Abstract
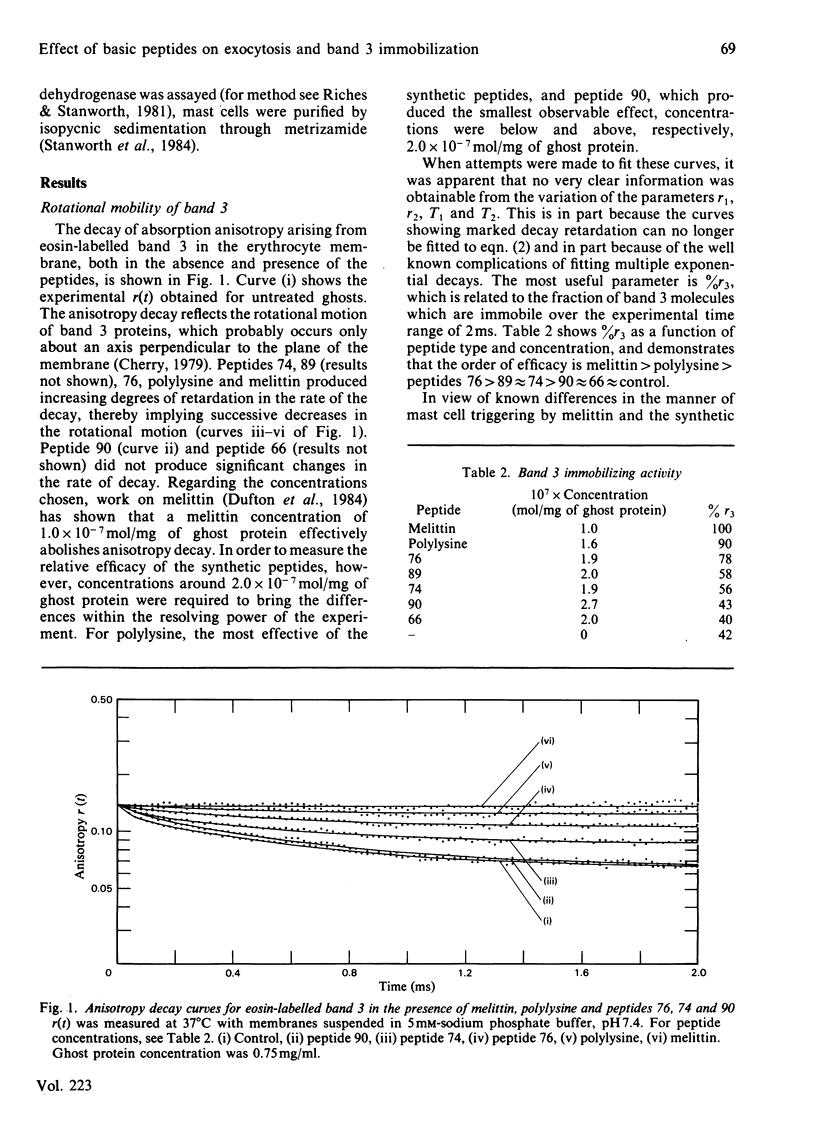
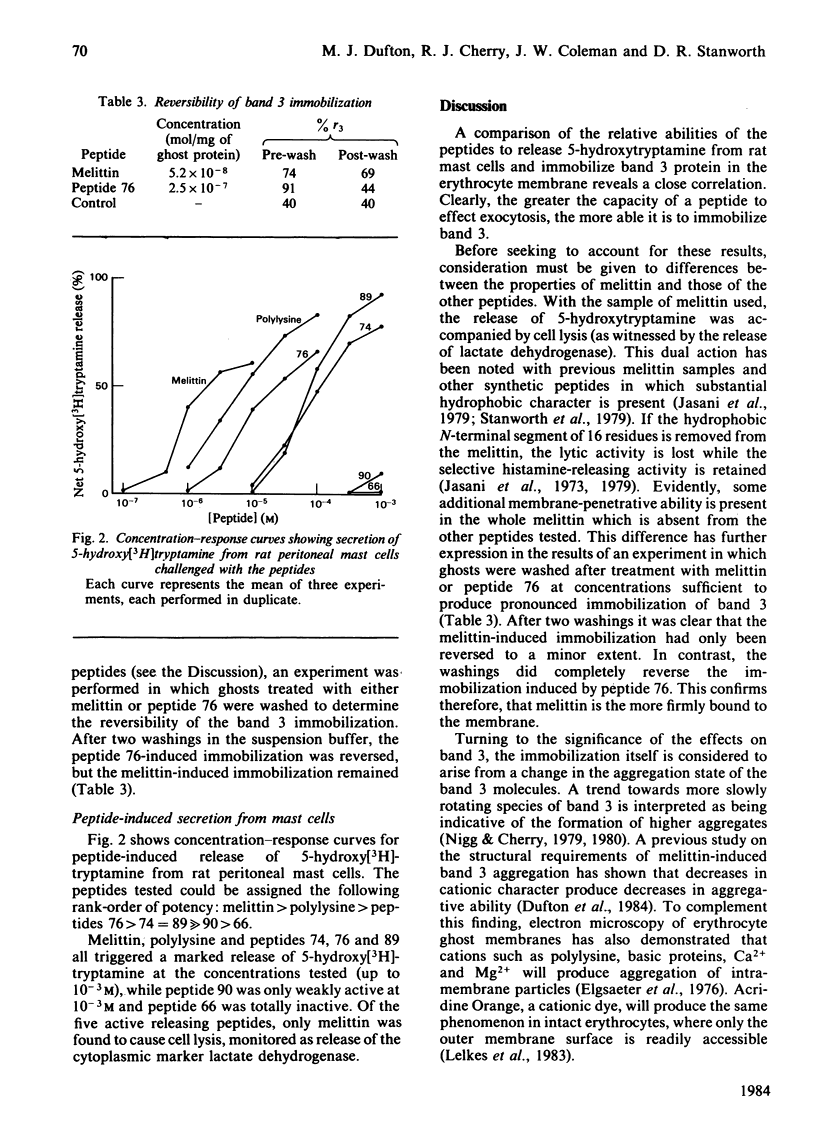
The effect of mast-cell-triggering peptides on the rotational properties of band 3, a protein component of the human erythrocyte membrane, was measured by observing flash-induced transient dichroism of the triplet probe eosin maleimide. In the presence of melittin, polylysine and five synthetic peptides, varying degrees of retardation in the rotational motion of band 3 were produced. When placed in order of band 3 immobilizing activity, the peptides formed a series identical with their order of efficacy in releasing 5-hydroxytryptamine from rat peritoneal mast cells. The correspondence in the abilities to immobilize band 3 in the erythrocyte and trigger mast cells is significant because structure-activity analyses of the peptides show both processes to have the same cationic, hydrophobic and stereochemical requirements. Probably, the immobilization of band 3 proteins reflects an ability of the basic peptides to aggregate anionic surface moieties, and therefore a similar mechanism is implied in mast-cell triggering.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Coleman J. W., Holgate S. T., Church M. K., Godfrey R. C. Immunoglobulin E decapeptide-induced 5-hydroxytryptamine release from rat peritoneal mast cells. Comparison with corticotropin-(1-24)-peptide, polyarginine, polylysine and antigen. Biochem J. 1981 Sep 15;198(3):615–619. doi: 10.1042/bj1980615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. W. Neuraminidase- and benzalkonium chloride-dependent inhibition of basic peptide-induced rat mast cell secretion. Immunol Lett. 1982 Oct;5(4):197–201. doi: 10.1016/0165-2478(82)90134-1. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Shotton D. M., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. II. The influence of spectrin aggregation. Biochim Biophys Acta. 1976 Feb 19;426(1):101–122. doi: 10.1016/0005-2736(76)90433-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Lichtenstein L. M. Induction of histamine secretion by polycations. Biochim Biophys Acta. 1980 May 22;629(3):587–603. doi: 10.1016/0304-4165(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Taurén G., Virtanen I., Wartiovaara J. Distribution of glycophorin on the surface of human erythrocyte membranes and its association with intramembrane particles: an immunochemical and freeze-fracture study of normal and En(a-) erythrocytes. J Supramol Struct. 1978;8(3):337–347. doi: 10.1002/jss.400080311. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Ishizaka T. The Robert A. Cooke memorial lecture. Analysis of triggering events in mast cells for immunoglobulin E-mediated histamine release. J Allergy Clin Immunol. 1981 Feb;67(2):90–96. doi: 10.1016/0091-6749(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Jasani B., Kreil G., Mackler B. F., Stanworth D. R. Further studies on the structural requirements for polypeptide-mediated histamine release from rat mast cells. Biochem J. 1979 Sep 1;181(3):623–632. doi: 10.1042/bj1810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasani B., Stanworth D. R. Studies on the mast cell triggering action of certain artificial histamine liberators. Int Arch Allergy Appl Immunol. 1973;45(1):74–81. doi: 10.1159/000231005. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973 Apr;142(4):1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Kaul R. K., Murthy S. N., Reddy A. G., Steck T. L., Kohler H. Amino acid sequence of the N alpha-terminal 201 residues of human erythrocyte membrane band 3. J Biol Chem. 1983 Jul 10;258(13):7981–7990. [PubMed] [Google Scholar]
- Lelkes G., Lelkes G., Merse K. S., Hollán S. R. Intense, reversible aggregation of intramembrane particles in non-haemolyzed human erythrocytes. A freeze-fracture study. Biochim Biophys Acta. 1983 Jul 13;732(1):48–57. doi: 10.1016/0005-2736(83)90185-2. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Bron C., Girardet M., Cherry R. J. Band 3-glycophorin A association in erythrocyte membrane demonstrated by combining protein diffusion measurements with antibody-induced cross-linking. Biochemistry. 1980 Apr 29;19(9):1887–1893. doi: 10.1021/bi00550a024. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: protein rotational diffusion measurements. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4702–4706. doi: 10.1073/pnas.77.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Studies on the possible involvement of complement component C3 in the initiation of acid hydrolase secretion by macrophages. I. Correlation between enzyme-releasing and complement-activating capacities of several secretagogues. Immunology. 1981 Sep;44(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Stanworth D. R., Coleman J. W., Khan Z. Essential structural requirements for triggering of mast cells by a synthetic peptide comprising a sequence in the C epsilon 4 domain of human IgE. Mol Immunol. 1984 Mar;21(3):243–247. doi: 10.1016/0161-5890(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Stanworth D. R., Kings M., Roy P. D., Moran J. M., Moran D. M. Synthetic peptides comprising sequences of the human immunoglobulin E heavy chain capable of releasing histamine. Biochem J. 1979 Jun 15;180(3):665–668. doi: 10.1042/bj1800665. [DOI] [PMC free article] [PubMed] [Google Scholar]


