Abstract
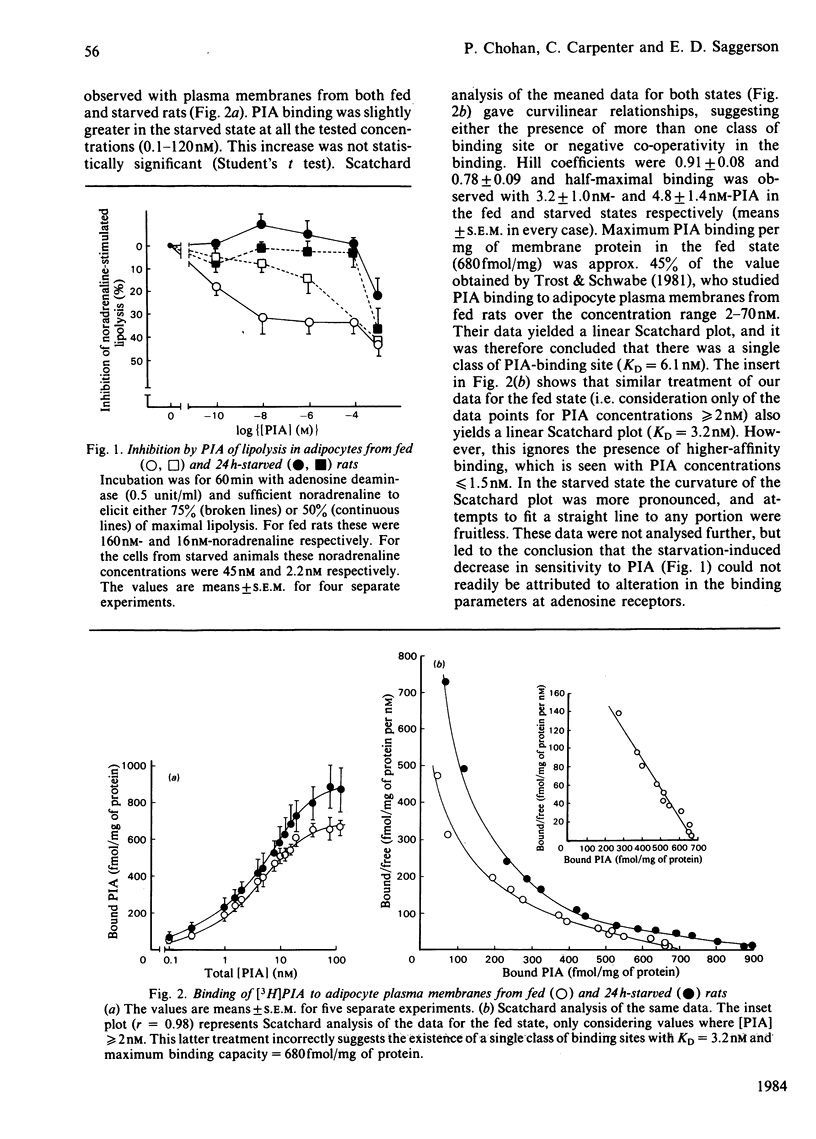
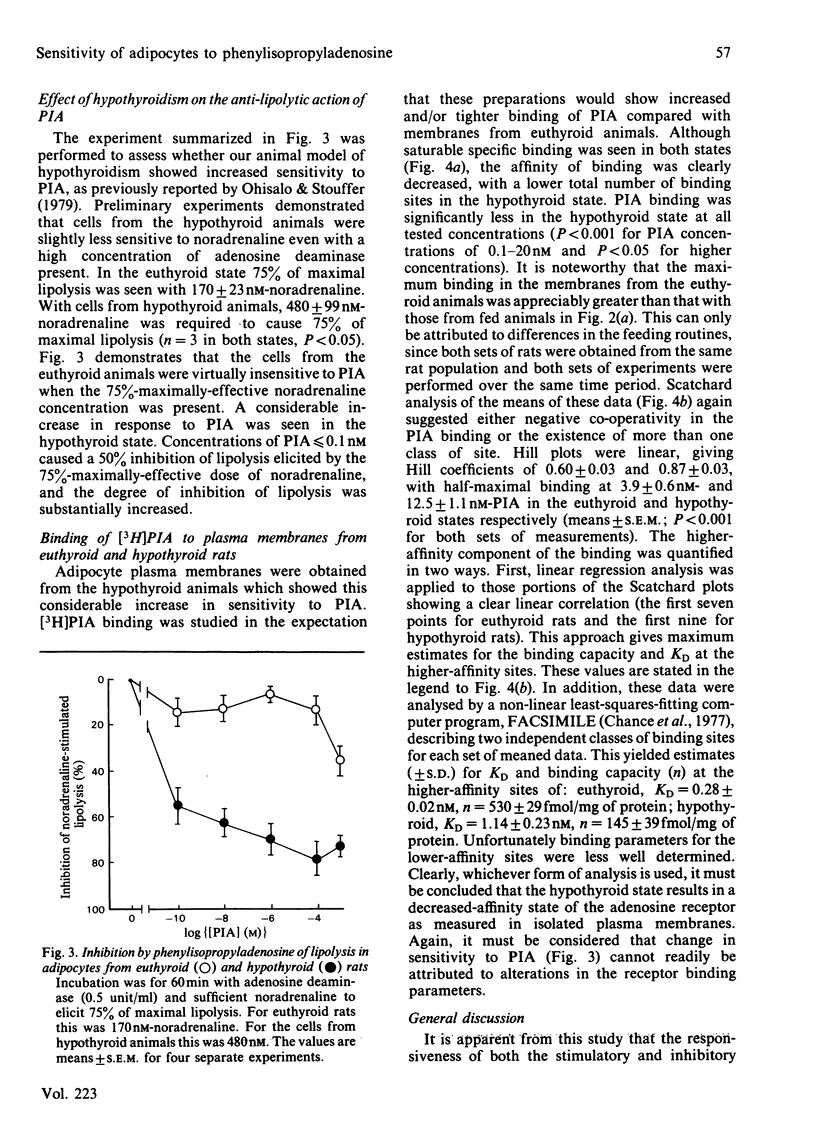
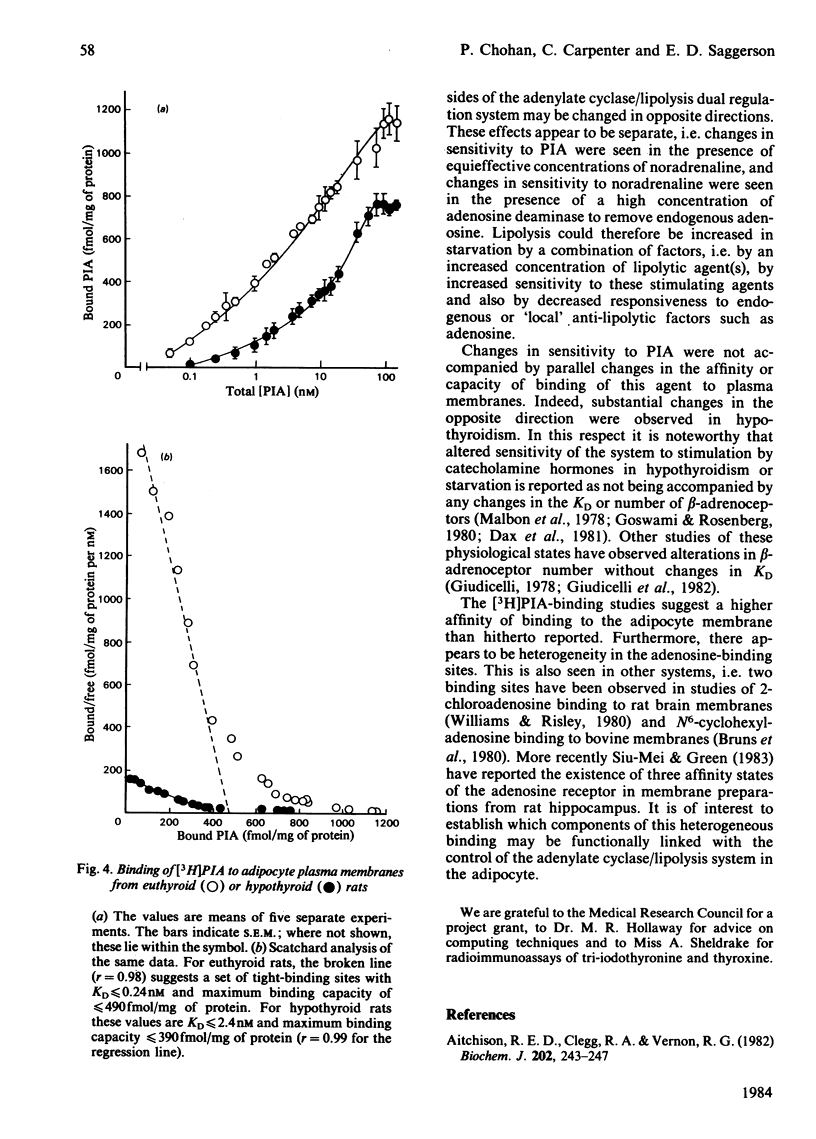
The anti-lipolytic effect of the adenosine analogue N6-L-phenylisopropyladenosine was studied with rat adipocytes incubated with a high concentration of adenosine deaminase (0.5 unit/ml, approx. 2.5 micrograms/ml) and concentrations of noradrenaline that were equieffective in different physiological states. These studies were performed to compare the fed and starved (24h) states and to compare a hypothyroid state (induced by feeding propylthiouracil + a low-iodine diet) with the euthyroid state. Starvation increased sensitivity of the cells to the lipolytic action of noradrenaline, while decreasing sensitivity to the antilipolytic action of phenylisopropyladenosine. Hypothyroidism resulted in decreased sensitivity to noradrenaline and increased sensitivity to phenylisopropyladenosine. Studies of the binding of [3H]phenylisopropyladenosine to adipocyte plasma membranes indicated heterogeneity of binding sites or negative co-operativity in the binding. Starvation did not change [3H]phenylisopropyladenosine binding to membranes, whereas hypothyroidism caused an unexpected decrease in both the number and affinity of the binding sites. These observations are discussed in terms of the dual regulation of adipose-tissue lipolysis by lipolytic and anti-lipolytic agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison R. E., Clegg R. A., Vernon R. G. Lipolysis in rat adipocytes during pregnancy and lactation. The response to noradrenaline. Biochem J. 1982 Jan 15;202(1):243–247. doi: 10.1042/bj2020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J., Denton R. M., Tanner M. J. Use of a novel rapid preparation of fat-cell plasma membranes employing Percoll to investigate the effects of insulin and adrenaline on membrane protein phosphorylation within intact fat-cells. Biochem J. 1980 Nov 15;192(2):457–467. doi: 10.1042/bj1920457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzipanteli K., Saggerson D. Streptozotocin diabetes results in increased responsiveness of adipocyte lipolysis to glucagon. FEBS Lett. 1983 May 2;155(1):135–138. doi: 10.1016/0014-5793(83)80225-7. [DOI] [PubMed] [Google Scholar]
- Chohan P., Saggerson D. Increased sensitivity of adipocyte adenylate cyclase to glucagon in the fasted state. FEBS Lett. 1982 Sep 20;146(2):357–360. doi: 10.1016/0014-5793(82)80952-6. [DOI] [PubMed] [Google Scholar]
- Correze C., Laudat M. H., Laudat P., Nunez J. Hormone-dependent lipolysis in fat-cells from thyroidectomized rats. Mol Cell Endocrinol. 1974 Oct;1(5):309–327. doi: 10.1016/0303-7207(74)90021-5. [DOI] [PubMed] [Google Scholar]
- Dax E. M., Partilla J. S., Gregerman R. I. Increased sensitivity to epinephrine stimulated lipolysis during starvation: tighter coupling of the adenylate cyclase complex. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1186–1192. doi: 10.1016/0006-291x(81)91573-4. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wieser P. B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. J Biol Chem. 1975 Feb 10;250(3):1027–1034. [PubMed] [Google Scholar]
- Fernandez B. M., Saggerson E. D. Alterations in response of rat white adipocytes to insulin, noradrenaline, corticotropin and glucagon after adrenalectomy. Correction of these changes by adenosine deaminase. Biochem J. 1978 Jul 15;174(1):111–118. doi: 10.1042/bj1740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B. Effect of adenosine, adenosine analogues and drugs inhibiting adenosine inactivation on lipolysis in rat fat cells. Acta Physiol Scand. 1978 Feb;102(2):191–198. doi: 10.1111/j.1748-1716.1978.tb06062.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol. 1981;313:351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Giudicelli Y., Lacasa D., Agli B. Alterations induced by a prolonged fasting: opposite effects on the beta-adrenergic receptor-coupled adenylate-cyclase system and on lipolysis in fat cells from rat. Eur J Biochem. 1982 Jan;121(2):301–308. doi: 10.1111/j.1432-1033.1982.tb05786.x. [DOI] [PubMed] [Google Scholar]
- Giudicelli Y. Thyroid-hormone modulation of the number of beta-adrenergic receptors in rat fat-cell membranes. Biochem J. 1978 Dec 15;176(3):1007–1010. doi: 10.1042/bj1761007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Thyroid hormone modulation of epinephrine-induced lipolysis in rat adipocytes: a possible role of calcium. Endocrinology. 1978 Dec;103(6):2223–2233. doi: 10.1210/endo-103-6-2223. [DOI] [PubMed] [Google Scholar]
- Honnor R. C., Saggerson E. D. Altered lipolytic response to glucagon and adenosine deaminase in adipocytes from starved rats. Biochem J. 1980 Jun 15;188(3):757–761. doi: 10.1042/bj1880757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Rodbell M. Receptor-mediated stimulation and inhibition of adenylate cyclases: the fat cell as a model system. Adv Cyclic Nucleotide Res. 1981;14:163–171. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Ohisalo J. J., Stouffer J. E. Adenosine, thyroid status and regulation of lipolysis. Biochem J. 1979 Jan 15;178(1):249–251. doi: 10.1042/bj1780249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olansky L., Myers G. A., Pohl S. L., Hewlett E. L. Promotion of lipolysis in rat adipocytes by pertussis toxin: reversal of endogenous inhibition. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6547–6551. doi: 10.1073/pnas.80.21.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D. Increased antilipolytic effect of the adenosine 'R-site' agonist N6-(phenylisopropyl)adenosine in adipocytes from adrenalectomized rats. FEBS Lett. 1980 Jun 16;115(1):127–128. doi: 10.1016/0014-5793(80)80741-1. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ebert R. Stimulation of cyclic adenosine 3',5'-monophosphate accumulation and lipolysis in fat cells by adenosine deaminase. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):33–44. doi: 10.1007/BF00647401. [DOI] [PubMed] [Google Scholar]
- Shechter Y. Evaluation of adenosine or related nucleosides as physiological regulators of lipolysis in adipose tissue. Endocrinology. 1982 May;110(5):1579–1583. doi: 10.1210/endo-110-5-1579. [DOI] [PubMed] [Google Scholar]
- Stock K., Prilop M. Dissociation of catecholamine-induced formation of adenosine 3'5'-monophosphate and release of glycerol in fat cells by prostaglandin E1, E2 and N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):15–31. doi: 10.1007/BF00647400. [DOI] [PubMed] [Google Scholar]
- Trost T., Schwabe U. Adenosine receptors in fat cells. Identification by (-)-N6-[3H]phenylisopropyladenosine binding. Mol Pharmacol. 1981 Mar;19(2):228–235. [PubMed] [Google Scholar]
- Vernon R. G., Finley E., Taylor E. Adenosine and the control of lipolysis in rat adipocytes during pregnancy and lactation. Biochem J. 1983 Oct 15;216(1):121–128. doi: 10.1042/bj2160121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Risley E. A. Biochemical characterization of putative central purinergic receptors by using 2-chloro[3H]adenosine, a stable analog of adenosine. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6892–6896. doi: 10.1073/pnas.77.11.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]
- Yeung S. M., Green R. D. Agonist and antagonist affinities for inhibitory adenosine receptors are reciprocally affected by 5'-guanylylimidodiphosphate or N-ethylmaleimide. J Biol Chem. 1983 Feb 25;258(4):2334–2339. [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Froesch E. R. Increased sensitivity of rat adipose tissue to the lipolytic action of epinephrine during fasting and its reversal during re-feeding. FEBS Lett. 1977 Apr 1;76(1):135–138. doi: 10.1016/0014-5793(77)80137-3. [DOI] [PubMed] [Google Scholar]
- Zumstein P., Zapf J., Waldvogel M., Froesch E. R. Increased sensitivity to lipolytic hormones of adenylate cyclase in fat cells of diabetic rats. Eur J Biochem. 1980 Mar;105(1):187–194. doi: 10.1111/j.1432-1033.1980.tb04488.x. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


