Abstract
The CXC chemokine gamma interferon (IFN-γ)-inducible protein CXCL10/IP-10 is markedly elevated in cerebrospinal fluid and brain of individuals infected with human immunodeficiency virus type 1 (HIV-1) and is implicated in the pathogenesis of HIV-associated dementia (HAD). To explore the possible role of CXCL10/IP-10 in HAD, we examined the expression of this and other chemokines in the central nervous system (CNS) of transgenic mice with astrocyte-targeted expression of HIV gp120 under the control of the glial fibrillary acidic protein (GFAP) promoter, a murine model for HIV-1 encephalopathy. Compared with wild-type controls, CNS expression of the CC chemokine gene CCL2/MCP-1 and the CXC chemokine genes CXCL10/IP-10 and CXCL9/Mig was induced in the GFAP-HIV gp120 mice. CXCL10/IP-10 RNA expression was increased most and overlapped the expression of the transgene-encoded HIV gp120 gene. Astrocytes and to a lesser extent microglia were identified as the major cellular sites for CXCL10/IP-10 gene expression. There was no detectable expression of any class of IFN or their responsive genes. In astrocyte cultures, soluble recombinant HIV gp120 protein was capable of directly inducing CXCL10/IP-10 gene expression a process that was independent of STAT1. These findings highlight a novel IFN- and STAT1-independent mechanism for the regulation of CXCL10/IP-10 expression and directly link expression of HIV gp120 to the induction of CXCL10/IP-10 that is found in HIV infection of the CNS. Finally, one function of IP-10 expression may be the recruitment of leukocytes to the CNS, since the brain of GFAP-HIV gp120 mice had increased numbers of CD3+ T cells that were found in close proximity to sites of CXCL10/IP-10 RNA expression.
Leukocyte infiltration of the central nervous system (CNS) is a central feature in the pathogenesis of diverse inflammatory neurological disorders, which range from bacterial and viral meningoencephalitis to multiple sclerosis, human immunodeficiency virus type 1 (HIV-)-associated dementia (HAD), cerebral malaria, and cerebral ischemia. The pathology of HAD is characterized by the presence of HIV-infected mononuclear phagocytes and activated T lymphocytes in the brain, pronounced gliosis, the presence of multinucleated giant cells, diffuse myelin pallor, and neuronal damage and loss (33, 48).
Chemokines are important in leukocyte migration and have been implicated in several of the inflammatory disorders cited above (for recent reviews, see references 5, 17, 18, and 40). Chemokines and their receptors are expressed by a wide variety of cells, including those intrinsic to the CNS. Besides their classical role in chemotaxis, some chemokine receptors, e.g., CCR5, CCR3, and CXCR4, are known to serve as important coreceptors with CD4 for the entry of HIV-1 into host cells (2, 12, 14, 16). Microglial expression of either CCR5 or CCR3 promotes efficient infection of these cells with HIV-1 (19) and is found to be associated with the progression of HAD in children and adults with HIV infection (67). The cause of HAD is not clear. It is known that macrophages or microglia and not neurons are the predominant CNS reservoir for the virus. The extensive expression of chemokine receptors in normal and HIV-infected human brain may not only provide a passage for HIV entry into the brain via macrophages-microglia, but also contribute to neuronal injury and loss (5, 43).
In addition to the chemokine receptors, cerebral expression of various chemokines is increased in HAD and could promote the recruitment of monocytes and lymphocytes that contribute to encephalitis and modulate HIV-1 entry into and spread in the brain (13, 22). For example, several chemokines, including CCL3/MIP-1α, CCL2/MCP-1, and CCL4/MIP-1β, are found elevated in the brains of AIDS patients (55). More recently, high levels of the CXC chemokine CXCL10/IP-10 and the CC chemokine CCL2/MCP-1 were detected in the cerebrospinal fluid (CSF) of individuals with HIV-1 infection (24) and in astrocytes in brain from individuals with HAD (51). Interestingly, a significant correlation was found between expression of CXCL10/IP-10 and the progression of neuropyschiatric impairment, implicating CXCL10/IP-10 in the pathogenesis of HAD (24).
CXCL10/IP-10 is a secreted polypeptide of 10 kDa that was first identified as an early response gene induced after gamma interferon (IFN-γ) treatment in a variety of cells (36, 37). CXCL10/IP-10 can also be induced by IFN-α and IFN-β as well as by lipopolysacharide. CXCL10/IP-10 belongs to the CXC (or -α) chemokine family. This chemokine is secreted by activated T cells, monocytes, endothelial cells, and keratinocytes (65) and exerts chemotactic activity towards human peripheral blood monocytes and activated T lymphocytes but not neutrophils (65). Other functions for CXCL10/IP-10 are now known and include inhibition of angiogenesis (3, 62), inhibition of hematopoietic progenitor cell and tumor cell growth (21, 52), and antiviral actions (38). CXCL10/IP-10 mediates its actions by interaction with CXCR3 (35), a common receptor for the other CXC chemokines CXCL9/Mig (monokine induced by IFN-γ) and CXCL11/I-TAC (IFN-inducible T-cell α-chemoattractant).
The cause of CXCL10/IP-10 gene expression in the brain in HIV infection is unknown but presumably may be indirect, involving host immune factors such as IFNs, and/or direct, involving viral factors such as the HIV-1 envelope glycoprotein gp120. To further explore the possible role of chemokines in the pathogenesis of HAD, we examined chemokine gene expression in the CNS of transgenic mice with astrocyte-targeted expression of HIV gp120LAV under the control of the glial fibrillary acidic protein (GFAP) promoter (66). GFAP-HIV gp120 transgenic mice develop electrophysiological and pathological changes, including temporal neurodegeneration and gliosis, that mimic aspects of the CNS alterations found in HAD (25, 44, 66).
MATERIALS AND METHODS
Animals.
The development and characterization of the GFAP-HIV gp120 and GFAP-IFN-α transgenic mice were described in detail previously (1, 66). Mice (C57BL/6 × 129s strain) deficient for STAT1, generated by homologous recombination using standard targeting techniques (41), were obtained from Robert Schreiber, Washington University School of Medicine, St. Louis, Mo. All mice were maintained under specific-pathogen-free conditions in the closed breeding colony of the Scripps Research Institute.
RNA isolation.
Anesthesized mice were killed at different ages, and organs were immediately removed, frozen in liquid nitrogen, and stored at −80°C until RNA preparation. Polyadenylated [poly(A)+] RNA was prepared according to a previously described method (8). Total RNA was extracted with Trizol reagent (Life Technologies, Grand Island, N.Y.) according to the manufacturer's instructions.
RPAs.
The RNase protection assay (RPA) for the detection of chemokine RNAs was performed as described previously (4). Two additional RPA probe sets were developed for these studies. The first included probes to CXCL9/Mig (nucleotides 102 to 401 [15]), CXCR3 (nucleotides 29 to 188 [57]), CXCL10/IP-10 (nucleotides 221 to 361 [68]), and CXCL11/I-TAC (nucleotides 165 to 291 [42]). A second probe set for the analysis of IFN-regulated genes included probes for IFN-inducible RNA-dependent protein kinase (PKR), IFN regulatory factor 1 (IRF-1), IFN regulatory factor 2 (IRF-2), protein kinase inhibitor p58 (PKIp58), T-cell GTPase (TGTP), and 2′,5′-oligoadenylate synthetase (OAS)-L2 (L2). Target sequences for these probes were murine PKR (nucleotides 130 to 450 [64]), IRF-1 (nucleotides 211 to 326; GenBank accession no. M21065), IRF-2 (nucleotides 431 to 561; GenBank accession no. J03168), PKIp58 (nucleotides 161 to 441; GenBank accession no. U28423), OAS-L2 (nucleotides 15 to 230; GenBank accession no. X58077), and TGTP (nucleotides 101 to 351; GenBank accession no L38444). All cDNA fragments flanked by 5′ EcoRI and 3′ HindIII restriction sequences were synthesized by reverse transcription (RT)-PCR and cloned into pGEM-4 (Promega, Madison, Wis.) as described (60). The correct sequence identity of all the RPA probes was verified prior to their use in the RPAs. For all probe sets, a fragment of the rpl32 gene was included and served as an internal loading control.
In situ hybridization.
Anesthetized control and transgenic mice were perfused transcardially with ice-cold saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). Brains were removed, postfixed in the same fixative overnight at 4°C before being processed, and embedded in paraffin. Sagittal sections (10 μm) were used for in situ hybridization, performed as described previously (4) with the exception that cRNA probes were labeled with [33P]UTP instead of [35S]CTP. For the probe, a CXCL10/IP-10 cDNA fragment (726 bp) flanked with 5′ XbaI and 3′ SalI sites was synthesized by RT-PCR and cloned in pGEM4 as previously described (4). For detection of HIV-1 gp120, a cDNA fragment (346 bp) was used as previously described (66, 70). Following hybridization with the CXCL10/IP-10 probe, some slides were colabeled by immunostaining for GFAP to identify astrocytes, for CD3 to identify T lymphocytes, for neurofilament to identify neurons, or for lectin binding to identify microglial cells. Briefly, after the final posthybridization wash, slides were transferred to PBS containing 5% goat serum for 1 h at room temperature to block nonspecific binding. Sections were then incubated for 2 h with either primary antibody against GFAP (diluted 1:2,000; Dako, Carpinteria, Calif.), phosphorylated neurofilament (SMI33, diluted 1:1,000; Sternberger, Lutherville, Md.), and CD3 (diluted 1:500; Dako), or with a lectin from Lycopersicon esculentum (biotin labeled, diluted 1:100; Sigma Chemical Co. St. Louis, Mo.). After extensive washing, sections were incubated with avidin-biotin-horseradish peroxidase complex (ABC kit; Vector, Burlingame, Calif.) used according to the manufacturer's instructions. Staining reactions were performed with 3,3-diaminobenzidine (Sigma) as the substrate. After dehydration through graded alcohols and air drying, slides were dipped in Kodak NTB-2 emulsion, dried, and stored in the dark for 1 to 2 weeks, after which the slides were developed, counterstained with Mayer's hematoxylin, and examined by dark- and bright-field microscopy.
Astrocyte cell culture.
Primary cultures of cerebral cortical astrocytes were prepared from newborn wild-type or STAT1-null mice as previously described (71). Briefly, cortices were removed aseptically from the skulls, and the meninges were carefully removed under a dissecting microscope. The cells were mechanically dissociated and enzymatically digested with a solution containing papain (30 U/ml), l-cysteine (0.24 mg/ml), and DNase I type IV (40 μg/ml) (all enzymes were from Sigma) in 1 ml of minimal essential medium-HEPES for 1 h at 37°C. Supernatants were plated at a density of one brain in three T25 flask (Falcon; Becton Dickinson, Franklin Lakes, N.J.) in Dulbecco's Modified Eagle's medium (Sigma) containing 10% fetal calf serum, l-glutamine, penicillin, and streptomycin (Sigma). As judged by GFAP immunostaining, these cultures were >95% enriched for astrocytes, with remaining cells consisting of microglia and fibroblasts. Once confluent, the cells were starved for 48 h without serum before treatment as described below.
The astrocyte cell cultures were incubated with medium or with different doses (0.1 to 1 nM) of soluble recombinant HIV-1 IIIB gp120 protein (gp120 IIIB; obtained from the NIH AIDS Research and Reference Reagent Program, Rockville, Md.) for 6 h at 37°C. To establish the specificity for any effect mediated via the gp120 IIIB interaction, the recombinant protein was incubated with an anti-gp120 neutralizing antibody (immunoglobulin G1b12 [IgG1b12] [10]) for 1 h at room temperature on a shaker and then added to the astrocyte cultures for 6 h at 37°C. A control isotype-matched antibody (human IgG1) was also used (kindly provided by Pascal Poignard, The Scripps Research Institute, La Jolla, Calif.). To examine the role of STAT1 wild-type and STAT1-null astrocyte cultures were treated with soluble recombinant HIV-1 IIIB gp120 protein or murine recombinant IFN-γ (R&D Systems, Minneapolis, Minn.) for 6 h at 37°C prior to RNA isolation. After incubation, cells were washed with saline solution, and total RNA was extracted using Trizol reagent according to the manufacturer's instructions.
RESULTS
Temporal analysis of cerebral chemokine and CXCR3 gene expression in GFAP-HIV gp120 transgenic mice.
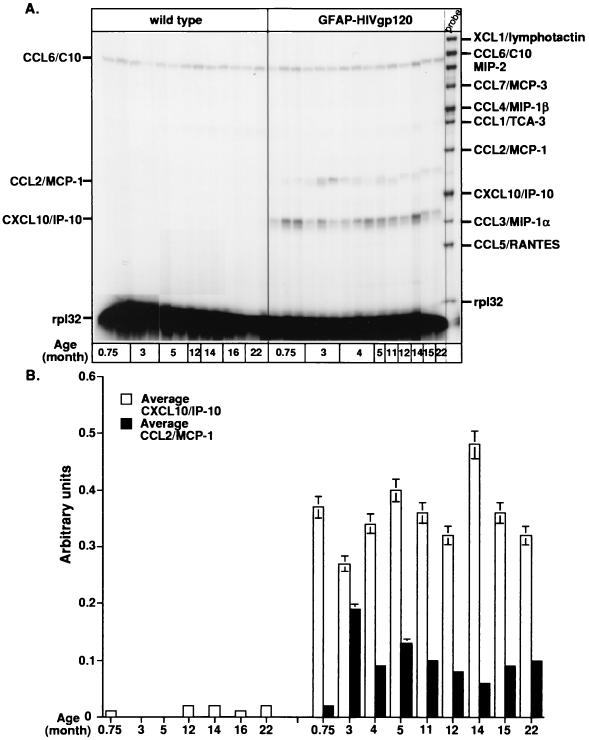
Initially, we examined CXC, CC, and C chemokine gene expression in the brains of mice at different ages by RPA. The CC chemokine CCL6/C10 was the only detectable chemokine found constitutively in the brain, and its level remained unchanged in both wild-type and GFAP-HIV gp120 mice at different ages (Fig. 1A). However, in the brain of the GFAP-HIV gp120 transgenic but not wild-type mice, both CCL2/MCP-1 and CXCL10/IP-10 mRNAs were also present. CXCL10/IP-10 mRNA was detectable at maximum levels by 3 weeks of age, and thereafter its expression remained relatively constant up to 12 months of age. In contrast, CCL2/MCP-1 mRNA expression was also detectable after 3 weeks of age but reached a maximum level at 3 months of age before declining over the remaining age points examined. Quantification of the signal intensities revealed that relative to background, there was up to a fourfold increase in CXCL10/IP-10 mRNA expression in the GFAP-HIV gp120 mice. At its peak, expression of CCL2/MCP-1 mRNA increased twofold compared to the background level (Fig. 1B).
FIG. 1.
(A) Chemokine mRNA expression in the brain of GFAP-HIV gp120 transgenic mice at different ages. In this representative experiment, poly(A)+ RNA was isolated from the whole brain of wild-type or transgenic mice, and 1 μg was analyzed by RPA as described in Materials and Methods. (B) Quantitative analysis of CXCL10/IP-10 and CCL2/MCP-1 gene expression. Densitometric analysis of each lane was performed on scanned autoradiographs using NIH Image 1.57 software. Expression of different chemokine mRNAs was normalized to the respective expression of rp132 mRNA, and the mean plus standard deviation was calculated using Microsoft Excel 98.
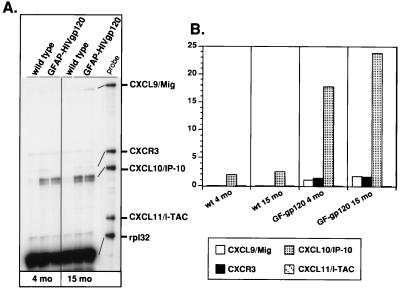
CXCL10/IP-10 belongs to the non-ELR CXC chemokine subfamily, and we therefore investigated whether other members of this subfamily were also expressed in the brain of the GFAP-HIV gp120 mice. Another multiprobe RPA was developed that, in addition to IP-10, permitted detection of the non-ELR CXC chemokine mRNAs for CXCL9/Mig and CXCL11/I-TAC and their common receptor, CXCR3. As shown in Fig. 2A, there was little if any detectable expression in the wild-type brain of these chemokine genes or the CXCR3 receptor. In contrast, in the brain of the GFAP-HIV gp120 transgenic mice, consistent with our previous finding, CXCL10/IP-10 mRNA expression was induced at 4 and 15 months of age. In addition, increased expression of CXCL9/Mig mRNA was also detectable. However, the levels of CXCL9/Mig RNA were clearly considerably lower than for CXCL10/IP-10 (Fig. 2B). CXCL11/I-TAC mRNA gene expression was not detectable in the brains of these mice. Expression of CXCR3 was also detectable at low levels in the brain of the GFAP-HIV gp120 mice at 5 and 15 months of age.
FIG. 2.
(A) Expression of mRNAs for CXCL9/Mig, CXCL10/IP-10, CXCL11/I-TAC, and their common receptor CXCR3 in the brain of GFAP-HIV gp120 transgenic mice. Poly(A) RNA was isolated from the whole brain of wild-type (wt) or transgenic mice at 4 and 15 month of age, and 1 μg was analyzed by RPA as described in Materials and Methods. (B) Quantitative analysis of RPA autoradiographs was performed as described for Fig. 1.
All in all, these findings revealed that in the brain of GFAP-HIV gp120 transgenic mice, CXCL10/IP-10, CCL2/MCP-1, and to a lesser extent CXCL9/Mig gene expression was induced, and there was differential regulation of the non-ELR CXC chemokines, where CXCL10/IP-10 gene expression was most prominent while CXCL11/I-TAC gene expression was not detectable.
Anatomical and cellular localization of IP-10 and HIV gp120 gene expression.
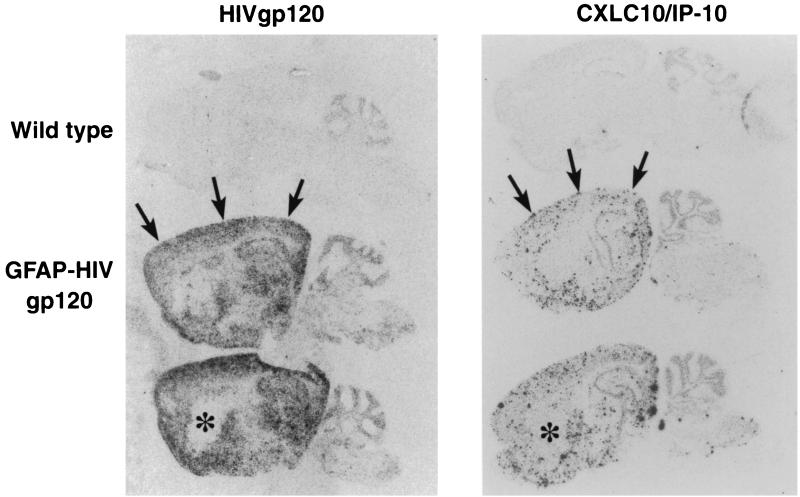
To further determine the relationship between the expression of the CXCL10/IP-10 gene and its localization in the brain of the GFAP-HIV gp120 transgenic mice, we performed in situ hybridization and compared IP-10 RNA expression to that of the transgene-encoded HIV gp120 RNA. Sagittal sections of brain from wild-type and GFAP-HIV gp120 mice were hybridized with either CXCL10/IP-10 or HIV gp120 antisense cRNA probes. No detectable hybridization above background levels was observed in brain from control litter mates for either HIV gp120 or CXCL10/IP-10 (Fig. 3, top panels). However, in brain from GFAP-HIV gp120 mice, a diffuse hybridization signal was observed for HIV gp120 RNA in many brain regions, including the cortex, hippocampus, and hypothalamus (Fig. 3, left panels). In adjacent sections, a highly punctate hybridization signal was observed for CXCL10/IP-10 RNA which showed an anatomic distribution similar to that of the HIV gp120 RNA (Fig. 3, right panels). For example, expression of both HIV gp120 and IP-10 was observed in the cortex (Fig. 3, arrows) but not in the basal ganglia (Fig. 3, asterisk).
FIG. 3.
Anatomical localization of CXCL10/IP-10 and HIV gp120 RNA in the brain of GFAP-HIV gp120 transgenic mice. Mice were anesthetized, and the brains removed, processed, and analyzed by in situ hybridization as described previously using 33P-labeled-gp120 IIIB and CXCL10/IP-10 riboprobes (4). Examples of overlap in the brain for the hybridization pattern of these two different probes are represented by arrows (expression) and asterisks (no expression).
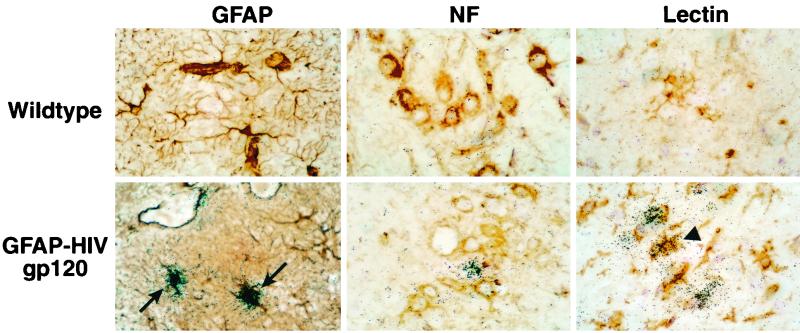
The punctate nature of the hybridization signal for CXCL10/IP-10 RNA would be consistent with its expression by scattered cells. To further investigate this possibility, we combined in situ hybridization with immunohistochemical staining for specific neural cell types. Microscopic analysis of the hybridized sections revealed background levels of hybridization in the wild-type brains (Fig. 4, top panels). In contrast, in brain sections from GFAP-HIV gp120 mice, expression of CXCL10/IP-10 RNA was found to be almost entirely localized to a subset of astrocytes (Fig. 4, bottom left panel, arrows). In addition, rare tomato lectin-positive microglial cells were also observed that expressed CXCL10/IP-10 RNA (Fig. 4, bottom right panel, arrowhead). In contrast, neurons identified by staining for neurofilament (NF) contained no detectable CXCL10/IP-10 RNA (Fig. 4, bottom middle panel). These findings therefore indicated that in the GFAP-HIV gp120 transgenic brain, there was considerable overlap in the anatomic sites for transgene-encoded HIV gp120 and CXCL10/IP-10 gene expression, and astrocytes and to a much lesser extent microglia were responsible for CXCL10/IP-10 RNA gene expression.
FIG. 4.
Cellular localization of CXCL10/IP-10 RNA in the brain of GFAP-HIV gp120 transgenic mice. After in situ hybridization, some sections were further subjected to dual-label analysis to identify the cellular localization for CXCL10/IP-10 RNA expression. Immunostaining for GFAP (to label astrocytes) and neurofilament (NF; to label neurons) or binding of tomato lectin (to label microglia) was performed after in situ hybridization as described in Materials and Methods. Top panels, immunohistochemistry for wild-type animals. In the GFAP-HIV gp120 transgenic mice, numerous cells positive for CXCL10/IP-10 colabeled with the GFAP-positive cells (left panel, arrows). Few cells positive for the tomato lectin and representing the microglia were colabeled with CXCL10/IP-10 RNA expression (right panel, arrowhead). In contrast, no neurofilament-positive cells that were also positive for CXCL10/IP-10 were detected (middle panel).
Analysis of IFN and IFN-regulated gene expression in the brain of GFAP-HIV gp120 transgenic mice.
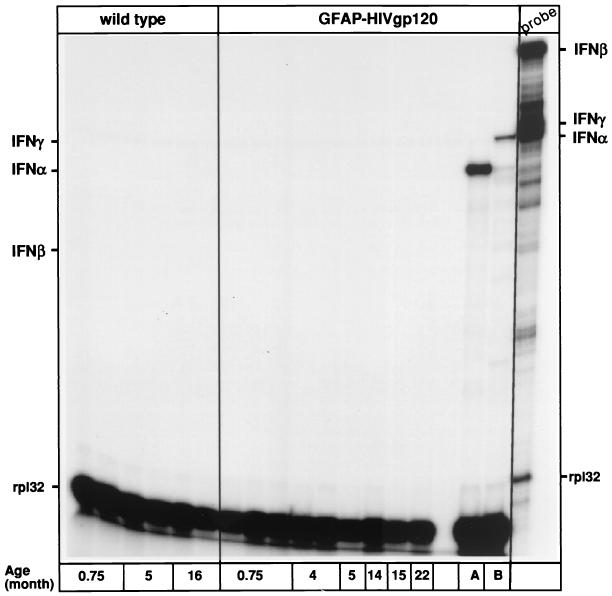
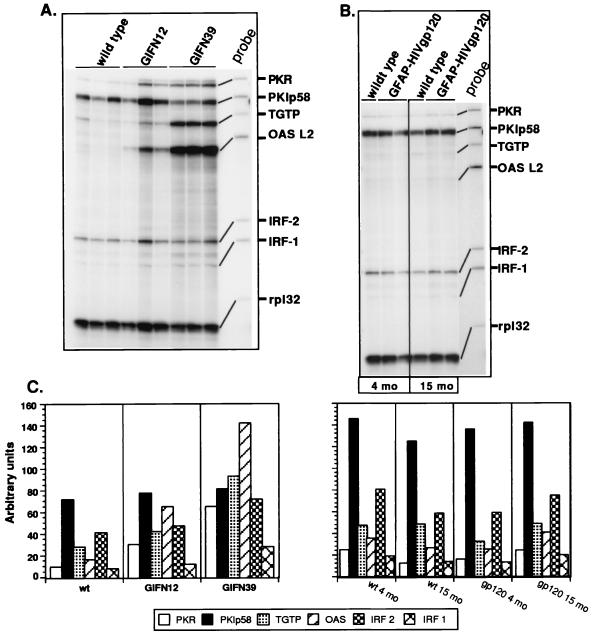
It is clear that CXCL10/IP-10 expression is induced by IFN-α/β and IFN-γ IFNs. Therefore we asked whether any of these IFNs were present in the brain of the GFAP-HIV gp120 mice. By RPA, there was no detectable expression of either type of IFN genes in the brain of either wild-type mice or GFAP-HIV gp120 transgenic mice at any age tested (Fig. 5). In contrast, in two positive control samples, brain from a GFAP-IFN-α transgenic mouse (Fig. 5, lane A) and spleen from a mouse infected with lymphocytic choriomeningitis virus (LCMV) (Fig 5, lane B), IFN-α and IFN-α and IFN-γ transcripts, respectively, were readily detected. In order to further confirm that there was an absence of IFN in the brain, we also examined for the expression of a number of other IFN-regulated genes (Fig. 6B). In comparing litter mate controls with the GFAP-HIV gp120 mice, no differences were seen in the cerebral expression of a number of prototypic IFN-regulated genes, including PKR, PKIp58, TGTP, OAS L2, and IRF-1 and -2. In contrast, in brain from transgenic mice that expressed different levels of IFN-α, a dose-dependent increase in the expression of a number of these IFN-regulated genes, including PKR, TGTP, and OAS L2, was observed (Fig. 6A). In addition, immunohistochemical staining of brain sections for the IFN-regulated proteins major histocompatibility complex (MHC) class I and MHC class II failed to reveal any significant differences in the levels of these molecules between wild-type and GFAP-HIV gp120 mice (not shown).
FIG. 5.
Analysis of IFN mRNA expression in brain from GFAP-HIV gp120 mice of different ages or GFAP-IFN-α transgenic mice (lane A) and the spleen from a mouse infected with LCMV and killed at day 3 after infection (lane B). Poly(A)+ RNA was isolated from the whole brain of wild-type or GFAP-HIV gp120 mice at 4 and 15 month of age and from symptomatic GFAP-IFN-α mice, and 1 μg was analyzed by RPA as described in Materials and Methods.
FIG. 6.
Expression of various IFN-regulated genes in the brain of GFAP-IFN-α (A) and GFAP-HIV gp120 (B) transgenic mice. Poly(A)+ RNA was isolated from the whole brain of wild-type (wt) or transgenic mice at 4 and 15 months of age for the GFAP-HIV gp120 mice and from symptomatic GFAP-IFN-α mice, and 1 μg was analyzed by RPA as described in Materials and Methods. Quantitative analysis of RPA autoradiographs (C) was performed as described above for Fig. 1.
In summary, the absence of detectable IFN gene expression and the lack of any changes in the expression of a number of IFN-regulated genes provide strong evidence that the induction of CXCL10/IP-10 gene expression in the brain of the GFAP-HIV gp120 mice was not due to an IFN.
Analysis of chemokine gene expression in astrocyte cell cultures incubated with soluble recombinant gp120 protein.
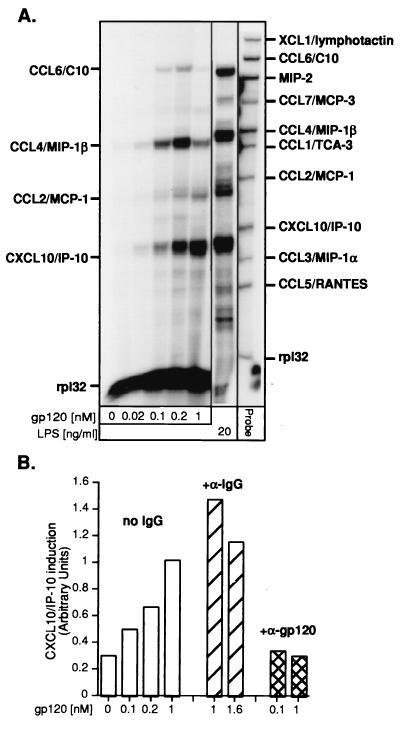
To further delineate the possible mechanisms responsible for the induction of CXCL10/IP-10 gene expression, we hypothesized that the HIV-1 envelope glycoprotein gp120 itself might be responsible. To investigate this possibility, the effect of gp120 IIIB on chemokine gene expression was studied in astrocyte cell cultures. Little chemokine gene expression was detectable in astrocyte cell cultures incubated with PBS. However, after incubation with different concentrations of gp120 IIIB for 6 h, expression of several chemokine genes was increased in a dose-dependant fashion (Fig. 7A and B). CCL4/MIP-1β and CXCL10/IP-10 mRNAs were expressed at significantly higher levels than the CCL6/C10 and CCL2/MCP-1 transcripts and peaked at 200 pM and 1 nM for CCL4/MIP-1β and CXCL10/IP-10, respectively, of gp120 IIIB. To further assess whether the increased expression of these chemokine genes was specific for gp120 IIIB, we absorbed gp120 IIIB with an excess of a specific neutralizing antibody before addition to the astrocyte culture. As shown in Fig. 7B, antibody to gp120 IIIB but not an isotype-matched control antibody significantly reduced the stimulation of CXCL10/IP-10 gene expression by HIV-1 gp120. Although not shown, the expression of the other gp120 IIIB-induced chemokine genes was also markedly suppressed by the gp120-specific antibody.
FIG. 7.
(A) Induction of CXCL10/IP-10 RNA expression in astrocyte cell culture by soluble recombinant HIV gp120 protein. The astrocyte cultures were incubated with medium alone or with different doses (0.02 to 1 nM) of HIV gp120 protein (gp120 IIIB) for 6 h. Specificity for gp120 IIIB was shown by incubation of gp120 IIIB with an anti-gp120 (α-gp120) neutralizing antibody (B) for 1 h at room temperature prior to addition to the astrocyte culture. A control isotype antibody (human IgG1) was used in parallel (α-IgG). LPS, lipopolysaccharide.
Role of STAT1 in gp120 IIIB-stimulated CXCL10/IP-10 gene expression.
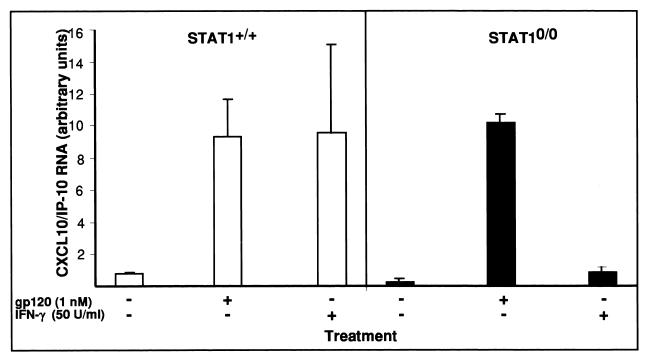
STAT1 is a crucial component of IFN-receptor signaling pathways (61) essential for IFN-γ-induced IP-10 gene expression (39). To examine the role of STAT1 signaling in the induction of CXCL10/IP-10 gene expression by HIV-1 gp120, astrocyte cell cultures were prepared from wild-type and STAT1-null mice and treated with gp120 IIIB. As shown in Fig. 8, and consistent with the findings discussed above, gp120 IIIB stimulated significant CXCL10/IP-10 gene expression by wild-type astrocytes. However, this response remained unchanged in astrocytes that lacked STAT1. By contrast, IFN-γ, which was a potent inducer of CXCL10/IP-10 gene expression in wild-type astrocytes, failed to induce this chemokine gene in astrocytes that lacked STAT1.
FIG. 8.
Examination of role of STAT1 in induction of CXCL10/IP-10 RNA expression by soluble recombinant HIV gp120 protein. Astrocyte cultures derived from wild-type or STAT1-null mice were incubated in medium alone or with gp120 IIIB or murine recombinant IFN-γ proteins for 6 h. Each treatment was done in triplicate. Following treatment, the astrocyte cultures were washed twice in PBS, and RNA was extracted with Trizol reagent according to the manufacturer's instructions. For RPA, 5 μg of total RNA was analyzed as described in Materials and Methods. Quantitative analysis of RPA autoradiographs was performed as described for Fig. 1.
In all, these findings indicated that gp120 IIIB induction of CXCL10/IP-10 gene expression by astrocytes is STAT1 independent.
Relationship of CXCL10/IP-10 gene expression to lymphocyte infiltration.
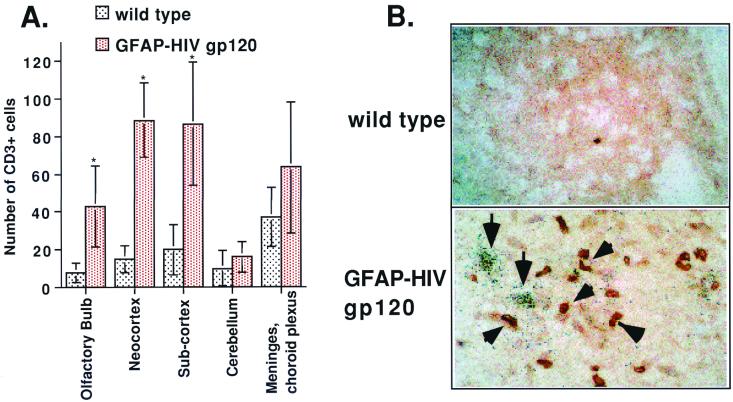
CXCL10/IP-10 is known to be a chemoattractant for lymphocytes and monocytes. To determine whether the expression of CXCL10/IP-10 was associated with increased T-cell accumulation in the brain of the GFAP-HIV gp120 mice, dual-label in situ analysis was performed. Immunostaining for the T-cell marker CD3 revealed a significant increase in the numbers of T cells in the brain of the GFAP-HIV gp120 mice compared to littermate controls (Fig. 9). The majority of the CD3-positive cells were localized in the neocortex and in the subcortex, with fewer numbers in other regions of the brain, such as the meninges, choroid plexus, and olfactory bulb (Fig. 9A). When combined with in situ hybridization for CXCL10/IP-10 RNA, the CD3+ T cells (Fig. 9A, arrowheads) were commonly observed in proximity to sites of CXCL10/IP-10 gene expression (Fig. 9B, arrows).
FIG. 9.
(A) Analysis of numbers of CD3+ cells in the brain. Six different brain sections containing specimens from nontransgenic littermates or GFAP-HIV gp120 mice were immunostained for CD3 and analyzed by two different individuals. The average cell count was calculated, and statistical significance was determined using the Student t-test. The presence of CD3+ T cells in the olfactory bulb (∗, P < 0.07), neocortex (∗, P < 0.00007), and subcortex (∗, P < 0.002) was significantly increased compared with the wild-type mice. (B) Colocalization of infiltrating CD3+ lymphocytes and CXCL10/IP-10 RNA in the brain of GFAP-HIV gp120 transgenic mice. Sections from a wild-type control and a GFAP-HIV gp120 mouse were hybridized with the CXCL10/IP-10 antisense probe and immunostained for CD3 as described in Materials and Methods. Original magnification, ×450.
These findings demonstrate for the first time a significant increase in CD3-positive T cells in the brain of the GFAP-HIV gp120 mice, many of which are found in areas of detectable CXCL10/IP-10 gene expression.
DISCUSSION
HIV-1 infects the brain, frequently causes dementia and related neurologic disorders in children and adults with AIDS, and is associated with infiltration of the brain by activated T lymphocytes and macrophages. Numerous chemokines, including CCL2/MCP-1, CCL3/MIP-1α, and CCL4/MIP-1β, were detected in the brain or CSF of HIV-1 patients and may play a direct role in fostering the recruitment of leukocytes (13, 22, 32, 55). Recently, Kolb et al. demonstrated the presence of CCL2/MCP-1 and CXCL10/IP-10 in CSF samples from all individuals infected with HIV-1 (24). Significantly, these authors reported that CXCL10/IP-10 levels were closely associated with the progression of HIV-1-related CNS infection and neuropyschiatric impairment (24). In an earlier study, Sanders et al. found that CXCL10/IP-10 was localized to astrocytes in HIV encephalitis (51). Additionally, increased expression of chemokines, notably CXCL10/IP-10 and its receptor CXCR3, was shown to correlate with simian immunodeficiency virus encephalitis in a primate model (53, 69). CXCL10/IP-10 is a pleiotropic chemokine that, in addition to stimulating leukocyte chemotaxis, is known to be an inhibitor of angiogenesis (3, 62) and to have antiviral actions (38). CXCL10/IP-10 may therefore play a significant role in the pathogenesis of HAD, and yet little is known concerning the CNS pathobiology of this chemokine. To further explore the possible role of CXCL10/IP-10 in the pathogenesis of HAD, we examined the expression of this and other chemokines in the CNS of transgenic mice with astrocyte-targeted expression of HIV gp120 under the control of the GFAP promoter. GFAP-HIV gp120 transgenic mice are known to replicate many of the features of HAD, including temporal neurodegeneration, gliosis, and electropathophysiological alterations (25, 44, 66). Here we showed that there is prominent CXCL10/IP-10 and to a lesser extent CCL2/MCP-1 gene expression in the CNS of the GFAP-HIV gp120 mice, with astrocytes being the major cellular source of the CXCL10/IP-10 gene expression. This chemokine gene expression picture in the CNS of the GFAP-HIV gp120 transgenic mice therefore partly mimics that in the HIV-1-infected human brain, allowing further assessment of the regulation and mechanism of action of these genes in the CNS.
CXCL10/IP-10 is an early response gene induced by IFN-γ treatment in a variety of cells (36, 37). CXCL10/IP-10 can also be induced by IFN-α and IFN-β as well as by lipopolysaccharide and some other proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) (45, 46). Consistent with these findings, CXCL10/IP-10 expression is markedly upregulated in the brain of transgenic mice with astrocyte-targeted expression of IFN-α but not interleukin-3 (IL-3) or IL-6 (7), and following infection with a number of different infectious agents and infections, including LCMV (4, 6), murine hepatitis virus (MHV) (28, 29), Theiler's murine encephalomyelitis virus (20), Borna disease virus (54), mouse adenovirus type 1 (11), bacterial meningitis (27, 58, 59), and toxoplasmosis encephalitis (23). However, the present study clearly showed that induction of CXCL10/IP-10 gene expression in the CNS of the GFAP-HIV gp120 mice did not involve the classical IFN pathway. First, there was no detectable expression of IFN genes in the brain of GFAP-HIV gp120 mice. Second, the expression of a number of prototypic IFN-regulated genes, such as PKR and OAS, was not detectable in the CNS of these mice. Finally, although the signal transduction molecule STAT1α has been shown previously, and confirmed here, to have an essential role in IFN-γ-induced CXCL10/IP-10 expression (39), the absence of STAT1 failed to affect HIV gp120-induced CXCL10/IP-10 expression by astrocytes. Finally, our previous studies established that the expression of a number of other proinflammatory cytokine genes, including IL-1 and TNF, is also not altered in the brain of the GFAP-HIV gp120 transgenic mice (44), making it improbable that other cytokines are responsible for the CNS induction of CXCL10/IP-10 gene expression. In consideration of these observations, we conclude that the induction of CXCL10/IP-10 gene expression by astrocytes in the CNS of the GFAP-HIV gp120 mice is independent of IFN or other cytokine receptor-mediated signaling.
Rather than an indirect mechanism, our findings argue in favor of the notion that the induction of CXCL10/IP-10 as well as CCL2/MCP-1 gene expression in the brain of the GFAP-HIV gp120 mice results from the direct action of gp120 IIIB. Consistent with this, in situ localization revealed concordance between the expression of HIV gp120 and CXCL10/IP-10 RNA transcripts. In our in vitro experiments, treatment of primary astrocyte cell cultures with gp120 IIIB resulted in marked upregulation of CXCL10/IP-10 gene expression which was effectively inhibited by an antibody against HIV gp120. Direct effects of HIV-1 gp120 on other parameters of astrocyte biology have been noted by others and include upregulation of GFAP (70) and ICAM-1 (56) gene expression. Thus, while astrocytes are unlikely to express CD4, the primary receptor for HIV-1 gp120 binding, other receptors probably serve this function. Possible candidates include the chemokine receptor CXCR4, which can serve as a coreceptor for HIV-1 gp120 (16, 31) and is found on astrocytes (30, 47, 63). Such alternative HIV gp120 binding sites may prove to be pathophysiologically significant, since recent studies indicate that astrocytes can host nonproductive HIV-1 infection in vitro and in vivo (9).
A comparison of the chemokine expression profiles between the brain of HIV gp120 mice and gp120 IIIB-treated astrocyte cultures revealed that a more extensive repertoire of chemokine genes was induced in the in vitro system. The ability to abrogate the stimulatory effect of gp120 IIIB on astroglial chemokine gene expression with an antibody against the HIV envelope protein indicated that the in vitro response was indeed specific. The reason for the dichotomy between the in vivo and in vitro responses to HIV gp120 likely relates to fundamental differences in the properties and relationship of the astrocytes to their milieu. Irrespective of this, we confirmed by combined in situ hybridization for CXCL10/IP-10 and immunostaining for GFAP that the expression of CXCL10/IP-10 RNA was induced in the cultured astrocytes following exposure to gp120 IIIB (data not shown).
A key issue concerns the function of CXCL10/IP-10 expressed in the brain of the GFAP-HIV gp120 mice as well as in HAD. In the present study, we were unsuccessful in detecting CXCL10/IP-10 protein in the CNS of the transgenic mice or in HIV gp120-treated astrocytes. Although we tested a number of commercial and noncommercial antibodies against IP-10, none of these proved to be effective for immunohistochemistry or immunoblotting. In the absence of confirmation of the presence of CXCL10/IP-10 protein, we sought indirect evidence for the presence of this chemokine by examining for markers of its downstream action. CXCL10/IP-10 is known to be an effective chemoattractant for the recruitment of activated T lymphocytes because expression of the CXCL10/IP-10 receptor CXCR3 is largely restricted to these cells (35). The significance of this process to the CNS is vividly illustrated by the recent report of Lane and colleagues (34), who showed that antibody-mediated blocking of CXCL10/IP-10 in vivo dramatically reduced CD4+ and CD8+ T-lymphocyte infiltration of the brain during acute MHV-induced encephalitis. Our results here suggest that CXCL10/IP-10 may indeed play a role in T-lymphocyte recruitment to the CNS in GFAP-HIV gp120 mice. First, while overt infiltration of the CNS of the GFAP-HIV gp120 mice is not evident from routine histological analysis, more sensitive and specific immunophenotypic analysis clearly revealed a significant increase in the number of CD3+ T lymphocytes present in the brain of these animals. Second, the T lymphocytes appeared in clusters around astrocytes expressing the CXCL10/IP-10 gene. Finally, expression of the CXCR3 receptor gene was only detectable in the brain of the GFAP-HIV gp120 mice. Since it has been reported that the CXCR3 receptor is only expressed by activated T lymphocytes (35, 49, 50), the finding of CXCR3 receptor expression in the brain of the GFAP-HIV gp120 mice implies that the infiltrating T lymphocytes are activated. The morphological appearance of these cells is also consistent with this view. In future studies using flow cytometry, we hope to be able to further clarify the phenotypic characteristics of these cells. In any case, our observations raise for the first time the possibility that an immunoinflammatory response may contribute to the degenerative disease observed in the GFAP-HIV gp120 mice. Ongoing studies with CXCR3 knockout mice crossed with the GFAP-HIV gp120 transgenic mice should allow us to elucidate the function of CXCL10/IP-10 and its receptor in the recruitment of the CD3+ T lymphocytes to the brain and their involvement in the development of neurodegenerative disease.
In conclusion, this study demonstrates the novel, IFN- and STAT1-independent HIV-1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes. These findings add the gp120 envelope glycoprotein to the transactivator TAT (26) of HIV-1 as HIV-1 gene products that may contribute directly to the increased production of CXCL10/IP-10 that is found in patients with HAD (24). We suggest that astrocytes may serve as an early and sensitive cellular monitor of HIV-1 infection in the brain that respond with robust production of CXCL10/IP-10. CXCL10/IP-10 likely then functions as a key component of the host response signaling the recruitment of protective antiviral T lymphocytes and mustering these cells to sites of HIV-1 infection in the brain.
ACKNOWLEDGMENTS
We thank Gabriele Werner-Felmayer for the gift of the plasmid containing I-TAC cDNA, Dennis Burton for the anti-gp120 antibody, and Lennart Mucke for the gp120 RPA probe.
Our studies were supported by USPHS grants MH47680 and MH62231 to I.L.C. and MH62261 to H.S.F. and I.L.C.
Footnotes
Manuscript 13725-NP from the Scripps Research Institute.
REFERENCES
- 1.Akwa Y, Hassett D E, Eloranta M L, Sandberg K, Masliah E, Powell H, Whitton J L, Bloom F E, Campbell I L. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo A L, Sgadari C, Taub D D, Liao F, Farber J M, Maheshwari S, Kleinman H K, Reaman G H, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asensio V C, Campbell I L. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J Virol. 1997;71:7832–7840. doi: 10.1128/jvi.71.10.7832-7840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asensio V C, Campbell I L. Chemokines and their receptors in the CNS: directing cellular communication. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 6.Asensio V C, Kincaid C, Campbell I L. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J Neurovirol. 1999;5:65–75. doi: 10.3109/13550289909029747. [DOI] [PubMed] [Google Scholar]
- 7.Asensio V C, Lassmann S, Pagenstecher A, Steffensen S C, Henriksen S J, Campbell I L. C10 is a novel chemokine expressed in experimental inflammatory demyelinating disorders that promotes the recruitment of macrophages to the central nervous system. Am J Pathol. 1999;154:1181–1191. doi: 10.1016/S0002-9440(10)65370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badley J E, Bishop G A, St. John T, Frelinger J A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988;6:114–116. [PubMed] [Google Scholar]
- 9.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 10.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W, Sawyer L S, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 11.Charles P C, Chen X, Horwitz M S, Brosnan C F. Differential chemokine induction by the mouse adenovirus type-1 in the central nervous system susceptible and resistant strains of mice. J Neurovirol. 1999;5:55–64. doi: 10.3109/13550289909029746. [DOI] [PubMed] [Google Scholar]
- 12.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 13.Conant K, Garzino-Demo A, Nath A, McArthur J C, Halliday W, Power C, Gallo R C, Major E O. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Farber J M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci USA. 1990;87:5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol. 2000;6(Suppl. 1):S24–S32. [PubMed] [Google Scholar]
- 18.Glabinski A R, Ransohoff R M. Chemokines and chemokine receptors in CNS pathology. J Neurovirol. 1999;5:3–12. doi: 10.3109/13550289909029740. [DOI] [PubMed] [Google Scholar]
- 19.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman L M, Fife B T, Begolka W S, Miller S D, Karpus W J. Central nervous system chemokine expression during Theiler's virus-induced demyelinating disease. J Neurovirol. 1999;5:635–642. doi: 10.3109/13550289909021292. [DOI] [PubMed] [Google Scholar]
- 21.Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, Farber J M, Liao F, Liu L, Tosato G. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukocyte Biol. 1998;64:384–392. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- 22.Kelder W, McArthur J, Nance-Sproson C, T, McClernon D, Griffin D E. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 23.Khan I A, MacLean J A, Lee F S, Casciotti L, DeHaan E, Schwartzman J D, Luster A D. IP-10 is critical for effector T-cell trafficking and host survival in Toxoplasma gondii. Infect Immun. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 24.Kolb S A, Sporer B, Lahrtz F, Koedel U, Pfister H-W, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-γ inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 25.Krucker T, Toggas S M, Mucke L, Siggins G R. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- 26.Kutsch O, Oh J, Nath A, Benveniste E N. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahrtz F, Piali L, Spanaus K S, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. doi: 10.1016/s0165-5728(97)00267-1. [DOI] [PubMed] [Google Scholar]
- 28.Lane T E, Asensio V C, Yu N, Paoletti A, Campbell I L, Buchmeier M J. Dynamic regulation of α-and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- 29.Lane T E, Liu M T, Chen B P, Asensio V C, Samawi R M, Paoletti A D, Campbell I L, Kunkel S L, Fox H S, Buchmeier M J. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavi E, Kolson D L, Ulrich A M, Fu L, Gonzalez-Scarano F. Chemokine receptors in the human brain and their relationship to HIV infection. J Neurovirol. 1998;4:301–311. doi: 10.3109/13550289809114531. [DOI] [PubMed] [Google Scholar]
- 31.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O'Connor M, Hoxie J A, Gonzalez-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 32.Letendre S L, Lanier E R, McCutchan J A. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J Infect Dis. 1999;180:310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- 33.Lipton S A, Gendelman H E. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 34.Liu M T, Chen B P, Oertel P, Buchmeier M J, Armstrong D, Hamilton T A, Lane T E. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 35.Loetscher M, Gerber B, Loetscher P, Jones S A, Piali L, Clarklewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luster A D, Ravetch J V. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luster A D, Unkeless J C, Ravetch J V. γ-Interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 38.Mahalingam S, Farber J M, Karupiah G. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity in vivo. J Virol. 1999;73:1479–1491. doi: 10.1128/jvi.73.2.1479-1491.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majumder S, Zhou L Z, Chaturvedi P, Babcock G, Aras S, Ransohoff R M. p48/STAT-1α-containing complexes play a predominant role in induction of IFN-γ-inducible protein, 10 kDa (IP-10) by IFN-γ alone or in synergy with TNF-α. J Immunol. 1998;161:4736–4744. [PubMed] [Google Scholar]
- 40.Mennicken F, Maki R, de Souza E B, Quirion R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci. 1999;20:73–78. doi: 10.1016/s0165-6147(99)01308-5. [DOI] [PubMed] [Google Scholar]
- 41.Meraz M A, White J M, Sheehan K C F, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, Carver-Moore K, DuBois R N, Clark R, Aguet M, Schreiber R D. Targeted disruption of the StatI gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 42.Meyer M, Erdel M, Duba H C, Werner E R, Werner-Felmaye G. Cloning, genomic sequence, and chromosome mapping of Scyb11, the murine homologue of SCYB11 (alias βR1/H174/SCYB9B/I-TAC/IP-9/CXCL11) Cytogenet Cell Genet. 2000;88:278–282. doi: 10.1159/000015538. [DOI] [PubMed] [Google Scholar]
- 43.Miller R J, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- 44.Mucke L, Masliah E, Campbell I L. Transgenic models to assess the neuropathogenic potential of HIV-1 proteins and cytokines. Curr Top Microbiol Immunol. 1995;202:187–205. doi: 10.1007/978-3-642-79657-9_13. [DOI] [PubMed] [Google Scholar]
- 45.Ohmori Y, Hamilton T A. Cell type and stimulus specific regulation of chemokine gene expression. Biochem Biophys Res Commun. 1994;198:590–596. doi: 10.1006/bbrc.1994.1086. [DOI] [PubMed] [Google Scholar]
- 46.Ohmori Y, Hamilton T A. The interferon-stimulated response element and a κB site mediate synergistic induction of murine IP-10 gene transcription by IFN-γ and TNF-α. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 47.Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, Satoh M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci Lett. 1998;249:163–166. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- 48.Price R W. Neurological complications of HIV infection. Lancet. 1996;348:445–452. doi: 10.1016/S0140-6736(95)11035-6. [DOI] [PubMed] [Google Scholar]
- 49.Qin S, Rottman J B, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch A E, Moser B, Mackay C R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders V J, Pittman C A, White M G, Wang G, Wiley C A, Achim C L. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- 52.Sarris A H, Broxmeyer H E, Wirthmueller U, Karasavvas N, Cooper S, Lu L, Krueger J, Ravetch J V. Human interferon-inducible protein 10: expression and purification of recombinant protein demonstrate inhibition of early human hematopoietic progenitors. J Exp Med. 1993;178:1127–1132. doi: 10.1084/jem.178.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasseville V G, Smith M M, Mackay C R, Pauley D R, Mansfield K G, Ringler D J, Lackner A A. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- 54.Sauder C, Hallensleben W, Pagenstecher A, Schneckenburger S, Biro L, Pertlik D, Hausmann J, Suter M, Staeheli P. Chemokine gene expression in astrocytes of borna disease virus-infected rats and mice in the absence of inflammation. J Virol. 2000;74:9267–9280. doi: 10.1128/jvi.74.19.9267-9280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidtmayerova H, Nottet H, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes-implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shrikant P, Benos D J, Tang L P, Benveniste E N. HIV glycoprotein 120 enhances intercellular adhesion molecule-1 gene expression in glial cells. Involvement of Janus kinase/signal transducer and activator of transcription and protein kinase C signaling pathways. J Immunol. 1996;156:1307–1314. [PubMed] [Google Scholar]
- 57.Soto H, Wang W, Strieter R M, Copeland N G, Gilbert D J, Jenkins N A, Hedrick J, Zlotnik A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci USA. 1998;95:8205–8210. doi: 10.1073/pnas.95.14.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spanaus K S, Nadal D, Pfister H W, Seebach J, Widmer U, Frei K, Gloor S, Fontana A. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J Immunol. 1997;158:1956–1964. [PubMed] [Google Scholar]
- 59.Sprenger H, Rosler A, Tonn P, Braune H J, Huffmann G, Gemsa D. Chemokines in the cerebrospinal fluid patients with meningitis. Clin Immunol Immunopathol. 1996;80:155–161. doi: 10.1006/clin.1996.0109. [DOI] [PubMed] [Google Scholar]
- 60.Stalder A K, Pagenstecher A, Kincaid C L, Campbell I L. Analysis of gene expression by multiprobe RNase protection assay. In: Harry J, Tilson H A, editors. Neurodegeneration methods and protocols. Totowa, N.J: Humana Press Inc.; 1999. pp. 53–66. [DOI] [PubMed] [Google Scholar]
- 61.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 62.Strieter R M, Kunkel S L, Arenberg D A, Burdick M D, Polverini P J. Interferon γ-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 63.Tanabe S, Heesen M, Berman M A, Fischer M B, Luo Y, Dorf M E. Murine astrocytes express a functional chemokine receptor. J Neurosci. 1997;17:6522–6528. doi: 10.1523/JNEUROSCI.17-17-06522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka H, Samuel C E. Sequence of the murine interferon-inducible RNA-dependent protein kinase (PKR) deduced from genomic clones. Gene. 1995;153:283–284. doi: 10.1016/0378-1119(94)00821-9. [DOI] [PubMed] [Google Scholar]
- 65.Taub D D, Lloyd A R, Conlon K, Wang J M, Ortaldo J R, Harada A, Matsushima K, Kelvin D J, Oppenheim J J. Recombinant human-interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toggas S M, Masliah E, Rockenstein E M, Rall G F, Abraham C R, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 67.Vallat A V, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T W, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 68.Vanguri P, Farber J M. Identification of CRG-2. An interferon-inducible mRNA predicted to encode a murine monokine. J Biol Chem. 1990;265:15049–15057. [PubMed] [Google Scholar]
- 69.Westmoreland S V, Rottman J B, Williams K C, Lackner A A, Sasseville V G. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am J Pathol. 1998;152:659–665. [PMC free article] [PubMed] [Google Scholar]
- 70.Wyss-Coray T, Masliah E, Toggas S M, Rockenstein E M, Brooker M J, Lee H S, Mucke L. Dysregulation of signal transduction pathways as a potential mechanism of nervous system alterations in HIV-1 gp120 transgenic mice and humans with HIV-1 encephalitis. J Clin Investig. 1996;97:789–798. doi: 10.1172/JCI118478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu N, Martin J L, Stella N, Magistretti P J. Arachidonic acid stimulates glucose uptake in cerebral cortical astrocytes. Proc Natl Acad Sci USA. 1993;90:4042–4046. doi: 10.1073/pnas.90.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]