Abstract
The COVID‐19 pandemic has emerged as a major global health crisis. Vitamin D, a crucial fat‐soluble vitamin, has been recommended for COVID‐19 patients, though evidence of its effectiveness is inconsistent. This systematic literature review and meta‐analysis aimed to evaluate the impact of vitamin D supplementation on COVID‐19‐related outcomes. A comprehensive search was conducted across PubMed, Scopus, Web of Science, Embase, and Cochrane databases. Primary outcomes included mortality and hospital length of stay, while secondary outcomes encompassed C‐reactive protein (CRP), ferritin, D‐dimer, hemoglobin (Hb) concentrations, and lymphocyte, neutrophil, and platelet counts. Data analysis was performed using Stata™ Version 14. A total of 16 trials were analyzed. The meta‐analysis revealed that vitamin D supplementation significantly reduced hospital length of stay (mean difference = −1.16; 95% confidence interval [CI]: −2.23, −0.09; p = .033) with significant heterogeneity (I 2 = 69.2%, p = .002). Subgroup analysis showed a more pronounced reduction in studies with vitamin D dosages ≤10 000 international units (IU) (mean difference = −1.27; 95% CI: −1.96, −0.57; p < .001) and in patients over 60 years old (mean difference = −1.84; 95% CI: −2.53, −1.14; p < .001). Additionally, vitamin D significantly reduced CRP concentrations in older adults (>60 years) (mean difference = −1.13; 95% CI: −2.07, −0.18; p = .019). No significant changes were found in ferritin, D‐dimer, Hb concentrations, or in lymphocyte, neutrophil, and platelet counts (p > .05). In conclusion, while vitamin D supplementation did not significantly affect most COVID‐19‐related biomarkers, however, it reduces the length of hospital stay.
Keywords: COVID‐19, meta‐analysis, systematic review, vitamin D
Selection process for eligible studies from all citations.
Abbreviations
- 25(OH)D
25‐hydroxyvitamin D
- ARDS
acute respiratory distress syndrome
- CIs
confidence intervals
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- Hb
hemoglobin
- ICU
intensive care unit
- IU
international units
- PICOS
population, intervention/exposure, comparator, outcome, and study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis
- SD
standard deviation
- WHO
World Health Organization
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) epidemic, which began in China in late 2019, quickly spread worldwide, infecting millions and causing numerous deaths. 1 , 2 , 3 According to the latest statistics from the World Health Organization (WHO) as of June 2023, over 767 million COVID‐19 cases have been confirmed, with approximately 7 million fatalities. 4 Since the outbreak began, extensive investigations have explored the effects of pharmacological interventions, herbal remedies, traditional medicine, and other factors in managing COVID‐19. 5 , 6 , 7 , 8
Nutritional factors are recognized as important in the prevention and treatment of COVID‐19. 9 , 10 Various researchers have focused on nutritional factors that can strengthen the immune system against COVID‐19 or support the treatment process, leading to numerous studies in this area. 11 , 12 Most of these studies have focused on antioxidant compounds or diets designed to increase the intake of antioxidants and immune system‐enhancing nutrients. 13
Vitamin D, a key modulator of the immune system, plays a crucial role in both innate and adaptive immunity. 14 , 15 Since the outbreak of COVID‐19, vitamin D has been extensively studied and is considered one of the most crucial nutrients. 16 Vitamin D insufficiency has been associated with worse outcomes, greater severity, and a higher incidence of comorbidities in respiratory infections. 17 Serum concentrations of 25‐hydroxyvitamin D [25(OH)D] <20 ng/mL have been shown to increase the risk of pneumonia by over 60%. 18 There is an inverse correlation between 25(OH)D concentrations and both the severity of the disease and specific clinical biomarkers in COVID‐19 patients. 19 , 20 , 21 Vitamin D may also mitigate the negative effects of COVID‐19 by regulating the renin‐angiotensin system and the production of angiotensin‐converting enzyme 2, which helps reduce lung leakage in acute respiratory distress syndrome (ARDS) animal models. 22
Several clinical trials have evaluated the effects of different doses of vitamin D on COVID‐19 outcomes, but the results have been contradictory. 23 , 24 To our knowledge, two recent meta‐analyses have examined the effects of vitamin D supplementation in COVID‐19 patients. 25 , 26 However, these studies faced issues such as incomplete inclusion of primary articles, inclusion of retracted articles, and methodological limitations. Additionally, the factors investigated differed from those examined in the current study. Therefore, this systematic review and meta‐analysis aimed to assess the effects of vitamin D supplementation on clinical outcomes in adult COVID‐19 patients. Our primary objective was to evaluate the impact of vitamin D supplementation on mortality and hospital length of stay. Secondary objectives included investigating changes in C‐reactive protein (CRP), ferritin, D‐dimer, hemoglobin (Hb) concentrations, and lymphocyte, neutrophil, and platelet counts following vitamin D supplementation.
2. METHODS
This meta‐analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 27 The study was registered in the Systematic Review Registration: PROSPERO (registration ID: CRD42023441017).
2.1. Search study
To find relevant articles, a systematic search was performed in PubMed, Scopus, Web of Science, Embase, and Cochrane databases. The search strategy involved two concept keywords: COVID‐19 and vitamin D supplementation. The details of the search strategy used in this search are shown in Table S1. We also conducted a manual search of references listed in relevant review articles, including backward and forward searches and queries using Google Scholar, to ensure that no relevant research was missed. The search was conducted without any language restrictions. In our systematic review, while our primary focus was on peer‐reviewed papers to ensure reliability and quality, we conducted a targeted search for gray literature. This involved exploring sources such as conference proceedings, dissertations, theses, government reports, and other relevant documents. Additionally, we did not include preprint studies in our analysis. After conducting a systematic search, the obtained records were transferred to the EndNote software to perform the screening process. After removing duplicate records, two researchers independently reviewed the titles and abstracts to exclude articles with unrelated titles from the review process. Any discrepancies between the findings of the two researchers were resolved through consultation with a third person.
2.2. Eligibility criteria
In the second stage, the screening process was carried out based on the population, intervention/exposure, comparator, outcome, and study design (PICOS) criteria. The PICOS framework was used for inclusion and exclusion criteria. The inclusion criteria include: (1) clinical trial studies with a control group conducted on adults over 18 years old; (2) vitamin D supplementation of at least one dose in patients with COVID‐19; (3) comparison of at least one of the outcomes considered in this study (mortality, length of hospital stay, CRP, ferritin, D‐dimer, Hb concentrations, and lymphocyte, neutrophil, and platelet counts) between the intervention and control groups reported at the beginning and end of the intervention. Studies that had a design other than a clinical trial, or were conducted on animal samples, or on children, were excluded. Additionally, studies that evaluated vitamin D simultaneously with other agents, where it was not possible to assess the independent effect of vitamin D, were excluded from the analysis. Detailed inclusion and exclusion criteria are described in Table S2.
2.3. Data extraction
Two researchers independently extracted the required data from the articles. This information includes the name of the first author, year of publication, country, study sample size and gender distribution, mean age of the participants, vitamin D dosage, duration of the intervention, control group, and the mean and standard deviation (SD) of the investigated variables. Any disagreement between the two researchers was resolved through consultation with a third person.
2.4. Assessment of the risk of bias and certainty of the evidence
The Cochrane risk‐of‐bias tool (RoB 2), specifically designed for randomized trials, was utilized to assess the risk of bias within this study. This methodology includes criteria for selection bias, detection bias, performance bias, reporting bias, attrition bias, and other potential biases. 28 The overall strength of the evidence was determined using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method. 29 , 30 According to our evaluation criteria, the estimates of biomarker effects were categorized into four quality tiers: high, moderate, low, and very low. The reviewers separately conducted GRADE assessments, and conflicts between reviewers were settled by a third reviewer.
2.5. Data analysis
The information obtained from the primary articles was first entered into Excel software and then transferred to Stata 14 software (Stata Corp, College Station, TX, USA) for statistical analysis. Intervention effects were determined as the mean differences and 95% confidence intervals (CIs) obtained for changes in serum concentrations of CRP and D‐dimer, as well as length of hospital stay. We used the following formula to compute SD change from SD baseline in both intervention and control groups: . The I 2 statistic and Cochran's chi‐square test (Q) were used for evaluation of studies heterogeneity. If the I 2 was above 50%, we used the random‐effect model, and if the I 2 was below 50%, we used the fixed‐effect model. Also, sensitivity analysis was used to evaluate the effect of removing each study on the results. Publication bias was assessed by funnel plot and Egger's and Begg's test. 31 Subgroup analyses were conducted based on participants' baseline vitamin D concentrations (≤22 ng/mL and >22 ng/mL), age (≤60 years and >60 years), and vitamin D dosage (≤10 000 international units [IU] and >10 000 IU). A p‐value of <.05 was, a priori, considered statistically significant, unless otherwise specified.
3. RESULTS
3.1. Literature search and characteristics of included studies
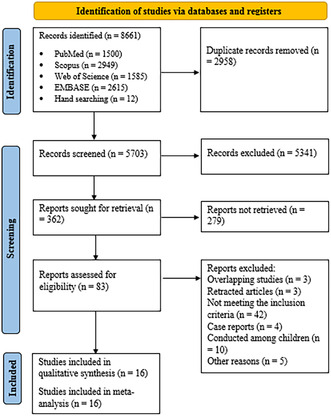
At the end of the systematic search process, 8661 records were identified, and after removing duplicate records, 5703 articles entered the screening phase. After a systematic search and two stages of screening, 16 studies met the necessary criteria to be included in this study. 23 , 24 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 The article selection process is displayed in the flowchart (Figure 1), following the PRISMA method. 27 From the included studies, two studies were conducted in Egypt, two studies in Brazil, three studies in Spain, and one study each in Croatia, Switzerland, India, Argentina, Israel, Russia, Saudi Arabia, Mexico, and France. The sample size in the evaluated studies ranged from 40 to 237 patients. The characteristics of the included studies are summarized in Table 1. The dose of vitamin D used in the studies varied from 2000 to 500 000 IU. In the comparison group, three studies used a low dose of vitamin D, 32 , 34 , 41 and in the remaining studies, placebo was used. No prevention trials were included.
FIGURE 1.
Selection process for eligible studies from all identified citations.
TABLE 1.
Characteristics of included studies.
| Study (year) | Study design | Country | Total sample size (intervention/control) | Age, mean (SD) | Baseline 25(OH)D concentrations | Vitamin D dosage/duration | Control | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Bugarin et al. (2023) 24 | Open‐label randomized clinical trial | Croatia | 152 (55/57) | 64.95 (31.85) | 24.4 (16.95–36.8) | 10 000 IU/14 days | Without vitamin D supplementation | Median number of days spent in the ICU, mean WHO clinical progression scale, CRP, neutrophil/lymphocyte ratio, fibrinogen |
| Jaun et al. (2023) 23 | Multicenter, randomized, placebo‐controlled double‐blind trial | Switzerland | 78 (39/39) | 60.49 (13.84) | 31.46 (10.95) | 140 000 IU single dose plus 800 IU daily/until discharge | Standard treatment | Length of hospital stay |
| Cervero et al. (2022) 32 | Multicenter, single‐blinded, prospective randomized pilot clinical trial | Brazil | 85 (44/41) | 65 (17.32) | 14.8 (6.2) | 10 000 IU cholecalciferol/14 days | 2000 IU cholecalciferol | Length of the hospital stay and ARDS development, LDH, D‐dimer, platelet count, ferritin |
| Rastogi et al. (2022) 33 | Randomized, placebo‐controlled, study | India | 40 (16/24) | 50 (11.11) | 8.6 (7.1–13.1) | 60 000 IU/7 days | Placebo (5 mL distilled water) | D‐dimer, fibrinogen, CRP, procalcitonin |
| Torres et al. (2022) 34 | Multicenter, single blind, prospective, randomized clinical trial | Spain | 85 (44/41) | 65.0 (53.0–74.0) | 22.4 (25.07) | 10 000 IU/14 days | 2000 IU cholecalciferol | Length of hospital stay, changes in the cytokine profile, distribution of CD4+ T cell |
| Soliman et al. (2022) 35 | Placebo‐controlled randomized prospective study | Egypt | 56 (40/16) | 71.3 (4.16) | 14.8 (6.2) | 200 000 IU single dose | Placebo | 6 Weeks mortality, D‐dimer, CRP, platelet count, Hb, lymphocytes |
| Sarhan et al. (2022) 36 | Prospective randomized controlled trial | Egypt | 116 (58/58) | 65 (15.55) | 18.33 (7.5) | 200 000 IU single dose | Standard COVID‐19 management | Ferritin, D‐dimer, LDH, CRP |
| Mariani et al. (2022) 37 | Multicenter, randomized, double‐blind, sequential, placebo‐controlled trial | Argentina | 218 (115/103) | 55.8 (10.70) | 32.5 (12.59) | 500 000 IU single dose | Matching placebo | rSOFA, change in SpO2, length of hospital stay |
| Elamir et al. (2022) 38 | Randomized pilot study | Israel | 50 (25/25) | 69 (18) | 25.8 (11.26) | 2000 IU/14 days | Matching placebo | Length of hospital stay, mortality, endotracheal intubation |
| Karonova et al. (2022) 39 | Randomized, open‐label, single‐center study | Russia | 110 (56/54) | 57 (11.11) | 17.8 (10.4) | 100 000 IU single dose | Placebo | Neutrophil and lymphocyte counts, CRP, frequencies of CD38++ CD27 |
| Murai et al. (2021) 40 | Multicenter, double‐blind, randomized, placebo‐controlled trial | Brazil | 237 (119/118) | 56.5 (13.8) | 21.2 (10.1) | 200 000 IU single dose | 10 mL of a peanut oil solution | Hospital length of stay, duration of mechanical ventilation, CRP |
| Sabico et al. (2021) 41 | Randomized clinical trial | Saudi Arabia | 69 (36/33) | 49.8 (14.3) | 53.4 (2.9) | 5000 IU/14 days | 1000 IU/14 days | IL‐6, D‐dimer |
| Sánchez‐Zuno et al. (2021) 42 | Open‐label randomized clinical trial | Mexico | 42 (22/20) | 43.0 (20–74) | 22.4 (12.1–45.9) | 10 000 IU/14 days | Standard COVID‐19 management | Mortality |
| Annweiler et al. (2020) 43 | Clinical trial | France | 48 (32/16) | 88.0 (5.5) | 65.2 (34.8) | 800 IU/3 months | Standard COVID‐19 management | Mortality |
| Caballero‐García et al. (2021) 45 | Double‐blind | Spain | 30 (15/15) | 60.6 (1.7) | 27.30 (10.5) | 2000 IU/6 weeks | Placebo | CRP, ferritin, Hb, lymphocytes |
| Castillo et al. (2020) 44 | Pilot randomized clinical study | Spain | 76 (50/26) | 53 (10) | 21.2 (1.4) | Loading dose of 532 μg, followed by 266 μg on days 3, 7, 14, 21, and 28 | Standard COVID‐19 management |
Mortality Need for ICU admission |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; ARDS, acute respiratory distress syndrome; CRP, C‐reactive protein; Hb, hemoglobin; ICU, intensive care unit; IL‐6, interleukin 6; IU, international units; LDH, lactate dehydrogenase; rSOFA, respiratory sepsis‐related organ failure assessment; WHO, World Health Organization.
3.2. Risk of bias assessment
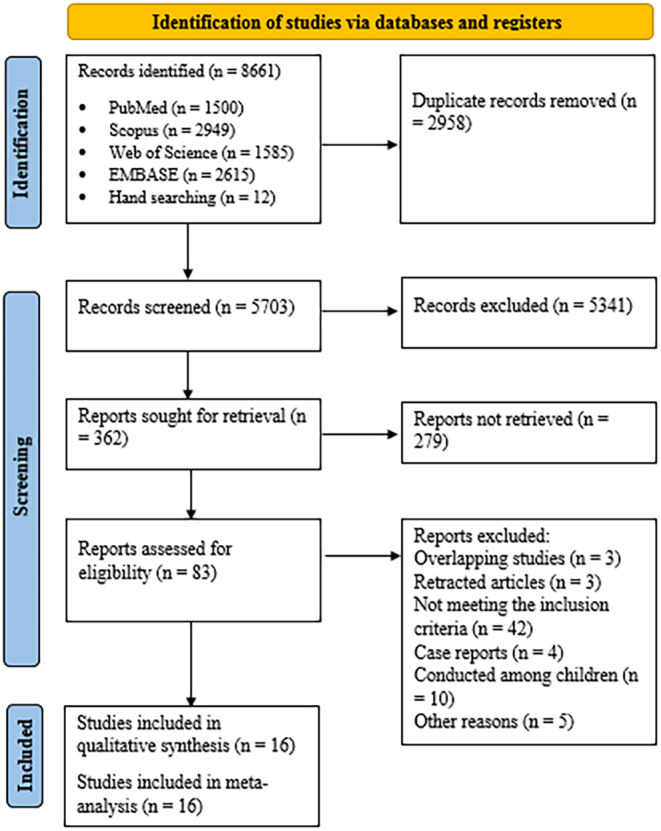
The results of the risk‐of‐bias assessment are shown in Table 2. All the trials used acceptable random sequence generation. In term of allocation concealment, six studies had acceptable conditions, 32 , 36 , 37 , 40 , 41 , 43 three studies had a high risk of bias, 39 , 42 , 43 and for the remaining studies, the risk of bias is unclear. Also, in terms of blinding, we found a high risk of bias in four studies, 10 trials had a low risk of bias, and in two studies, the risk‐of‐bias assessment was unclear. Moreover, only four studies received a low risk‐of‐bias grade in terms of blinding of outcome assessment. 23 , 37 , 41 , 44 Except for two studies whose status was unclear, 34 , 36 the rest of the studies were in a good condition in terms of attrition bias. Finally, except for one trial, 44 all other studies provided a low risk of bias in terms of selection bias and other sources of bias.
TABLE 2.
Study quality and risk‐of‐bias assessment of included studies in the meta‐analysis.
|
Note:  : low risk,
: low risk,  : high risk and
: high risk and  : unclear.
: unclear.
3.3. Effects of vitamin D supplementation on COVID‐19‐related mortality
Overall, nine trials considered the effects of vitamin D supplementation on mortality rate. 24 , 34 , 35 , 36 , 37 , 38 , 40 , 43 , 44 Due to high heterogeneity in the reporting of deaths, meta‐analysis was not possible. One study showed a non‐significant difference between the vitamin D and control group in terms of all‐cause mortality on day 60 (26.2% vs. 40.6% mortality rate). 24 Similar findings were observed in Murai et al. (7.6% vs. 5.1%), 40 and Torres et al. studies (2.44% vs. 2.27%). 34 Soliman et al., in a trial among diabetic elderly patients, did not find any significant differences in mortality rate between the vitamin D and placebo groups (17.5% vs. 18.8%). 35 In line with the results of this study, there were no significant differences between vitamin D and control groups in terms of COVID‐19 mortality in other studies, including Sarhan et al. (45% vs. 51%, p = .49), 36 Mariani et al. (4.3% vs. 1.9%, p = .451), 37 and Elamir et al. (three deaths in the control group and none in the vitamin D group, p = .23). 38 However, in one study conducted among older adult patients with COVID‐19, it was reported that a single oral high dose of cholecalciferol led to a significant improvement in overall mortality at day 14 (adjusted hazard ratio = 0.39 [95% CI: 0.16, 0.99], p = .049). 43
3.4. Effects of vitamin D supplementation on hospital length of stay
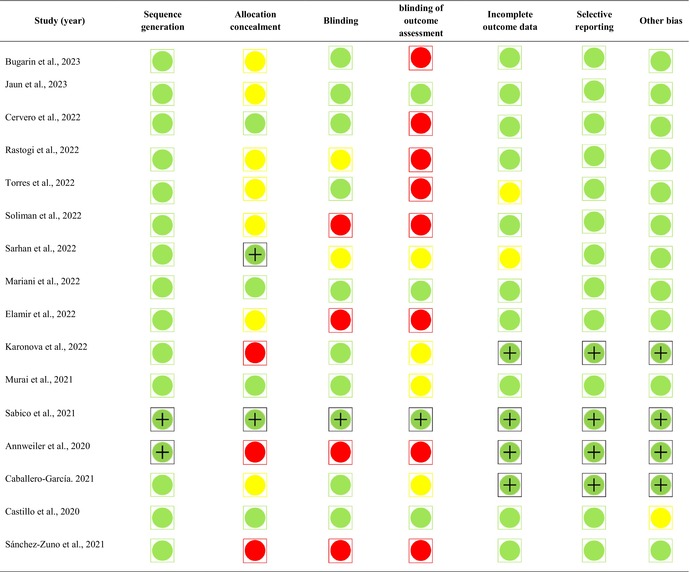
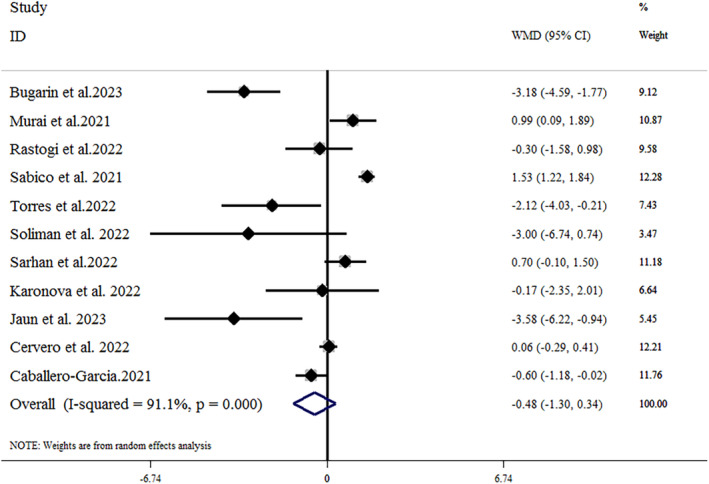
Eight trials, including 944 patients (471 treated and 473 controls), provided data related to the effects of vitamin D supplementation on hospital length of stay. 23 , 24 , 34 , 35 , 37 , 38 , 40 , 43 As shown in Figure 2, vitamin D supplementation led to a significant reduction in hospital length of stay (mean difference = −1.16 [95% CI: −2.23, −0.09]; p = .033), with a significant heterogeneity (I 2 = 69.2%, p = .002). In the subgroup analysis, we found that vitamin D supplementation reduced the length of hospital stay in studies where the vitamin D dosage was ≤10 000 IU (mean difference = −1.27 [95% CI: −1.96, −0.57]; p < .001) and in older adult patients over 60 years old (mean difference = −1.84 [95% CI: −2.53, −1.14]; p < .001). The results of the subgroup analysis are summarized in Table 3. The leave‐one‐out sensitivity analysis showed that leaving each of the trials resulted in a range from −0.809 [95% CI: −1.79, −0.17] by Torres et al. to −1.40 [95% CI: −2.58, −0.23] by Mariani et al., with no significant effect on the pooled effect size. 34 , 37 A funnel plot (Figure 3A) indicated no substantial evidence of publication bias (Egger's test p = .784; Begg's test p = .71).
FIGURE 2.
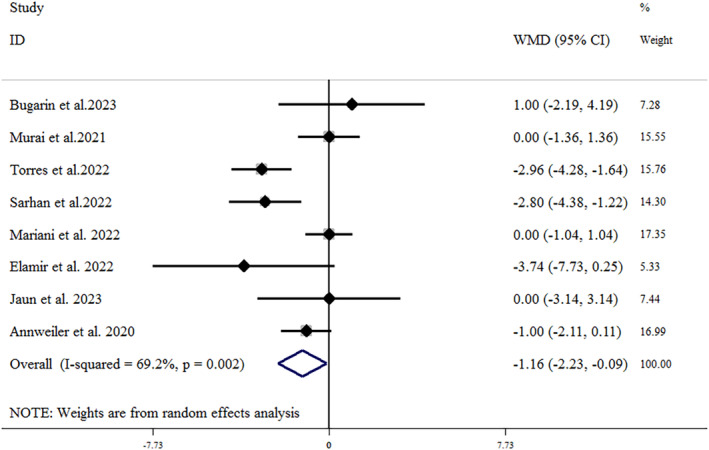
Forest plot detailing weighted mean difference and 95% CIs for the impact of vitamin D supplementation on hospital length of stay. CI, confidence interval; WMD, weight mean difference.
TABLE 3.
Subgroup analyses for the effect of vitamin D on COVID‐19‐related outcomes.
| Number of effect sizes | WMD (95% CI) | p Effect | p Within subgroup a | I 2 (%) | p Between subgroups b | |
|---|---|---|---|---|---|---|
| Subgroup analyses of vitamin D on hospital length of stay | ||||||
| 25(OH)D concentrations (ng/mL) | .643 | |||||
| ≤22 | 4 | −1.19 (−1.93, −0.45) | .002 | .035 | 65.2 | |
| >22 | 4 | −0.94 (−1.71, −0.17) | .016 | .003 | 78.4 | |
| Age (years) | .001 | |||||
| ≤60 | 2 | 0.00 (−0.83, 0.83) | 1 | 1 | 0.0 | |
| >60 | 6 | −1.84 (−2.53, −1.14) | .000 | .04 | 57 | |
| Vitamin D dosage (IU) | .390 | |||||
| ≤10 000 | 4 | −1.27 (−1.96, −0.57) | .000 | .01 | 69.9 | |
| >10 000 | 4 | −0.79 (−1.63, 0.05) | .065 | .013 | 77 | |
| Subgroup analyses of vitamin D on CRP concentrations | ||||||
| 25(OH)D concentrations (ng/mL) | <.001 | |||||
| ≤22 | 7 | 0.07 (−0.45, 0.59) | .788 | .025 | 58.6 | |
| >22 | 4 | −1.74 (−4.86, 1.39) | .276 | <.001 | 95.4 | |
| Age (years) | <.001 | |||||
| ≤60 | 4 | 0.78 (−0.11, 1.67) | .086 | .017 | 70.6 | |
| >60 | 7 | −1.13 (−2.07, −0.18) | .019 | <.001 | 84.3 | |
| Vitamin D dosage (IU) | .108 | |||||
| ≤10 000 | 6 | −0.35 (−1.42, 0.71) | .518 | <.001 | 94.8 | |
| >10 000 | 5 | −0.74 (−2.14, 0.66) | .299 | .013 | 64.6 | |
| Subgroup analyses of vitamin D on D‐dimer concentrations | ||||||
| 25(OH)D concentrations (ng/mL) | <.001 | |||||
| ≤22 | 5 | 0.02 (−0.27, 0.32) | .871 | .004 | 73.5 | |
| >22 | 2 | 1.48 (0.08, 2.88) | .038 | <.001 | 95.1 | |
| Age (years) | .277 | |||||
| ≤60 | 3 | 0.62 (−1.07, 2.31) | .474 | <.001 | 97.5 | |
| >60 | 4 | 0.18 (−0.32, 0.68) | .484 | <.001 | 86.2 | |
| Vitamin D dosage (IU) | <.001 | |||||
| ≤10 000 | 4 | 0.86 (−0.01, 1.73) | .052 | <.001 | 96.4 | |
| >10 000 | 3 | −0.43 (−1.25, 0.4) | .309 | .019 | 74.6 | |
| Subgroup analyses of vitamin D on lymphocyte numbers | ||||||
| 25(OH)D concentrations (ng/mL) | ‐ | |||||
| ≤22 | 50 (−190, 280) | .707 | <.001 | 82.1 | ||
| >22 | −1600 (−1790, −1410) | <.001 | ‐ | ‐ | ||
| Age (years) | <.001 | |||||
| ≤60 | −630 (−1640, 380) | .221 | <.001 | 98.5 | ||
| >60 | −2.53 (−9.37, 4.30) | .351 | .001 | 91.5 | ||
| Vitamin D dosage (IU) | <.001 | |||||
| ≤10 000 | −600 (−1570, 370) | .228 | <.001 | 99.1 | ||
| >10 000 | −270 (−930, 390) | .467 | <.001 | 89.7 | ||
| Subgroup analyses of vitamin D supplementation on ferritin concentrations | ||||||
| 25(OH)D concentrations (ng/mL) | ‐ | |||||
| ≤22 | −3.09 (−10.06, 3.89) | .386 | .032 | 62 | ||
| >22 | 4.40 (−0.82, 9.62) | .098 | ‐ | ‐ | ||
| Age (years) | .156 | |||||
| ≤60 | −0.87 (−10.20, 8.45) | .854 | .115 | 53.7 | ||
| >60 | −1.09 (−11.44, 9.27) | .21 | .007 | 80 | ||
| Vitamin D dosage (IU) | .350 | |||||
| ≤10 000 | 4.35 (0.11, 8.60) | .044 | .99 | 0.0 | ||
| >10 000 | −8.23 (−10.99, −5.48) | <.001 | .729 | 0.0 | ||
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; CI, confidence interval; CRP, C‐reactive protein; IU, international units; WMD, weighted mean difference.
p For heterogeneity, within subgroup.
p For heterogeneity, between subgroups.
FIGURE 3.
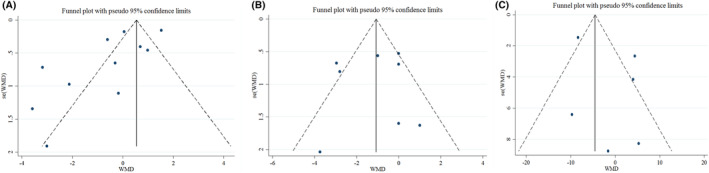
Funnel plots detailing publication bias in the studies selected for analysis. (A) Hospital length of stay; (B) CRP; (C) ferritin. Visual inspection of funnel plots indicating that there is no publication bias among studies. CI, confidence interval; CRP, C‐reactive protein; WMD, weight mean difference.
3.5. Effects of vitamin D supplementation on inflammatory markers
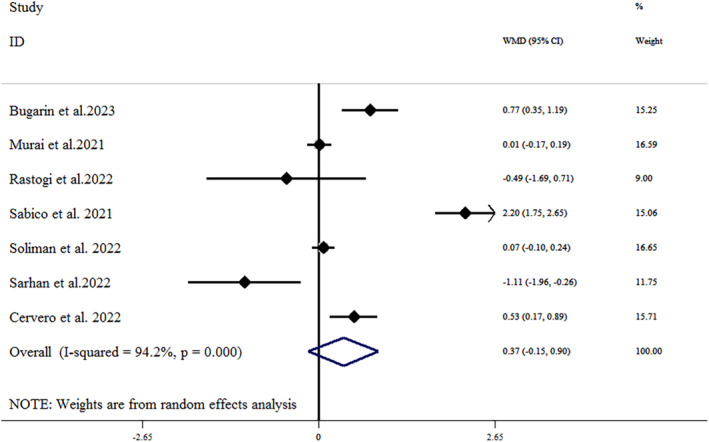
The effect of vitamin D supplementation on CRP concentrations in patients with COVID‐19 was investigated in 11 studies. 23 , 24 , 32 , 33 , 34 , 35 , 36 , 39 , 40 , 41 , 45 The results showed that vitamin D supplementation did not lead to a significant decrease in CRP concentrations compared to placebo (mean difference = −0.48 [95% CI: −1.30, 0.34]; p = .255), with a significant heterogeneity (I 2 = 91.1%, p < .001) (Figure 4). The subgroup analysis reported that vitamin D supplementation led to a significant reduction in serum concentrations of CRP in older adult patients over 60 years old (mean difference = −1.13 [95% CI: −2.07, −0.18]; p = .019). The results of sensitivity analysis showed that removing each of the trials in a range from −0.28 [95% CI: −1.1, 0.53] by Jaun et al. to −0.67 [95% CI: −1.57, 0.22] by Murai et al., did not change the significance of the results. 23 , 40 There was no substantial evidence of publication bias based on the funnel plot (Figure 3B) and Egger's test (Egger's test p = .132; Begg's test p = .484).
FIGURE 4.
Forest plot detailing weighted mean difference and 95% CIs for the impact of vitamin D supplementation on CRP concentrations. CI, confidence interval; CRP, C‐reactive protein; WMD, weight mean difference.
Overall, six studies provided sufficient data on the effect of vitamin D on ferritin concentrations. 32 , 33 , 36 , 39 , 41 , 45 According to the meta‐analysis, there were no significant effects of vitamin D on ferritin concentrations (mean difference = −1.24 [95% CI: −8.27, 5.80]; p = .730; I 2 = 79.5%, p < .002) (Figure 5). Subgroup analysis could not identify the source of heterogeneity. Also, the results of the sensitivity analysis showed that removing any of the studies had no effect on the results. Moreover, based on the publication bias test, there was no substantial evidence of publication bias between studies (Egger's test p = .322; Begg's test p = .851) (Figure 3C).
FIGURE 5.
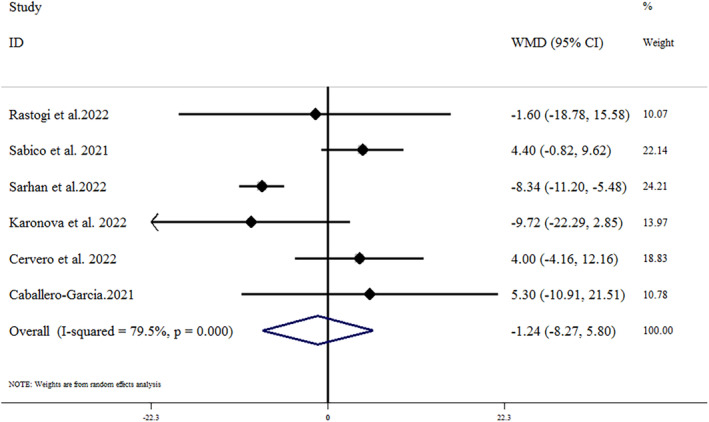
Forest plot detailing weighted mean difference and 95% CIs for the impact of vitamin D supplementation on Ferritin concentrations. CI, confidence interval; WMD, weight mean difference.
Seven studies compared the effects of vitamin D supplementation on D‐dimer concentrations. 24 , 32 , 33 , 35 , 36 , 40 , 41 The results of the pooled analysis showed that vitamin D did not significantly change the D‐dimer concentrations (mean difference = 0.37 [95% CI: −0.15, 0.9]; p = .166), with significant heterogeneity (I 2 = 94.2%, p < .001) (Figure 6). Subgroup analysis reported that vitamin D had a significant effect on D‐dimer concentrations among patients with baseline 25(OH)D concentrations greater than 22 ng/mL (mean difference = 1.48 [95% CI: 0.08, 2.88]; p = .038). Sensitivity analysis suggested no difference in the results following the exclusion of any of the trials. Additionally, no significant evidence of publication bias was found (Egger's test p = .538; Begg's test p = .652).
FIGURE 6.
Forest plot detailing weighted mean difference and 95% CIs for the impact of vitamin D supplementation on D‐dimer concentrations. CI, confidence interval; WMD, weight mean difference.
3.6. Effects of vitamin D supplementation on hematological parameters
Four trials with 447 participants investigated the effect of vitamin D supplementation on platelet count. 32 , 35 , 40 , 41 There were no significant differences between the vitamin D and control groups in terms of platelets counts (mean difference = −1.82 [95% CI: −61.62, 57.96]; p = .952), with a considerable heterogeneity (I 2 = 98.1%, p < .001). No new findings were observed in the sensitivity analysis and publication bias test.
Four trials reported the results of investigating the effect of vitamin D supplementation on Hb concentrations. 35 , 40 , 41 , 45 The results showed that vitamin D supplementation did not significantly change Hb concentrations in COVID‐19 patients (mean difference = −0.11 [95% CI: −0.26, 0.04]; p = .145; I 2 = 0.0%). Sensitivity analysis suggests no difference in the results following the exclusion of any of the trials. Additionally, no significant evidence of publication bias was found (Egger's test p = .988; Begg's test p = .734).
3.7. Effects of vitamin D supplementation on lymphocytes and neutrophil count
Five studies reported the effect of vitamin D supplementation on lymphocyte numbers. 35 , 39 , 40 , 41 , 45 It was found that vitamin D supplementation did not significantly change lymphocyte numbers compared to the control group (mean difference = −270 [95% CI: −930, 390]; p = .421), with a significant heterogeneity (I 2 = 98.4%, p < .001). The results of the sensitivity analysis showed that removing any of the studies had no significant effect on the results. Additionally, neither Begg's test (p = .806) nor Egger's test (p = .748), nor a visual inspection of the funnel plot showed any publication bias.
The effect of vitamin D on neutrophil count was evaluated in three studies. 39 , 40 , 41 According to the meta‐analysis, vitamin D supplementation did not significantly increase neutrophil count (mean difference = 36.39 [95% CI: −2231.57, 2304.26]; p = .976). No new findings were observed in the sensitivity analysis and publication bias test.
3.8. Grading of evidence
We used the GRADE framework to evaluate the quality of evidence. Based on the GRADE framework, the quality of evidence for hospital length of stay was moderate. The evidence for CRP, ferritin, Hb, and lymphocyte was downgraded to low. Finally, evidence regarding D‐dimer, platelet, and neutrophil was identified as very low quality (Table 4).
TABLE 4.
GRADE profile of vitamin D supplementation for COVID‐19‐related outcomes in adults.
| Outcomes | Quality assessment | Summary of findings | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Number of intervention/control | WMD (95% CI) | Heterogeneity (I 2) | Quality of evidence | |
| Hospital length of stay | No serious limitations | Serious limitations | No serious limitations | No serious limitations | No serious limitations | 471/473 | −1.16 (−2.23, −0.09) | 69.2 |
Moderate |
| CRP | No serious limitations | Very serious limitations | No serious limitations | No serious limitations | No serious limitations | 522/496 | −0.48 (−1.30, 0.34) | 91.1 |
Low |
| Ferritin | No serious limitations | Very serious limitations | No serious limitations | No serious limitations | No serious limitations | 225/225 | −1.24 (−8.27, 5.80) | 79.5 |
Low |
| D‐dimer | No serious limitations | Very serious limitations | Serious limitations | No serious limitations | No serious limitations | 368/347 | 0.37 (−0.15, 0.9) | 94.2 |
Very Low |
| Platelet | No serious limitations | Very serious limitations | No serious limitations | Serious limitations | No serious limitations | 239/208 | −1.82 (−61.62, 57.96) | 98.1 |
Very Low |
| Hb | No serious limitations | No serious limitations | Serious limitations | Serious limitations | No serious limitations | 210/205 | −11 (−0.26, 0.04) | 0.0 |
Low |
| Lymphocyte | No serious limitations | Very serious limitations | No serious limitations | No serious limitations | No serious limitations | 266/236 | −270 (−930 380) | 98.4 |
Low |
| Neutrophil | No serious limitations | No serious limitations | Serious limitations | Very serious limitations | No serious limitations | 211/205 | 36.39 (−2231.57, 2304.26) | 0.0 |
Very Low |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; Hb, hemoglobin; WMD, weighted mean difference.
4. DISCUSSION
Vitamin D, as a critical fat‐soluble vitamin, plays an important role in a large number of metabolic processes within the body. Due to the importance of this vitamin in metabolic processes as well as immune system enhancement, it was one of the main nutritional supplements recommended during the COVID‐19 pandemic. However, the evidence regarding the efficacy of this vitamin in managing COVID‐19 symptoms remains contradictory. 46 , 47 The results of the present systematic review and meta‐analysis showed that vitamin D supplementation led to a significant reduction in length of hospital stay. Additionally, the results showed that vitamin D supplementation in elderly patients caused a significant decrease in CRP concentrations. However, we did not find any significant effect from vitamin D supplementation in terms of other hematological and immune system biomarkers.
The results of epidemiological studies showed that vitamin D deficiency significantly increases the risk of ARDS. 48 , 49 Additionally, some studies indicated that an improvement in vitamin D serum concentrations was associated with a reduction in the duration of mechanical ventilation among critically ill patients, particularly those with COVID‐19. 50 , 51 Various reasons can be proposed to explain the mechanisms involved in the shortening of hospital stay after vitamin D supplementation. It has been shown that vitamin D exerts antimicrobial effects by stimulating the production of compounds such as nitric oxide and superoxide. 52 , 53 Also, some studies have suggested that vitamin D can improve the antimicrobial activity of other proteins, such as cathelicidin. 54 Some studies have shown that vitamin D can strengthen antiviral immunity, which is effective in managing the symptoms of COVID‐19 and shortening the length of hospitalization. This includes several concurrent antibacterial processes, such as the activation of cathelicidin and defensins, which can prevent viral entry into cells and decrease viral multiplication. 55 Enhancing autophagy is another characteristic of vitamin D related to both antibacterial and antiviral processes. Autophagy is a crucial biological mechanism that preserves cellular homeostasis by encasing malfunctioning organelles and improperly folded proteins inside the cell membrane. 56
Unlike the duration of hospitalization, most of the studies reviewed in this article reported that vitamin D supplementation did not have a significant effect on reducing the risk of mortality in COVID‐19 patients. In line with our findings, the results of another meta‐analysis showed that vitamin D supplementation had no significant effect on reducing the risk of mortality in COVID‐19 patients. 26
In the present study, we could not find significant effects of vitamin D on CRP concentrations, as well as on lymphocyte and neutrophil counts. However, in the subgroup analysis, CRP concentrations in individuals over 60 years old were significantly reduced following vitamin D supplementation. The evaluation of these factors was important because, theoretically, part of the positive effect of vitamin D against COVID‐19 is due to its ability to strengthen the host's immune system and suppress inflammatory cytokines in the body. 57 , 58 However, given that the studied patients differed in terms of their initial vitamin D concentrations and the severity of the disease, this may impact the accuracy of the results. Also, due to the small number of studies, it was not possible to subgroup the studies based on the severity of the COVID‐19 disease.
The results of the meta‐analysis showed no significant effect of vitamin D supplementation on Hb, ferritin, and D‐dimer concentrations, or on platelet counts. It has been reported that D‐dimer concentrations >1 μg/L were an independent predictor of mortality in COVID‐19 disease. 59 The current study focused on the relevance of blood inflammatory indicators, such as CRP, homocysteine, and D‐dimer concentrations, in the prediction of COVID‐19. The results of several studies have shown that ferritin concentrations, as an indicator of immune system response, increase in critically ill COVID‐19 patients. Elevated ferritin concentrations could trigger a cytokine storm due to their direct immunosuppressive and pro‐inflammatory effects. 60 , 61 , 62
According to our knowledge, this study was the first meta‐analysis that examined the effect of vitamin D supplementation on factors such as length of hospital stay and inflammatory and hematological biomarkers among patients with COVID‐19. Previous meta‐analyses focused mostly on mortality, intensive care unit length of stay, and risk of infection. 25 , 26 Also, another strength of this study compared to previous meta‐analyses was that the strength of the evidence was also examined based on the GRADE framework.
The present study had several limitations that should be considered when interpreting the results. First, there was significant variation among the participants regarding COVID‐19 severity, duration, medications, and baseline vitamin D concentrations, which could influence outcomes and contribute to observed heterogeneity. Second, the types and dosages of vitamin D supplementation varied widely across studies, with some using mega doses and others daily doses, complicating direct comparisons and conclusions about optimal dosing. Third, despite our extensive search and rigorous criteria, the number of studies meeting inclusion criteria was relatively small, limiting our ability to conduct detailed subgroup analyses, especially regarding COVID‐19 severity. Fourth, focusing exclusively on peer‐reviewed papers might have missed relevant findings from preprints or ongoing studies, affecting the comprehensiveness of our meta‐analysis. Fifth, most included studies reported high heterogeneity due to differences in study design, population characteristics, intervention protocols, and outcome measures. Although we used random effects models and conducted sensitivity analyses to address this, variability remains a challenge. Lastly, reliance on published data without access to individual patient data limited our ability to perform detailed analyses and adjust for potential confounders at the patient level.
5. CONCLUSIONS
Our study focused on the impact of vitamin D supplementation in hospitalized COVID‐19 patients, aiming to evaluate its effects on various factors. The results revealed a significant reduction in hospital length of stay among patients who received vitamin D supplementation, particularly in those who received a dosage of ≤10 000 IU and in older adult patients over 60 years old. Additionally, we observed a noteworthy decrease in CRP concentrations in older adults aged over 60 years. Despite these positive outcomes, no significant effects of vitamin D were observed on biomarkers such as ferritin, D‐dimer, and Hb concentrations, or on lymphocyte, neutrophil, and platelet counts.
AUTHOR CONTRIBUTIONS
FS, Z‐a‐sG, and JC participated in the design and coordination of the study. MRZR conceived the study and drafted the manuscript. NA, MN, and MRZR searched for the studies, collected, and analyzed the data. MN participated in the design of this study and edited the manuscript. Z‐a‐sG, JC, MN, and MRZR did the data management and analyzed the data. All authors read and approved the final manuscript.
FUNDING INFORMATION
No funding was received for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
No ethical approval and patient consent are required since this study data is based on published literature. This meta‐analysis review was registered with PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, registration No. CRD42023441017).
Supporting information
Table S1.
Table S2.
ACKNOWLEDGMENTS
The authors have nothing to report.
Ghoreshi Z‐a‐s, Charostad J, Arefinia N, Nakhaie M, Rezaei Zadeh Rukerd M, Salajegheh F. Effect of vitamin D supplementation on clinical outcomes in adult patients with COVID‐19: A GRADE‐assessed systematic review and meta‐analysis of randomized controlled trials. Pharmacol Res Perspect. 2024;12:e70013. doi: 10.1002/prp2.70013
DATA AVAILABILITY STATEMENT
All data analyzed for this study are included in the manuscript and supplementary tables.
REFERENCES
- 1. Sahu KK, Mishra AK, Lal A. COVID‐2019: update on epidemiology, disease spread and management. Monaldi Arch Chest Dis. 2020;90(1):1292. [DOI] [PubMed] [Google Scholar]
- 2. Shafieipour S, Mohammadi E, Rukerd MRZ, et al. Gastrointestinal bleeding: prevalence, etiology, and outcomes in COVID‐19 inpatients. GOVARESH. 2023;28(1):30‐35. [Google Scholar]
- 3. Nakhaie M, Ghoreshi ZA, Rukerd MRZ, Askarpour H, Arefinia N. Novel mutations in the non‐structure protein 2 of SARS‐CoV‐2. Mediterr J Hematol Infect Dis. 2023;15(1):e2023059. doi: 10.4084/MJHID.2023.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weekly epidemiological update on COVID‐19 – 25 May 2023. Accessed July 14, 2023. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐25‐may‐2023
- 5. García‐Lledó A, Gómez‐Pavón J, del Castillo JG, et al. Pharmacological treatment of COVID‐19: an opinion paper. Rev Esp Quimioter. 2022;35(2):115‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heustess AM, Allard MA, Thompson DK, Fasinu PS. Clinical management of COVID‐19: a review of pharmacological treatment options. Pharmaceuticals. 2021;14(6):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boozari M, Hosseinzadeh H. Natural products for COVID‐19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother Res. 2021;35(2):864‐876. [DOI] [PubMed] [Google Scholar]
- 8. Shafieipour S, Rezaei Zadeh Rukerd M, Shamsizadeh Meymandi T, et al. The effect of intravenous tocilizumab therapy on the prognosis of patients with COVID‐19: a case‐control study. Iranian J Med Microbiol. 2023;17(2):243‐250. [Google Scholar]
- 9. Silverio R, Gonçalves DC, Andrade MF, Seelaender M. Coronavirus disease 2019 (COVID‐19) and nutritional status: the missing link? Adv Nutr. 2021;12(3):682‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naja F, Hamadeh R. Nutrition amid the COVID‐19 pandemic: a multi‐level framework for action. Eur J Clin Nutr. 2020;74(8):1117‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Flora S, Balansky R, La Maestra S. Antioxidants and COVID‐19. J Prev Med Hyg. 2021;62(1 Suppl 3):13185‐13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soto ME, Guarner‐Lans V, Soria‐Castro E, Manzano Pech L, Pérez‐Torres I. Is antioxidant therapy a useful complementary measure for Covid‐19 treatment? An algorithm for its application. Medicina. 2020;56(8):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pisoschi AM, Pop A, Iordache F, et al. Antioxidant, anti‐inflammatory and immunomodulatory roles of vitamins in COVID‐19 therapy. Eur J Med Chem. 2022;232:114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson CR, Thacher TD. Vitamin D: immune function, inflammation, infections and auto‐immunity. Paediatr Int Child Health. 2023;43:1‐11. [DOI] [PubMed] [Google Scholar]
- 15. Wu Z, Liu D, Deng F. The role of vitamin D in immune system and inflammatory bowel disease. J Inflamm Res. 2022;15:3167‐3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kow CS, Hadi MA, Hasan SS. Reply: vitamin D supplementation in influenza and COVID‐19 infections. Comment on: evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients 2020, 12 (4), 988. Nutrients. 2020;12(6):1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jolliffe DA, Camargo CA, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta‐analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276‐292. [DOI] [PubMed] [Google Scholar]
- 18. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community‐acquired pneumonia: a meta‐analysis of observational studies. Medicine. 2019;98(38):e17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siuka D, Saletinger R, Uršič J, et al. The effect of vitamin D levels on the course of COVID‐19 in hospitalized patients–a 1‐year prospective cohort study. F1000Research. 2023;12(254):254. [Google Scholar]
- 20. Paiz N, Alonso P, Portillo AL. Vitamin D status: can it affect the risk of infection and the severity of COVID‐19 symptoms? Curr Trop Med Rep. 2021;8:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang H, Zheng J, Liu Y, Zhou Q, Peng D. Effect of vitamin D status on adult COVID‐19 pneumonia induced by delta variant: a longitudinal, real‐world cohort study. Front Med. 2023;10:1121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kong J, Zhu X, Shi Y, et al. VDR attenuates acute lung injury by blocking Ang‐2‐Tie‐2 pathway and renin‐angiotensin system. Mol Endocrinol. 2013;27(12):2116‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaun F, Boesing M, Luethi‐Corridori G, et al. Effect of single high dose vitamin D substitution in hospitalized COVID‐19 patients with vitamin D deficiency on length of hospital stay. Biomedicine. 2023;11(5):1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bugarin JD, Dosenovic S, Ilic D, et al. Vitamin D supplementation and clinical outcomes in severe COVID‐19 patients—randomized controlled trial. Nutrients. 2023;15(5):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varikasuvu SR, Thangappazham B, Vykunta A, et al. COVID‐19 and vitamin D (Co‐VIVID study): a systematic review and meta‐analysis of randomized controlled trials. Expert Rev Anti‐Infect Ther. 2022;20(6):907‐913. doi: 10.1080/14787210.2022.2035217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kümmel LS, Krumbein H, Fragkou PC, et al. Vitamin D supplementation for the treatment of COVID‐19: a systematic review and meta‐analysis of randomized controlled trials. Front Immunol. 2022;13:1023903. doi: 10.3389/fimmu.2022.1023903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med. 2013;6(1):50‐54. doi: 10.1111/jebm.12018 [DOI] [PubMed] [Google Scholar]
- 30. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cervero M, López‐Wolf D, Casado G, et al. Beneficial effect of short‐term supplementation of high dose of vitamin D3 in hospitalized patients with COVID‐19: a multicenter, single‐blinded, prospective randomized pilot clinical trial. Front Pharmacol. 2022;13:863587. doi: 10.3389/fphar.2022.863587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rastogi A, Bhansali A, Khare N, et al. Short term, high‐dose vitamin D supplementation for COVID‐19 disease: a randomised, placebo‐controlled, study (SHADE study). Postgrad Med J. 2022;98(1156):87‐90. doi: 10.1136/postgradmedj-2020-139065 [DOI] [PubMed] [Google Scholar]
- 34. Torres M, Casado G, Vigón L, et al. Changes in the immune response against SARS‐CoV‐2 in individuals with severe COVID‐19 treated with high dose of vitamin D. Biomed Pharmacother. 2022;150:112965. doi: 10.1016/j.biopha.2022.112965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soliman AR, Abdelaziz TS, Fathy A. Impact of vitamin D therapy on the progress COVID‐19: six weeks follow‐up study of vitamin D deficient elderly diabetes patients. Proc Singap Healthc. 2021;31:20101058211041405. doi: 10.1177/20101058211041405 [DOI] [Google Scholar]
- 36. Sarhan N, Abou Warda AE, Sarhan RM, et al. Evidence for the efficacy of a high dose of vitamin D on the hyperinflammation state in moderate‐to‐severe COVID‐19 patients: a randomized clinical trial. Medicina. 2022;58(10):1358. doi: 10.3390/medicina58101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mariani J, Antonietti L, Tajer C, et al. High‐dose vitamin D versus placebo to prevent complications in COVID‐19 patients: multicentre randomized controlled clinical trial. PLoS One. 2022;17(5):e0267918. doi: 10.1371/journal.pone.0267918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elamir YM, Amir H, Lim S, et al. A randomized pilot study using calcitriol in hospitalized COVID‐19 patients. Bone. 2022;154:116175. doi: 10.1016/j.bone.2021.116175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karonova TL, Golovatyuk KA, Kudryavtsev IV, et al. Effect of cholecalciferol supplementation on the clinical features and inflammatory markers in hospitalized COVID‐19 patients: a randomized, open‐label, single‐center study. Nutrients. 2022;14(13):2602. doi: 10.3390/nu14132602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID‐19: a randomized clinical trial. JAMA. 2021;325(11):1053‐1060. doi: 10.1001/jama.2020.26848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sabico S, Enani MA, Sheshah E, et al. Effects of a 2‐week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate Covid‐19: a randomized clinical trial. Nutrients. 2021;13(7):2170. doi: 10.3390/nu13072170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sánchez‐Zuno GA, González‐Estevez G, Matuz‐Flores MG, et al. Vitamin D levels in COVID‐19 outpatients from Western Mexico: clinical correlation and effect of its supplementation. J Clin Med. 2021;10(11):2378. doi: 10.3390/jcm10112378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Annweiler C, Beaudenon M, Gautier J, et al. High‐dose versus standard‐dose vitamin D supplementation in older adults with COVID‐19 (COVIT‐TRIAL): a multicenter, open‐label, randomized controlled superiority trial. PLoS Med. 2022;19(5):e1003999. doi: 10.1371/journal.pmed.1003999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caballero‐García A, Pérez‐Valdecantos D, Guallar P, et al. Effect of vitamin D supplementation on muscle status in old patients recovering from COVID‐19 infection. Medicina (Kaunas). 2021;57(10):1079. doi: 10.3390/medicina57101079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: vitamin D and COVID‐19. Eur J Endocrinol. 2020;183(5):R133‐R147. doi: 10.1530/EJE-20-0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitchell F. Vitamin‐D and COVID‐19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dancer RCA, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617‐624. doi: 10.1136/thoraxjnl-2014-206680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Notz Q, Herrmann J, Schlesinger T, et al. Vitamin D deficiency in critically ill COVID‐19 ARDS patients. Clin Nutr. 2022;41(12):3089‐3095. doi: 10.1016/j.clnu.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angelidi AM, Belanger MJ, Lorinsky MK, et al. Vitamin D status is associated with in‐hospital mortality and mechanical ventilation: a cohort of COVID‐19 hospitalized patients. Mayo Clin Proc. 2021;96(4):875‐886. doi: 10.1016/j.mayocp.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He M, Cao T, Wang J, Wang C, Wang Z, Abdelrahim MEA. Vitamin D deficiency relation to sepsis, paediatric risk of mortality III score, need for ventilation support, length of hospital stay, and duration of mechanical ventilation in critically ill children: a meta‐analysis. Int J Clin Pract. 2021;75(4):e13908. doi: 10.1111/ijcp.13908 [DOI] [PubMed] [Google Scholar]
- 52. Gough ME, Graviss EA, May EE. The dynamic immunomodulatory effects of vitamin D3 during Mycobacterium infection. Innate Immun. 2017;23(6):506‐523. doi: 10.1177/1753425917719143 [DOI] [PubMed] [Google Scholar]
- 53. Hübel E, Kiefer T, Weber J, Mettang T, Kuhlmann U. In vivo effect of 1,25‐dihydroxyvitamin D3 on phagocyte function in hemodialysis patients. Kidney Int. 1991;40(5):927‐933. doi: 10.1038/ki.1991.296 [DOI] [PubMed] [Google Scholar]
- 54. White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121(1–2):234‐238. doi: 10.1016/j.jsbmb.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 55. Teymoori‐Rad M, Marashi SM. Vitamin D and Covid‐19: from potential therapeutic effects to unanswered questions. Rev Med Virol. 2021;31(2):e2159. doi: 10.1002/rmv.2159 [DOI] [PubMed] [Google Scholar]
- 56. Gilani SJ, Bin‐Jumah MN, Nadeem MS, Kazmi I. Vitamin D attenuates COVID‐19 complications via modulation of proinflammatory cytokines, antiviral proteins, and autophagy. Expert Rev Anti‐Infect Ther. 2022;20(2):231‐241. doi: 10.1080/14787210.2021.1941871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bikle DD. Vitamin D regulation of immune function during covid‐19. Rev Endocr Metab Disord. 2022;23(2):279‐285. doi: 10.1007/s11154-021-09707-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID‐19. Virus Res. 2021;292:198235. doi: 10.1016/j.virusres.2020.198235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID‐19: a meta‐analysis. Int J Infect Dis. 2020;96:467‐474. doi: 10.1016/j.ijid.2020.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang Z, Shi J, He Z, et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID‐19) patients. Aging (Albany NY). 2020;12(7):6037‐6048. doi: 10.18632/aging.102999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tan C, Huang Y, Shi F, et al. C‐reactive protein correlates with computed tomographic findings and predicts severe COVID‐19 early. J Med Virol. 2020;92(7):856‐862. doi: 10.1002/jmv.25871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vargas‐Vargas M, Cortés‐Rojo C. Ferritin levels and COVID‐19. Rev Panam Salud Publica. 2020;44:e72. doi: 10.26633/RPSP.2020.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Data Availability Statement
All data analyzed for this study are included in the manuscript and supplementary tables.





 ◯
◯
 ◯⃝
◯⃝
 ◯⃝
◯⃝ ◯⃝⃝⃝
◯⃝⃝⃝ ◯⃝⃝⃝
◯⃝⃝⃝
 ◯⃝
◯⃝
 ◯⃝
◯⃝ ◯⃝⃝⃝
◯⃝⃝⃝