3-hydroxyanthranilic acid was metabolized by ferrous or ferric haemoglobin and that ferrous and ferric haemoglobins were oxidized and reduced by the compound respectively. The significance of the metabolism of 3-hydroxyanthranilic acid and the oxidoreductive reactions of haemoglobin with this compound may be associated with the pathological conditions with increased 3-hydroxyanthranilic acid levels in the blood of diabetic subjects.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azmitia E. C., Jr, McEwen B. S. Corticosterone regulation of tryptophan hydroxylase in midbrain of the rat. Science. 1969 Dec 5;166(3910):1274–1276. doi: 10.1126/science.166.3910.1274. [DOI] [PubMed] [Google Scholar]
- Ishiguro I., Nagamura Y., Hara A. [Studies on the formation of phenoxazine-pigment from o-aminophenol derivatives by hemoglobin. I. Conversion of 3-OH-anthranilic acid into cinnabarinic acid in the presence of Mn]. Yakugaku Zasshi. 1971 Jul;91(7):760–765. doi: 10.1248/yakushi1947.91.7_760. [DOI] [PubMed] [Google Scholar]
- Khattab M., Abul-Fadl M., Khalafallah A., Hamza S. Studies on the urinary excretion of certain tryptophan metabolites in diabetics. J Egypt Med Assoc. 1972;55(7):531–541. [PubMed] [Google Scholar]
- Leklem J. E. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr. 1971 Jun;24(6):659–672. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- Savage N., Prinz W. Cinnabarinate synthase from baboon (Papio ursinus) liver. Identity with catalase. Biochem J. 1977 Mar 1;161(3):551–554. doi: 10.1042/bj1610551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Matsukawa S., Takeshita M., Yoneyama Y. Effect of inositol hexaphosphate on hemoglobin oxidation by nitrite and ferricyanide. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1469–1474. doi: 10.1016/0006-291x(77)90607-6. [DOI] [PubMed] [Google Scholar]
- Tomoda A., Takeshita M., Yoneyama Y. Characterization of intermediate hemoglobin produced during methemoglobin reduction by ascorbic acid. J Biol Chem. 1978 Oct 25;253(20):7415–7419. [PubMed] [Google Scholar]



